1. Background
Sodium arsenite (NaAsO2) is a common mineral contaminant in drinking water in numerous parts of the world. Abundant scientific evidence establishes a strong correlation between arsenic exposure and kidney-related ailments. The World Health Organization has set a permissible limit for arsenic concentration, capping it at 10 parts per billion (ppb) (1, 2). Studies indicate that millions of people worldwide are exposed to high arsenic concentrations through drinking water (3, 4). Available literature suggests that both acute and chronic arsenic exposure are strongly linked to various diseases, including kidney problems (5, 6). The precise molecular mechanism of arsenic's nephrotoxicity remains incompletely understood. Several reports have described the relationship between arsenic metabolism and its adverse health effects. Additionally, some studies have indicated that methylated arsenic are less toxic and more easily excreted through urine. However, these studies have also supported the role of oxidative stress and inflammation in tissue damage caused by arsenic exposure (7). The primary hypothesis proposed to understand NaAsO2 toxicity revolves around oxidative stress induced by arsenic (8). NaAsO2-induced oxidative stress primarily stems from reactive oxygen species (ROS) production and interaction with sulfhydryl groups of proteins/enzymes, potentially disrupting various cell signaling pathways that may contribute to NaAsO2-induced diseases. Given the association between oxidative stress and arsenic toxicity, researchers are exploring the utilization of antioxidant properties found in compounds and extracts of various plants to mitigate complications caused by NaAsO2 (9).
Citicoline (Cit), also known as cytidine diphosphate-choline (CDP-choline), is a naturally occurring compound found in the cells of both plants and animals. It serves as an essential intermediate in the synthesis of phospholipids, crucial components of cell membranes. In nature, citicoline can be obtained from dietary sources rich in choline, such as eggs, milk, liver, and certain vegetables. Upon consumption, the body can enzymatically convert choline into Cit (10, 11). Citicoline is an endogenous nucleotide comprising cytidine and choline. Studies have demonstrated that Cit exerts cardiovascular, metabolic, neuroendocrine, and urinary effects in the body (12). Various research findings indicate that this substance prevents the elevation of thiobarbituric acid reactive substances (TBARS) levels and may also decrease levels of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in individuals exposed to NaAsO2 poisoning (7). Citicoline's impact on parameters related to oxidative stress appears to be central to its protective effect against nephrotoxicity.
2. Objectives
As there is no existing study on the effect of Cit on NaAsO2-induced nephrotoxicity, the present study aims to investigate Cit's potential protective effect against NaAsO2-induced nephrotoxicity.
3. Methods
3.1. Chemicals
Sodium arsenite, citicoline, thiobarbituric acid (TBA), Ellman's Reagent, phosphate buffered saline, trichloroacetic acid, phosphoric acid, and Bradford solution reagent, were purchased from Sigma Aldrich Company. The kits utilized for glutathione peroxidase (GPx) and superoxide dismutase (SOD), nitric oxide (NO), IL-6, and TNF-α tests were obtained from ZellBio Company. Additionally, kits for measuring serum factors were purchased from Pars Azmoon Company.
3.2. Animals
Adult male NMRI mice, weighing between 23 to 27 g and aged 8 to 12 weeks, were sourced from the research center and animal house at Ahvaz Jundishapur University of Medical Sciences (AJUMS). Mice were provided unlimited access to food and water and were housed in controlled conditions for one week. These conditions included a 12:12-hour light/dark cycle, relative humidity of 50 - 60%, and a temperature range of 22 - 25°C. The study received approval from the Ethical Committee of Ahvaz Jundishapur University of Medical Sciences, in accordance with NIH protocols for the care and use of laboratory animals.
3.3. Ethical Considerations
The procedures for animal experiments and euthanasia were approved by the AJUMS ethics committee, following standard methods of animal care and use. (Ethics approval ID: IR.AJUMS.ABHC.REC.1400.090).
3.4. Experimental Method
The mice were divided into six experimental groups, each consisting of 8 animals. These groups comprised a control group, a group treated with NaAsO2 at a concentration of 50 ppm, a group treated with Cit at a dosage of 1000 mg/kg, and three groups treated with NaAsO2 50 ppm co-administered with Cit at doses of 250, 500, and 1000 mg/kg, respectively (13). Over the course of an eight-week study, mice were exposed to NaAsO2 at a concentration of 50 ppm in their drinking water (14). Meanwhile, the control group received a vehicle solution in their drinking water. In the final two weeks of the study, the mice received purified citicoline orally to achieve the desired dosage. Fresh drinking water containing NaAsO2 was prepared daily throughout the experiment.
