1. Background
Acute diarrhea is the second leading cause of morbidity in children, particularly in developing countries (1). It has been reported that 15 % of children under the age of 5 years die from diarrhea (2). Although the World Health Organization (WHO) does not recommend probiotics, they are increasingly being used to treat acute diarrhea in some countries (3). Previous research has demonstrated the efficacy of probiotics in treating acute diarrhea (4). Various types of probiotics have been utilized in previously published studies. In the current study, a combination of Lactobacillus rhamnosus, Lactobacillus reuteri, Bifidobacterium infantis bacteria, and Fructooligosaccharides (FOS) was administered to treat acute diarrhea. This probiotic mixture is available in the market in our country, prompting our decision to evaluate this combination in our study. However, the efficacy of single strains versus multi-strain formulations has been reported in some studies (5).
2. Objectives
Regarding the appropriate effect of probiotics in treating acute diarrhea and the high prevalence of non-bacterial acute diarrhea among children in the population, the present study is conducted to evaluate the effect of synbiotics in treating acute diarrhea in children.
3. Methods
3.1. Study Design
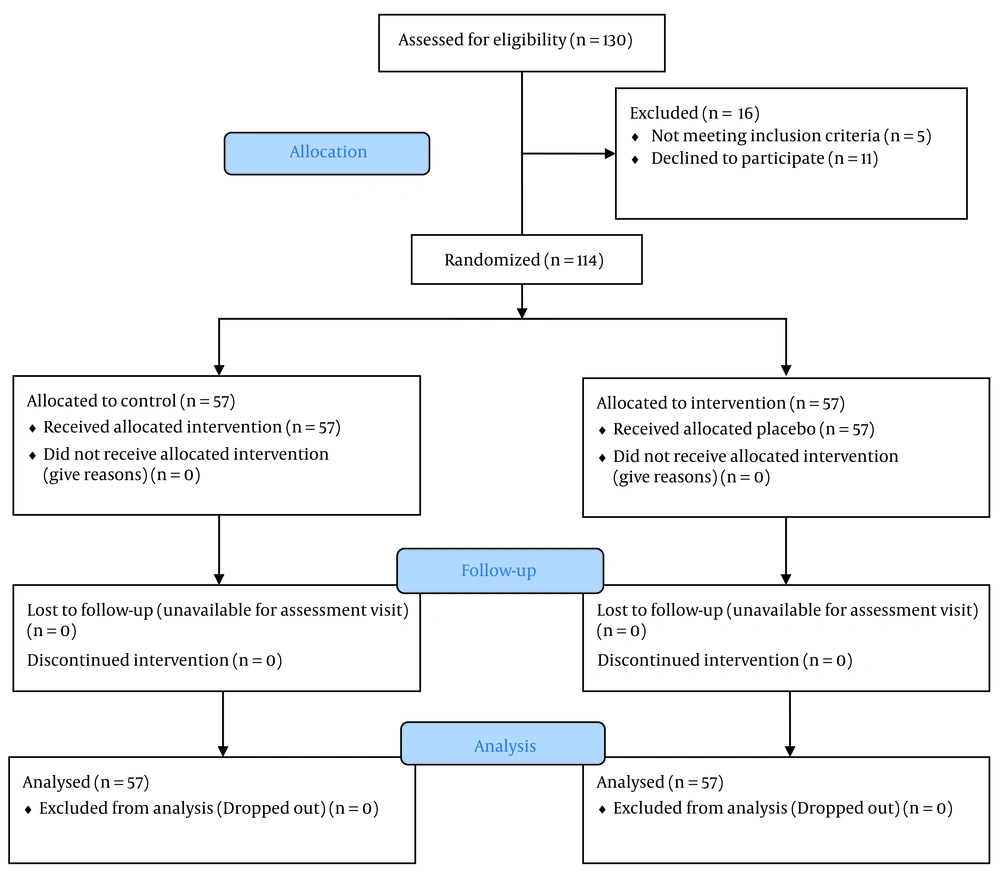
This was a prospective double-blinded and randomized-controlled clinical trial on children with diarrhea referring to the children’s medical center of the university. All data relating to diarrhea patterns and descriptions were collected in person in the presence of the interviewer and family in the clinical environment. All the mentioned subjects of the study were identified with especial aims after total collection and subject definition. The study flowchart is seen in Figure 1.
