1. Background
Premature birth stands as a significant cause of infant and child mortality, accounting for the majority of deaths in the neonatal period and being the second leading cause of death in children under five years of age (1). It is also associated with a spectrum of disabilities, including learning and motor disabilities as well as vision and hearing impairments, contributing to nearly half of all childhood disabilities. While the incidence of preterm birth has seen a decrease in the United States over the last decade, the global rate has been on the rise (1-3). A prematurely born infant faces additional challenges, including an increased risk of hemodynamic disorders. The myocardium of a premature infant exhibits an inefficient contractile mechanism, leading to systolic and diastolic dysfunction (4, 5). Moreover, the incidence of respiratory distress syndrome (RDS) and, as a consequence, bronchopulmonary dysplasia (BPD) is significantly higher among premature infants. Most very low birth-weight premature infants require some form of respiratory support early in life due to various reasons, including premature respiratory failure, apnea of prematurity, RDS, transmission disorders, and persistent pulmonary hypertension (6).
Apnea of prematurity is a prevalent condition characterized by a failure in the respiratory control system and unstable respiratory movements in premature infants. Repeated episodes of apnea can lead to respiratory failure, pulmonary hemorrhage, abnormal cardiac and pulmonary function, intracranial hemorrhage, impaired nervous system development, and sudden death (7). Thus, early and effective clinical intervention can significantly reduce infant disability and mortality rates (8). Caffeine, as a methylxanthine, exerts stimulatory effects on the respiratory system and is the standard pharmacological treatment for apnea in premature infants. The standard initial and maintenance doses of caffeine citrate are 20 mg/kg and 5–10 mg/kg per day, respectively (9, 10). The therapeutic plasma concentration of caffeine ranges between 3 to 84 mg/L. Caffeine's protective effects on the brain and lungs are its primary benefits, with few side effects for premature infants. It enhances the strength of respiratory muscles and diaphragm activity, and it is associated with reduced incidence of BPD, as well as decreased duration of need for continuous positive airway pressure and mechanical ventilation in premature infants (9). Caffeine (1,3,7-trimethyl xanthine), (C8H10N4O2), a plant alkaloid, structurally resembles adenosine and acts as a competitive antagonist to G protein-coupled adenosine receptors, specifically A1 and A2a receptors, at micromolar concentrations. Approximately 10 - 30% of caffeine binds reversibly to plasma proteins. Caffeine is widely consumed by children and adults globally. Its effects on humans are varied, including changes in sleep and mood, alterations in cardiovascular function, arrhythmia, increased blood pressure, and catecholamine levels, stimulation of gastric acid production, adverse effects on the female reproductive system, increased caloric intake leading to obesity, and increased urine production and enuresis (11). The efficacy of high doses of caffeine is controversial. It is crucial to continue discussions on the need for a therapeutic maintenance dose of caffeine to determine the optimal dose and address safety concerns regarding increased doses. Previous research highlights the heritability of apnea of prematurity and its occurrence, spurring interest in exploring the influence of genetic factors on the condition and the effectiveness of caffeine therapy. The success of caffeine treatment depends on the body's processing of caffeine, known as pharmacokinetics and genetic factors (12). Previous studies have investigated the effects of caffeine, but given the influence of genetic and environmental factors on drug pharmacokinetics, there is a need for this study in the target population.
2. Objectives
The objective of this study was to assess the effectiveness and side effects of maintenance doses of caffeine citrate in aiding the removal of tracheal tubes and in decreasing the frequency of subsequent apnea episodes in premature infants admitted to neonatal intensive care units. The research aimed to evaluate the impact of varying maintenance doses of caffeine citrate on both the ease of tracheal tube removal in premature infants and the incidence of apnea following tube removal. This comparison may assist healthcare professionals in identifying the most suitable maintenance dose of caffeine citrate for the care of premature infants in neonatal intensive care settings.