Following ethical guidelines for working with laboratory animals and under the observation of designated observers from AJUMS, the mice were administered intraperitoneal anesthesia using a combination of ketamine and xylazine at a dosage of 90/10 mg/kg. Blood samples were obtained by puncture, and the resulting sera were used to measure the activity of kidney enzymes such as blood urea nitrogen (BUN) and creatinine (Cr). Kidney tissue was also collected. Right kidneys were preserved in a solution called phosphate-buffered formalin for histological analysis by a histologist, while left kidneys were frozen at -70°C for later evaluation of kidney markers.
The protein concentration of the samples was determined using the Bradford method. The samples were then homogenized in phosphate-buffered saline at a specific ratio and speed, followed by centrifugation to separate the tissues and collect the supernatants in microtubes. Precautions such as working on ice and using a protease inhibitor were taken throughout the process to ensure sample integrity and prevent damage.
3.5. Biochemical Assays
3.5.1. Kidney Serum Biomarkers
Once the serum samples were collected, the levels of BUN and Cr were evaluated following the instructions provided by the manufacturer's kit protocols.
3.5.2. Kidney Oxidative Stress
The measurement of total thiol (TT) level (15), TBARS level (16), and catalase (CAT) activity (17) were performed according to the studies mentioned.
3.5.3. Kidney Cytokine
To measure IL-6 and TNF-α in the kidney supernatant samples, ZellBio kit methods were utilized.
3.6. Histological Procedure
For histopathological examination, kidney tissue was preserved in a formalin solution and then embedded in paraffin. The samples were embedded in paraffin and were cut into 4 μm thick sections. These sections were mounted on glass slides and subjected to H&E staining. Three slides were prepared for each animal tissue, and five random microscopic fields were evaluated and scored.
3.7. Statistical Method
The data are presented as the mean ± SD. The dataset of this study was analyzed using a one-way analysis of variance (ANOVA) with Tukey's multiple comparisons as a post hoc test. Statistical significance was determined by P-values less than 0.05.
4. Results
4.1. Citicoline Effects on Serum Biomarkers
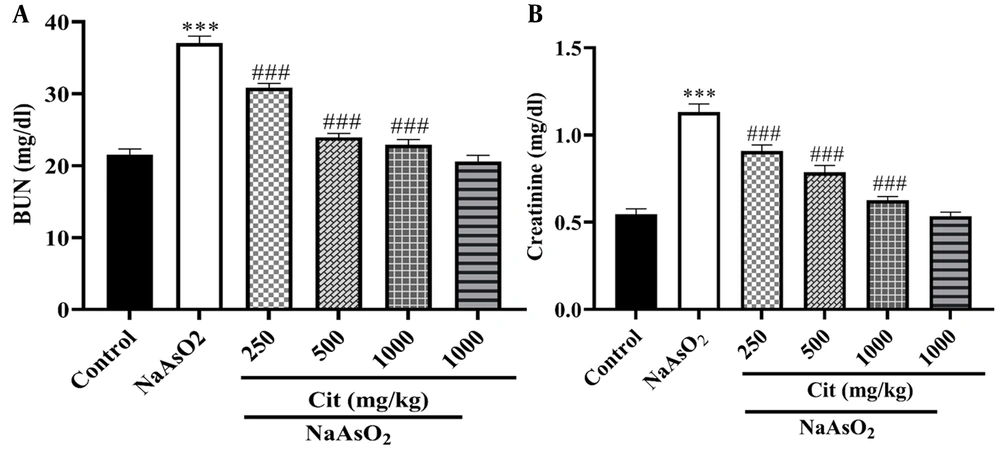
The NaAsO2 group showed significantly higher levels of BUN and Cr activities compared to the control group (P < 0.001). However, in the NaAsO2 co-treated Cit groups (250, 500, and 1000 mg/kg), there was a significant decrease in serum activity levels compared to the NaAsO2 group (P < 0.001), as depicted in Figure 1.