This prospective double-blinded, randomized controlled clinical trial was conducted using the quadrimodal blocks method. The patients were assigned to two groups: the treatment group receiving synbiotics and the control group receiving a placebo. The study was double-blinded, meaning both the researcher and the patients were unaware of the study group assignments. After selecting patients for inclusion in the study, they were randomly allocated to one of the two groups using quadrimodal blocks. Neither the researchers nor the patients were informed about the group assignments.
3.2. Blinding
None of the patients (or their parents) and evaluators were provided information about the drug group. To ensure blinding, all necessary medications for five days of consumption were provided in identical packages. The medications were administered only after randomization, and the groups were designated with letters A or B. Group A received PediLact drops (5 drops daily) as the synbiotic source, while group B received maltodextrin as a placebo. Both were presented in vials of similar shapes and sizes. PediLact drops contained Lactobacillus rhamnosus, Lactobacillus reuteri, and Bifidobacterium infantis with 109 CFU. Instructions for consumption were provided to the patients in written form, and a physician who was unaware of the drug group explained them if necessary.
In the case group, patients consumed synbiotic PediLact drops, including Lactobacillus rhamnosus, Lactobacillus reuteri, Bifidobacterium infantis with 109 CFU, and FOS, mixed with milk or lukewarm food for five days, in addition to receiving routine hospital care. Patients in the placebo group (receiving maltodextrin) received routine hospital care. The process of preparing the placebo and implementing double-blinding was conducted by a pharmacologist as a collaborator.
The evaluated parameters for each patient included comparison of the number of hospitalization days, daily excreted diarrhea, duration of diarrhea, and reduction in the number of bowel movements between patients receiving synbiotics and those in the control group.
During hospitalization, the researcher visited the patients daily and monitored their condition for two weeks after the end of the study. If any discrepancies in the objectives of the clinical trial were identified, efforts were made to revisit the patients to complete the data. If this was not feasible, the patient was excluded from the study. Patients were assigned to two groups based on random quadrimodal permutation, and both the patient and the physician, as well as the supporting medical staff, were blinded to the group assignments.
3.3. Inclusion Criteria
Breastfeeding and supplementary feeding with non-dairy products are recommended for children between the ages of 6 and 24 months who are experiencing non-bloody acute diarrhea lasting less than two days. Additionally, the children should exhibit mild to moderate dehydration and should not have taken any antibiotics in the past two weeks.
3.4. Exclusion Criteria
Failure to thrive, severe dehydration, antibiotic consumption during recent 2 weeks, severe vomiting, presence of inflammatory cells in a stool sample, history of different types of endocrine and metabolic disorders (diabetes, obesity, thyroid), history of blood diseases, and anemia, history of different types of gastrointestinal diseases (gastric ulcers, chronic diarrheas), formula-fed babies and previous probiotic or prebiotic supplement were excluded. The primary outcome measurement was the duration of hospitalization. The secondary outcome measurement was a pharmaceutical allergy.
3.5. Statistical Analysis
Data analysis will employ descriptive statistical methods, including frequency tables, charts, and numerical indices, to describe the intended variables. The correlation between variables will be assessed using a chi-square statistical test and an independent sample T-test, with regression methods utilized if necessary. A significance level of P < 0.05 will be applied. The data analysis will be conducted using SPSS software version 22.