3. Methods
3.1. Study Design and Population
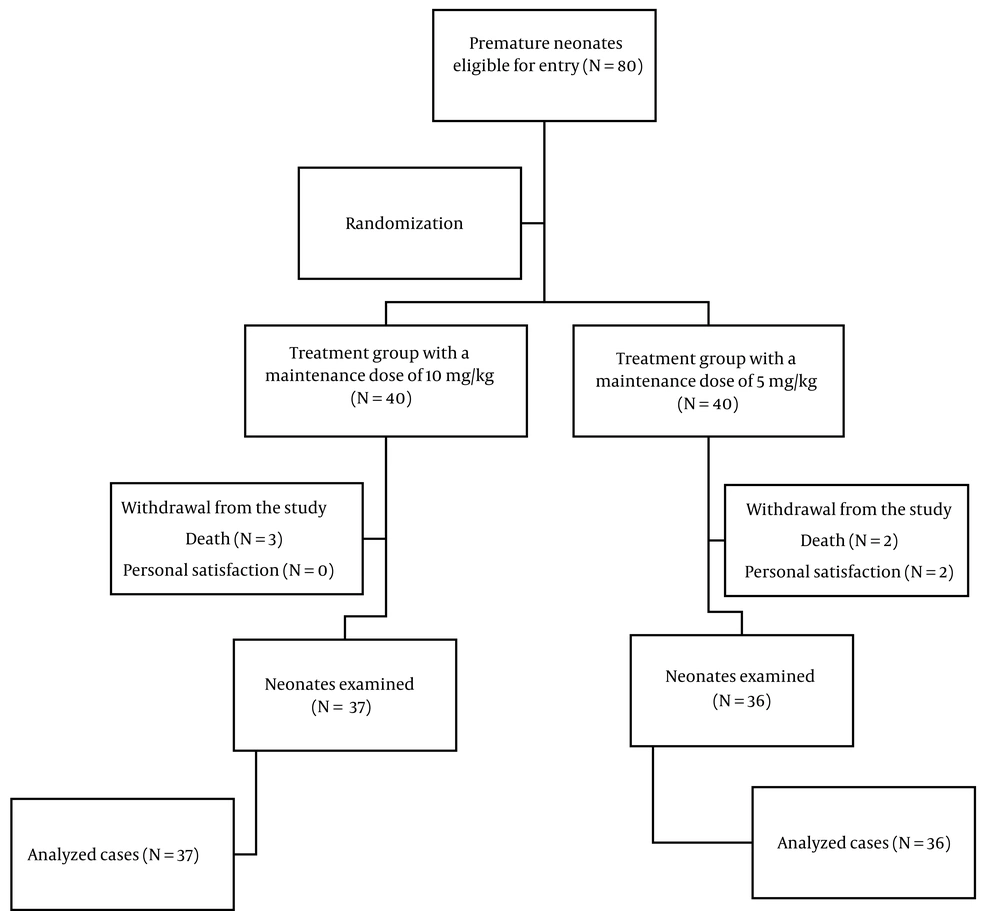
This study was a clinical trial with parallel groups, randomized into two groups, involving 80 neonates (as detailed in section 3.2). The study was initiated with approval from the Ethics Committee of Kerman University of Medical Sciences under code IR.KMU.AH.REC.1401.247, and registration at the Clinical Trial Registration Center of Iran with trial ID 69550 and code IRCT20230408057850N1. It was conducted on neonates hospitalized in the NICU of Afzalipur Hospital in Kerman, Iran, in 2023.
3.1.1. Inclusion Criteria
An available sampling method was employed. Based on the random allocation rule, 80 neonates who met the study's entry criteria were selected. Then, the randomization was carried out using the rand function in Microsoft Office Excel® (2019) software, dividing the neonates into two groups. Included were neonates with a gestational age of less than 35 weeks and who demonstrated hemodynamic stability within the first 48 hours after receiving mechanical ventilation. A thorough examination of the neonates was conducted by a doctor.
3.1.2. Exclusion Criteria
Infants diagnosed with congenital gastrointestinal obstruction and perforation, gastroschisis, omphalocele, congenital diaphragmatic hernia, cyanotic heart diseases, and other congenital anomalies were excluded from the study. During the study, five infants (6.25%) succumbed to their conditions (three due to sepsis and two due to other causes), and two infants (2.5%) were discharged and voluntarily withdrew from the study, resulting in the completion of the study with 73 infants (Figure 1).
The first group comprised neonates who received caffeine citrate with an initial dose of 20 mg/kg and a maintenance dose of 5 mg/kg until the infant exhibited no apnea for at least 7 days following the removal of the tracheal tube (the low maintenance dose group). The second group included neonates who were administered caffeine citrate with an initial dose of 20 mg/kg and a maintenance dose of 10 mg/kg until the infant showed no signs of apnea for at least 7 days post tracheal tube removal (the high maintenance dose group). Caffeine treatment commenced 48 hours post-intubation, and once hemodynamic stability was achieved. The caffeine citrate used in this study, supplied in ampoules (caffeine (as citrate) Cemidarou® 30 mg / 3 ml Amp), was manufactured by the Cemidarou Pharmaceutical Company of Iran.