4.2. Citicoline Effects on Oxidative Stress Factors
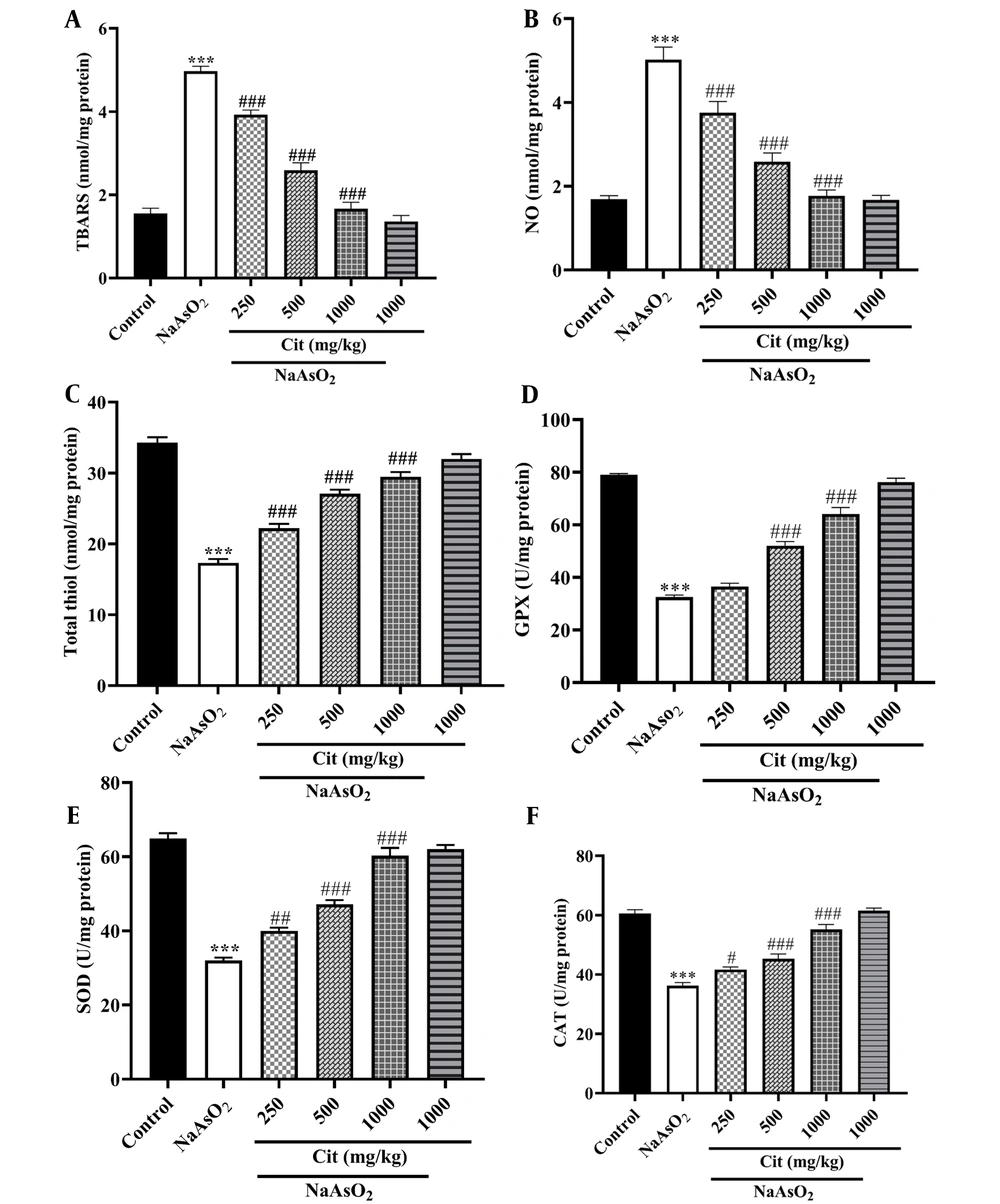
The NaAsO2 group exhibited higher levels of TBARS and NO compared to the control group (P < 0.001). According to Figure 2, administration of NaAsO2 significantly decreased TT levels and the activity of GPx, SOD, and CAT enzymes compared to the control group (P < 0.001). However, when the NaAsO2 group was co-treated with Cit (250, 500, and 1000 mg/kg), there was a significant reduction in NO and TBARS levels and an increase in the activity of antioxidant enzymes GPx, SOD, CAT, and TT levels compared to the NaAsO2 -administered group.
A, the effects of citicoline (Cit) on the level of nitric oxide (NO); B, thiobarbituric acid reactive substances (TBARS); C, total thiol (TT); D, glutathione peroxidase (GPx); E, superoxide dismutase (SOD); and F, catalase (CAT) in NaAsO2-induced kidney injury in mice. *** P < 0.001 compared to the control group and # P < 0.05, ## P < 0.01 and ### P < 0.001 compared to the NaAsO2 group.