4. Results
4.1. Demographic Characteristics
In the present study, 114 patients were randomly categorized into two groups: one receiving placebo and the other synbiotic. The synbiotic-fed group included 24 boys (44.44 %) and 33 girls (55.56 %), while the placebo-fed group included 22 boys (38.60 %) and 35 girls (61.4 0%). No significant difference was observed between the two groups in terms of gender (P > 0.05). Moreover, the average age of patients in the synbiotic-fed group was 12.59 ± 1.49 months, and in the placebo-fed group, it was 11.60 ± 0.86 months, with no significant difference between the two groups in this aspect (P > 0.05).
4.2. Birth Weight and Weight Alterations
The results indicated that there were no significant differences in birth weight, weight at the beginning of hospitalization, and weight at discharge between both groups of patients (P > 0.05; Table 1).
| Parameter | Synbiotic-fed Group (n = 57) | Placebo-fed Group (n = 57) | P-Value |
|---|---|---|---|
| Residence | Urban = 48, Rural = 9 | Urban = 49, Rural = 8 | |
| Birth weight (g) | 3076 ± 73.31 | 3036 ± 94.59 | 0.73 |
| Weight at the beginning of hospitalization (g) | 8899 ± 303.1 | 8536 ± 242.8 | 0.35 |
| Weight at discharge (gr) | 8929 ± 281.2 | 8413 ± 240.2 | 0.16 |
Comparison of Weight at Various Times in Both Studied Groups a
4.3. Dehydration and Diarrhea Status
The results indicated that the mean hospitalization duration, dehydration percentage, and time between diarrhea onset and recovery were significantly higher in the placebo group compared to the synbiotic group (P < 0.05). Conversely, the rate of decrease in the number of diarrhea episodes was significantly higher in the synbiotic group compared to the placebo group (P < 0.05; see Table 2).
| Parameter | Synbiotic Group | Placebo Group | P-Value |
|---|---|---|---|
| Hospitalization duration (days) | 3.87 ± 0.9 | 4.26 ± 0.12 b | 0.001 |
| The time between diarrhea to recovery (days) | 5.74 ± 0.33 | 6.43 ± 0.36 b | 0.032 |
| Number of defecations | 1.89 ± 0.13 | 2.52 ± 0.18 b | 0.014 |
| Frequency of number of evacuations in 24 hours | |||
| 2 – 3 | 1 | 0.73 | 0.75 |
| 4 – 5 | 10 | 0.35 | 0.013 |
| 6 – 8 | 17 | 9 | 0.54 |
| > 8 | 30 | 39 | 0.68 |
Dehydration Status, Recovery Time, and Other Treatment-Related Criteria in Both Evaluated Groups a
5. Discussion
Recent estimates indicate that diarrheal mortalities have decreased to 2.5 million individuals annually. However, despite these recent findings, diarrhea remains the primary cause of death among children under five years old (15 %).
Current strategies for controlling diarrhea include preventing and managing dehydration through oral or intravenous fluids, as well as sustaining breastfeeding for infants. However, in recent years, there has been increasing recognition of the hygiene benefits of probiotics, supported by scientific research. Currently, strong evidence exists for their use in preventing and treating certain human diseases. Several studies have demonstrated the preventive effects of certain probiotics in reducing gastrointestinal complications. Therefore, the present study aims to evaluate the effect of a synbiotic containing probiotics, specifically L. rhamnosus, L. reuteri, Bifidobacterium infantis bacteria, and FOS, on acute diarrhea in children.
The results of the present study showed that the mean dehydration percentage and the time between diarrhea and recovery were significantly lower in the placebo group compared to the synbiotic group (P < 0.05). On the other hand, the decreasing rate of diarrhea frequency was significantly higher in the synbiotic group compared to the placebo group (P < 0.05). However, there was no significant difference between the two groups for hospitalization duration, weight at the beginning of hospitalization, and weight at discharge (P > 0.05). Various studies have yielded contradictory results regarding the application of probiotic compounds in the treatment of childhood diarrhea, as some have reported positive effects of these compounds, while others have stated that these compounds have no positive effect on the process of childhood diarrhea.
In several studies, L. rhamnosus was recommended to treat acute gastroenteritis (6, 7).