3.2. Sample Size Calculation
The significance level (α) was set at 0.05. Based on a prior study (13) and considering the equation and a potential 15% sample dropout rate during the study, the sample size for each group was determined to be 40 neonates.
With correction n = 40.
Where, δ = 0.8, α = 0.05, β = 0.1, Z (1 - α/2) = 1.96, Z (1 - β) =1.28, and Z (1-β) = 1.28.
3.3. Data Collection
Variables such as gestational age, sex, weight, and Apgar scores at the first and fifth minutes after birth were examined in both groups. The primary outcomes investigated included the removal of tracheal tubes and the reduction in the incidence of subsequent apnea. Secondary outcomes such as patent ductus arteriosus (PDA), necrotizing enterocolitis (NEC), BPD, feeding intolerance, and tachycardia were also evaluated in both groups. Neonates were closely monitored and examined until they were discharged from the hospital in good condition. The diagnostic criteria for BPD were based on the 2001 criteria of the US National Institutes of Health, distinguishing it as either present or absent. The presence of NEC was determined through a combination of clinical, laboratory, and radiological findings, categorized as either present or absent. Initial clinical symptoms included mild changes in vital signs, new or increased apnea attacks, feeding intolerance, increased gastric residual and nausea, progressing to hypotonia, respiratory distress, and cardiovascular instability. Abdominal examinations revealed distension, sensitivity to touch, discoloration of the abdominal wall, and absence of bowel sounds. Laboratory findings indicated neutropenia, thrombocytopenia, and metabolic acidosis, while radiological signs showed air in the intestinal wall, rupture of the abdominal viscera with free air in the abdomen, and air in the portal vein. The diagnosis of PDA was based on color Doppler echocardiography, showing the anatomical openness, direction, and velocity of blood flow through the duct during the cardiac cycle. Tachycardia in infants was defined as a sinus heart rate exceeding 160 beats per minute, classified as present or absent.
3.4. Statistical Analysis
Data were analyzed using IBM SPSS Statistics 26® software. The Shapiro-Wilk test was applied to assess the normality of the data. Given the data's non-normality, non-parametric chi-square tests (Fisher's exact difference test and Pearson's chi-square test) were utilized to compare means, and the chi-Square test was employed to examine qualitative variables and relationships (P-value < 0.05).
4. Results
As shown in Table 1 the study analyzed 39 male infants (53.4%) and 34 female infants (46.6%). According to Fisher's exact test, the distribution of gender across the two study groups was similar, with no statistically significant difference observed (P-value = 0.484). The average weight of the infants in this study was 1715 ± 465 g. Pearson's chi-square test revealed no statistically significant difference in the distribution of infants based on birth weight (P-value = 0.935) or the gestational age of their mothers (P-value = 0.504) between the two groups. The Apgar scores at the first minute for the two groups were 7.72 ± 1.42 and 7.10 ± 1.85, respectively, while at the fifth minute, they were 8.89 ± 0.91 and 8.37 ± 1.9, respectively; no significant difference was noted between the groups (P-value = 0.069 and P-value = 0.358, respectively).
| Variable | Treatment Group with a Dose of 5 mg/kg | Treatment Group with a Dose of 10 mg/kg | Total | P-Value |
|---|---|---|---|---|
| Gender | 0.484 | |||
| Male | 21 (58.3) | 18 (51.4) | 39 (53.4) | |
| Female | 15 (41.7) | 19 (48.6) | 34 (46.6) | |
| Gestational age, week | 0.504 | |||
| 28 - 30 | 14 (38.9) | 10 (27.0) | 24 (32.9) | |
| 30 - 32 | 6 (16.7) | 9 (24.4) | 15 (20.5) | |
| 32 - 34 | 16 (44.4) | 18 (48.6) | 34 (46.6) | |
| Baby's weight, g | 0.935 | |||
| < 1500 | 17 (47.2) | 16 (43.2) | 33 (45.2) | |
| 1500 - 2500 | 16 (44.4) | 18 (48.6) | 34 (46.6) | |
| > 2500 | 3 (8.4) | 3 (8.2) | 6 (8.2) | |
| Apgar first minute | 7.72 ± 1.42 | 7.10 ± 1.85 | 0.069 | |
| Apgar fifth minute | 8.89 ± 0.91 | 8.37 ± 1.90 | 0.358 |
a Values are expressed as mean ± SD or No. (%).