4.3. Citicoline Effects on Inflammatory Factors
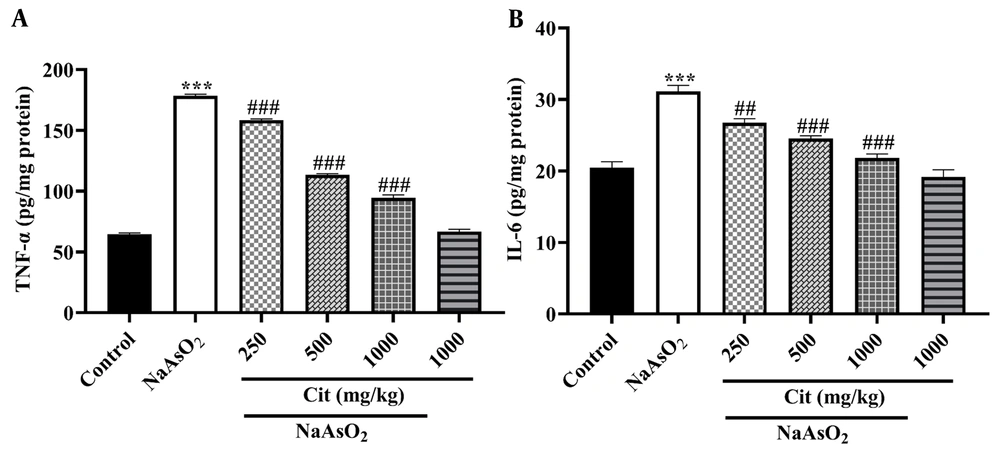
TNF-α and IL-6 levels were significantly increased in the NaAsO2 group compared to the control group (P < 0.001). In experimental Cit pretreated groups, a significant decrease in TNF-α and IL-6 cytokines was observed compared to the NaAsO2 group (Figure 3).
4.4. Histopathologic Findings
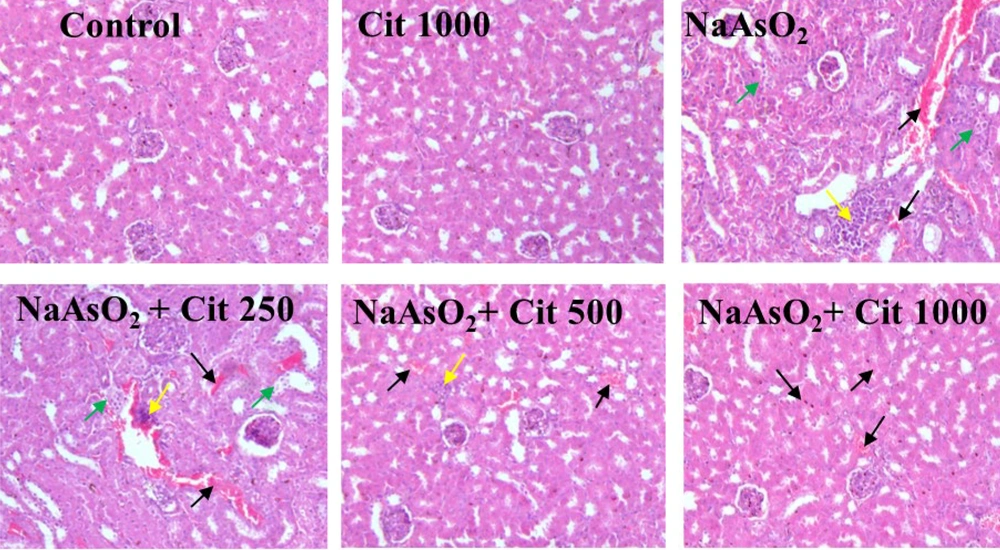
Slides of kidney sections taken from control animals showed normal structure, conversely, in the group exposed to as normal tissue patterns were disrupted. The NaAsO2 + Cit 1000 mg/kg group significantly inhibited NaAsO2-induced histological damage, such as inflammatory cell infiltration and congestion of blood vessels. Histological criteria were classified into four categories and given specific grades: Normal (-), weak (+), moderate (++), or intense (+++). The recorded ratings for each criterion were then averaged to obtain overall evaluations (Figure 4 and Table 1).