Canani et al. observed that the consumption of L. rhamnosus GG could decrease the daily frequency of bowel movements and the duration of diarrhea in children compared to the control group (8).
Unlike the above studies, in which the positive effects of probiotic consumption were mentioned, some studies have shown that these compounds have almost no positive effect in treating diarrhea. Costa-Ribeiro et al. examined the effect of L. rhamnosus GG in their study in Brazil involving 124 male infants (1 to 24 months) hospitalized with moderate to severe diarrhea (9). They found no significant decrease in diarrhea duration or the amount of bowel movements in children receiving L. rhamnosus GG compared to the control group (9).
In a meta-analysis by Szajewska et al., published in 2007, L. rhamnosus GG was found to have moderate clinical benefit in the treatment of acute diarrhea (10). Several review studies have assessed the effect of probiotics on diarrhea. Szajewska and Mrukowicz further evaluated acute diarrhea lasting more than three days in 731 infants and children in a systematic review of 8 randomized controlled studies (11). This analysis showed that diarrhea lasting 3 days or more in the group receiving L. rhamonosus GG probiotic was decreased by 40 % compared to the control group. In a recently published systematic review by Szajewska et al., they found that L. rhamnosus reduced diarrhea duration (with a greater impact in European countries) and hospitalization (12). However, in the study by Schnadower et al., the administration of L. rhamnosus GG had no effect on the outcome of children with gastroenteritis (13).
Probiotics also significantly decreased the duration of diarrhea compared to the control group. The meta-analysis by Van Niel et al. evaluated randomized studies of seven species of lactobacillus in 675 children (14). Probiotics reduced diarrhea duration to 0.7 days and decreased the frequency of diarrhea from 2 days to 6.1 excretions (14).
In the recent study by Szymanski and Szajewska, L. reuteri DSM17938 showed no significant efficacy in treating acute gastroenteritis (15). However, in the study by Francavilla et al., L. reuteri DSM17938 exhibited significant efficacy in reducing the duration, frequency, and recrudescence rate of acute gastroenteritis (16). In the recent systematic review, the use of L. reuteri was associated with a reduced duration of diarrhea and hospitalization (17). Bifidobacterium infantis may reduce diarrhea among healthy infants (18).
Fructooligosaccharides also has a beneficial effect in the treatment of rotavirus-induced diarrhea (19). The study by Vandenplas et al. on a synbiotic supplement containing FOS and probiotic strains of Streptococcus thermophilus, L. rhamnosus, L. acidophilus, Bifidobacterium lactis, and Bifidobacterium infantis resulted in earlier normalization of stool consistency in acute infectious diarrhea (20). More recently, a meta-analysis showed a potential beneficial role of probiotics in the treatment of acute diarrhea in children (21). In another study, multi-strain probiotics improved the efficacy of standard diarrhea treatment regardless of etiology (22). Additionally, a mixture of Streptococcus thermophilus, L. rhamnosus, L. acidophilus, Bifidobacterium lactis, Bifidobacterium infantis, and FOS normalized stool consistency more rapidly than placebo (23). However, in the recent study, a combination of L. rhamnosus-L. helveticus probiotic did not prevent the development of moderate-to-severe gastroenteritis in children (24).
5.1. Conclusions
The results of the present study showed that the concurrent application of synbiotic drops, including Lactobacillus reuteri, Lactobacillus rhamnosus, Bifidobacterium infantis, and FOS in the treatment of non-infectious diarrhea in children aged 6 - 24 months is effective in reducing the time of recovery, number of evacuations, and duration of hospitalization. However, these results are not definitive, and further studies with larger sample sizes are needed to determine the efficacy, optimal dosage, time required for effectiveness, and mechanism of action of various probiotic species and strains. Additionally, the strains and effective treatment doses for each disease should be specified to utilize probiotics as adjunct treatment alongside conventional diarrhea treatments in children.
5.2. Limitations of the Study
Include single-center study and follow-up of the cases with a limited sample size.