Given the non-normal distribution of the data, non-parametric chi-square tests were used to compare the frequencies of quantitative outcomes. As indicated in Table 2, there was no significant difference in the duration of hospitalization between the group receiving a maintenance dose of 5 mg/kg of caffeine citrate and the group receiving 10 mg/kg (P-value = 0.795), with durations of 24.67 ± 11.94 and 24.97 ± 14.99 days in the NICU, respectively. Similarly, the time to disconnection from the ventilator showed no significant difference between the two groups (P-value = 0.660).
| Outcome | Treatment Group with a Dose of 10 mg/kg | Treatment Group with a Dose of 5 mg/kg | Test Statistics | P-Value |
|---|---|---|---|---|
| Duration of hospitalization, day | 24.67 ± 11.94 | 24.97 ± 14.99 | -0.260 | 0.795 |
| Duration of disconnection from the ventilator, day | 6.56 ± 7.15 | 5.16 ± 4.81 | -0.440 | 0.660 |
a Values are expressed as mean ± SD.
Non-parametric chi-square tests were also applied to compare the frequencies of qualitative outcomes due to the non-normal distribution of the data. As demonstrated in Table 3, the occurrence of tachycardia was 2 (5.6%) in the first group and 9 (24.3%) in the second group, statistically indicating a higher incidence of tachycardia in the high-dose treatment group (P-value = 0.026). The frequency of oral feeding intolerance was identical in both groups (12 cases; P-value = 1.000). Two infants in the first group and one in the second group had PDA, showing no statistically significant difference (P-value = 0.615). The occurrence of NEC was 5 and 6 in the respective groups, with no significant difference (P-value = 1.000). Apnea of prematurity was observed in 8 and 7 infants of each group, respectively, and 9 infants in each group were diagnosed with BPD, indicating no significant difference between the groups (P-value = 1.000 and P-value = 0.778).
| Outcome | Treatment Group with a Dose of 5 mg/kg | Treatment Group with a Dose of 10 mg/kg | Total | P-Value |
|---|---|---|---|---|
| Tachycardia | 2 (5.6) | 9 (24.3) | 11 (15.1) | 0.026 |
| Oral feeding intolerance | 12 (33.3) | 12 (32.4) | 24 (32.9) | 1.000 |
| Patent ductus arteriosus | 2 (5.6) | 1 (2.7) | 3 (4.1) | 0.615 |
| Necrotizing enterocolitis | 5 (13.9) | 6 (16.2) | 11 (15.1) | 1.000 |
| Bronchopulmonary dysplasia | 9 (25.0) | 9 (24.3) | 18 (24.7) | 1.000 |
| Apnea of prematurity | 8 (22.2) | 7 (18.9) | 15 (20.5) | 0.778 |
a Values are expressed as No. (%).
5. Discussion
This study initially included 80 neonates, but after accounting for attrition, it focused on 39 male infants (53.4%) and 34 female infants (46.6%). The findings indicate no significant difference in efficacy between the maintenance doses of 10 mg/kg and 5 mg/kg of caffeine. However, the group receiving the higher maintenance dose of 10 mg/kg exhibited a higher incidence of tachycardia, highlighting it as a potential side effect of caffeine.
Methylxanthines such as theophylline, aminophylline, and caffeine are primary treatments for apnea of prematurity (14), with caffeine recognized for its superior therapeutic benefits (14, 15), fewer side effects, and longer half-life compared to other methylxanthines. It is notably effective in infants unresponsive to theophylline. Since its introduction in developed countries in 1970, caffeine citrate has been a preferred treatment for apnea of prematurity (15-17). Its adoption in China occurred in 2013, gradually favoring it over aminophylline. Despite confirmed therapeutic effects in multiple studies (18, 19), selecting an appropriate maintenance dose for treating apnea in preterm infants remains a challenge due to their unique physiological characteristics, potential renal failure, and organ underdevelopment, particularly the liver (12, 20, 21).