The effects of citicoline (Cit) on histopathological changes and lesions induced by sodium arsenite (NaAsO2) in kidney tissues of mice. The effects of Cit are evident in H&E staining. Green arrows: Proximal tube damage, yellow arrows: Inflammation, and black arrows: Congestion of blood vessels. Magnification: × 100.
| Groups | Congestion of Blood Vessels | Inflammation | Proximal Tube Damage |
|---|---|---|---|
| Control | - | - | - |
| Citicoline (1000 mg/kg) | - | - | - |
| NaAsO2 | (++) to (+++) | (+++) | (+) to (++) |
| NaAsO2 + citicoline (250 mg/kg) | (++) to (+++) | (++) to (+++) | (+) to (++) |
| NaAsO2 + citicoline (500 mg/kg) | (+) to (++ | (+) | (+) |
| NaAsO2 + citicoline (1000 mg/kg) | (-) to (+) | (-) to (+) | (-) to (+) |
Histology Assessments in the Control and Experimental Groups
5. Discussion
Due to the increased use of agricultural pesticides and metals, environmental levels of arsenic are continuously rising, attributing chronic and long-term toxic effects to NaAsO2. Long-term exposure to NaAsO2 has detrimental effects on the nervous system, heart, reproductive organs, liver, and kidneys, leading to various health complications (18-20). Researchers have recently focused on finding safe and effective ways to treat and prevent heavy metal poisoning. A suggested approach is to use chelating agents to bind to antioxidant compounds. Another effective mechanism to prevent arsenic poisoning is to increase the concentration of intracellular antioxidants (21).
Elevated levels of BUN and serum Cr are recognized as crucial indicators of renal impairment (22). Numerous studies have documented that exposure to arsenic can lead to an increase in BUN and Cr levels (23, 24). In our research, kidney injury induced by arsenic led to elevated levels of urea nitrogen and serum Cr. However, the administration of Cit demonstrated substantial alleviation of these adverse effects, consistent with the results of the Al-Hafyan et al. study (25).
One of the most important and fundamental damages is related to oxidative stress, wherein NaAsO2 acts as a toxic agent against the body's antioxidant system. Kidney cells have specialized antioxidant enzyme systems like SOD, CAT, GPx, and TT to combat oxidative stress. SOD converts superoxide, a byproduct of oxidative stress, into hydrogen peroxide (H2O2). CAT breaks down H2O2 into oxygen and water, and GPx helps neutralize harmful lipid peroxides and H2O2 in the cells. Together, these enzymes defend kidney cells against oxidative stress (25). In the present study, the antioxidant system, including CAT, GPx, SOD, and TT, was reduced in exposure to NaAsO2, and Cit, as an antioxidant, was able to reduce the toxicity caused by NaAsO2, consistent with many studies (19, 26). Oxidative stress increases pro-inflammatory cytokines, which ultimately cause widespread inflammation. TNF-α and IL-6 are two important pro-inflammatory cytokines in the process of inflammation, which cause the activation of inflammatory cells at the site of injury. Also, by stimulating inducible nitric oxide synthase (iNOS), TNF-α can increase the amount of NO, which is one of the harmful factors (27).
In a study conducted by our research team, NaAsO2 was found to elevate the levels of pro-inflammatory factors TNF-α and IL-6. However, the administration of Cit demonstrated its potential to decrease these pro-inflammatory factors and mitigate the associated complications. This outcome closely aligns with the findings of Zhang et al. and Al-Brakati et al. studies (24, 28). Additionally, in a study conducted by Nikravesh et al., Cit decreased factors such as TNF-α and IL-6 (7). Clearly, excessive production of TBARS and NO contributes to the damage caused by NaAsO2. In this study, according to the obtained results, Cit was able to reduce the toxicity caused by NaAsO2 by decreasing these harmful factors. Remarkably, these findings align with a prior study conducted by Al-Brakati et al. (24).
Overall, Cit exhibited the potential to mitigate the harmful effects of NaAsO2 by targeting TBARS and NO production. According to the histological examination, Cit effectively improved the kidney's pathological conditions caused by NaAsO2 toxicity. On the other hand, NaAsO2 caused significant damage to the renal tissue, including congestion of blood vessels, proximal tube damage, and inflammation. Based on the functional and biochemical findings, it can be concluded that Cit exerted its protective effects by acting as an antioxidant and anti-inflammatory agent.
5.1. Conclusions
Citicoline, known for its strong antioxidant properties, has shown promising results in reducing the damage caused by NaAsO2. The results of our research indicate that citicoline was able to reduce the damage caused by NaAsO2, suggesting that Cit can be used to control and prevent chronic poisoning caused by NaAsO2.