In this study, gender distribution across the two groups showed no significant difference, as confirmed by Fisher's exact test. The average weight of the infants was 1715 ± 465 g, with Pearson's chi-square test indicating no significant disparity in birth weight distribution or gestational age between the two groups. Furthermore, the study found no significant difference in hospitalization duration between the groups receiving 5 mg/kg and 10 mg/kg maintenance doses of caffeine citrate. Similarly, no statistical difference was observed in the duration of mechanical ventilation between both groups. Contrary to our findings, a study by Steer et al., which included 234 premature infants (gestational age less than 30 weeks) and compared high-dose caffeine citrate (initial 80 mg/kg and maintenance 20 mg/kg per day) with a low dose (initial 20 mg/kg and maintenance 5 mg/kg per day), found that high-dose caffeine significantly improved the success rate of tracheal extubation and reduced both the number of apneas and the duration of respiratory support (22). This discrepancy could be attributed to the differences in the dosage levels used in Steer's study compared to the lower, more standard doses employed in our study (initial 20 mg/kg and maintenance 5 and 10 mg/kg per day).
Evidence from retrospective studies and meta-analyses suggests that the administration of caffeine earlier and at higher doses may significantly benefit lung health. The advantages of administering caffeine early in cases of BPD could be linked to the distinct physiology of fetuses and preterm infants. Caffeine's ability to diminish the need for medical intervention for PDA implies that it can positively influence PDA hemodynamics and closure by stabilizing systemic blood pressure, potentially offering significant benefits to the cerebral circulation. In the study by Patel et al., 140 infants with a birth weight under 1250 g were divided into two groups: Those receiving caffeine early (before the third day of birth) and those receiving it late (after the third day of birth). This study demonstrated a more pronounced effect of early caffeine initiation on reducing BPD among the groups. However, the study did not specify the caffeine dosage used (23). In our research, the incidence of BPD between the two examined groups showed no significant difference, which could be attributed to variations in the administered dose of caffeine citrate.
In the research conducted by Taha et al., infants weighing less than 1250 g and treated with caffeine within the first 10 days of life were assessed. Comparisons were made between infants who started receiving caffeine from birth to two days old and those who began receiving caffeine later (3 - 10 days old). Early caffeine administration was associated with a reduced occurrence of BPD and improved other respiratory outcomes. Additionally, the prevalence of severe intraventricular hemorrhage, PDA, and the duration of hospitalization were lower in infants treated early with caffeine. Nonetheless, early caffeine use was linked to a higher risk of NEC. The study by Taha et al. did not specify the caffeine dose administered to the two groups, and the variance in findings compared to our study could result from differences in the received caffeine citrate doses (24).
Previous studies have reported that high doses of caffeine can lead to tachycardia, heart palpitations, and a swift increase in blood pressure in adults, likely due to its effect on and activation of the sympathetic system. In the research by Ulanovsky et al., 21 premature infants were analyzed. These infants received caffeine citrate with a loading dose of 15 - 20 mg/kg and a maintenance dose of 5 - 10 mg/kg, and the study examined caffeine's effects on their cardiac system. no changes in heart rate, blood pressure, or autonomic nervous system tone were observed following caffeine administration. The discrepancies between the findings of this study and our current research might stem from the small sample size used by Ulanovsky et al. and the fact that infants received varying doses of caffeine without an examination of its effects, focusing solely on individual pre- and post-caffeine administration comparisons (25).
A total of 13 clinical studies involving 1515 patients were reviewed. In the high-dose group, a higher success rate for tracheal tube removal and a lower rate of BPD were observed. However, the incidence of tachycardia was elevated in this group, with no significant differences in other side effects, including patient mortality (20). The findings of the current study also indicated an increased occurrence of tachycardia in the group receiving a maintenance dose of 10 mg/kg. The effects of varying caffeine doses and the duration of its use on lung function have shown mixed results, with some benefits and harms only studied in animal research. These studies have yet to clarify the primary mechanism by which caffeine affects lung function. Nonetheless, its action on adenosine receptors at low doses and phosphodiesterase inhibition at higher doses, coupled with the duration of administration and the distinct pharmacodynamics of caffeine in infants, could contribute to understanding its clinical lung effects.
5.1. Conclusions
The findings suggest that tachycardia was more common in the group administered a maintenance dose of 10 mg/kg. However, no significant difference in the effectiveness of the two doses was observed. Thus, the results recommend considering a lower dose of 5 mg/kg of caffeine as a potentially more suitable treatment option. This insight is crucial for healthcare professionals when determining caffeine dosage for patients at risk of tachycardia. Due to time and financial constraints, the long-term side effects of caffeine citrate, including its impact on neurological, behavioral, and developmental outcomes, were not explored. It is recommended that future research include a prospective study to investigate the long-term effects of caffeine citrate.