1. Background
Multiple sclerosis (MS) is one of the most disabling neurological diseases and has been investigated worldwide for decades, but there are still undiscovered layers and no proven treatments for this disease. Patients with MS are categorized into four types. The majority of these patients are detected as a clinically isolated syndrome, and later on, they enter the “secondary progressive MS” phase. The next stage would be “primary progressive multiple sclerosis”. Some patients may later progress and enter the last stage, which is called “progressive-relapsing MS”. The main aspect of this debilitating disease is the continuous breakdown of myelin (1). Anti-inflammatory herbs such as cinnamon may be effective in reducing inflammation in patients with MS.
Cinnamon (Cinnamomum zeylanicum) is a well-known herb around the world and is used either as a spice in foods and drinks or as herbal medicine. This herb is collected from the inner bark of an evergreen plant (genus Cinnamomum) and has two varieties; C. zeylanicum (CZ) and C. cassia (CC). All parts of this plant have medicinal uses (2). C. zeylanicum, also known as “true cinnamon”, is naturally grown in Sri Lanka and southern India (3). Cinnamon polyphenol extract can alter immune responses by regulating anti- and pro-inflammatory cytokines in mouse macrophages, and it can also increase tumor necrosis factor (4). Cinnamon aqueous extract is capable of reducing lipopolysaccharide-induced tumor necrosis factor in human serum (5). Cinnamon has been shown to have a positive effect on nonalcoholic fatty liver disease characteristics such as elevated liver enzymes and high-sensitivity C-reactive protein (hs-CRP) (6). Cinnamon can suppress inflammation and regulate the expression of myelin genes as well as inhibit demyelination in the central nervous system. Oral consumption of cinnamon (C. verum) in female PLP-TCR transgenic mice and adoptive transfer mouse model can reduce the symptoms of relapsing-remitting experimental allergic encephalomyelitis (7). Oral administration of cinnamon powder is thought to be a cost-effective and safe treatment in patients with MS (7). Like any other herb, cinnamon may have some side effects. It might lower blood sugar and might interfere with blood sugar control during and after surgery; therefore, the blood sugar levels should be monitored regularly. Despite various studies so far, the benefit of cinnamon for patients with MS in terms of immune markers remains unknown. Furthermore, there are few studies that target the effect of this herb on the most irritating problem in patients with MS, namely pain
2. Objectives
This study aimed to evaluate the effect of cinnamon on inflammatory factors, pain, and anthropometric indices in patients with MS.
3. Methods
3.1. Study Design and Population
This was a randomized controlled trial to evaluate the effect of cinnamon on inflammatory factors, pain, and anthropometric indices in patients with MS. Sixty patients who had medical records in Shiraz Multiple Sclerosis Center were recruited by screening 200 patients who were referred to this center (covering all regions of Shiraz City). The recruitment of patients started in early March and was completed by the end of March 2016.
Inclusion criteria were as follows: age between 20 and 70 years, diagnosed with progressive-relapsing multiple sclerosis, good mental health (self-reported), and having no allergies or food intolerance to cinnamon. Exclusion criteria were as follows: patients who had rheumatoid arthritis, gout, liver or kidney diseases, breast fibroids, breast cancer and statin use, athletes who exercised regularly (because regular exercising may alter the inflammation status of the patient), people who followed any kind of therapeutic or weight control diet, following any lifestyle or eating habit modification program, people who took lipid-lowering medication over the past month, or abused drugs or alcohol, and people with metabolic or gastrointestinal disease. According to a similar previous study (8), the sample size was calculated as 20 in each group and considering attrition, 30 patients were recruited for each group (power: 80% and α: 5%).
3.2. Ethical Considerations
Informed written consent was obtained from all participants, and the Ethical Committee of Shiraz University of Medical Sciences approved this study in 2016 (ref no.: IR.SUMS.REC.1395.16). The study protocol conformed to the ethical guidelines of the 2008 Declaration of Helsinki, and it has been registered at the Iranian Registry of Clinical Trials (code: IRCT2016072529041N1).
3.3. Randomization and Blinding
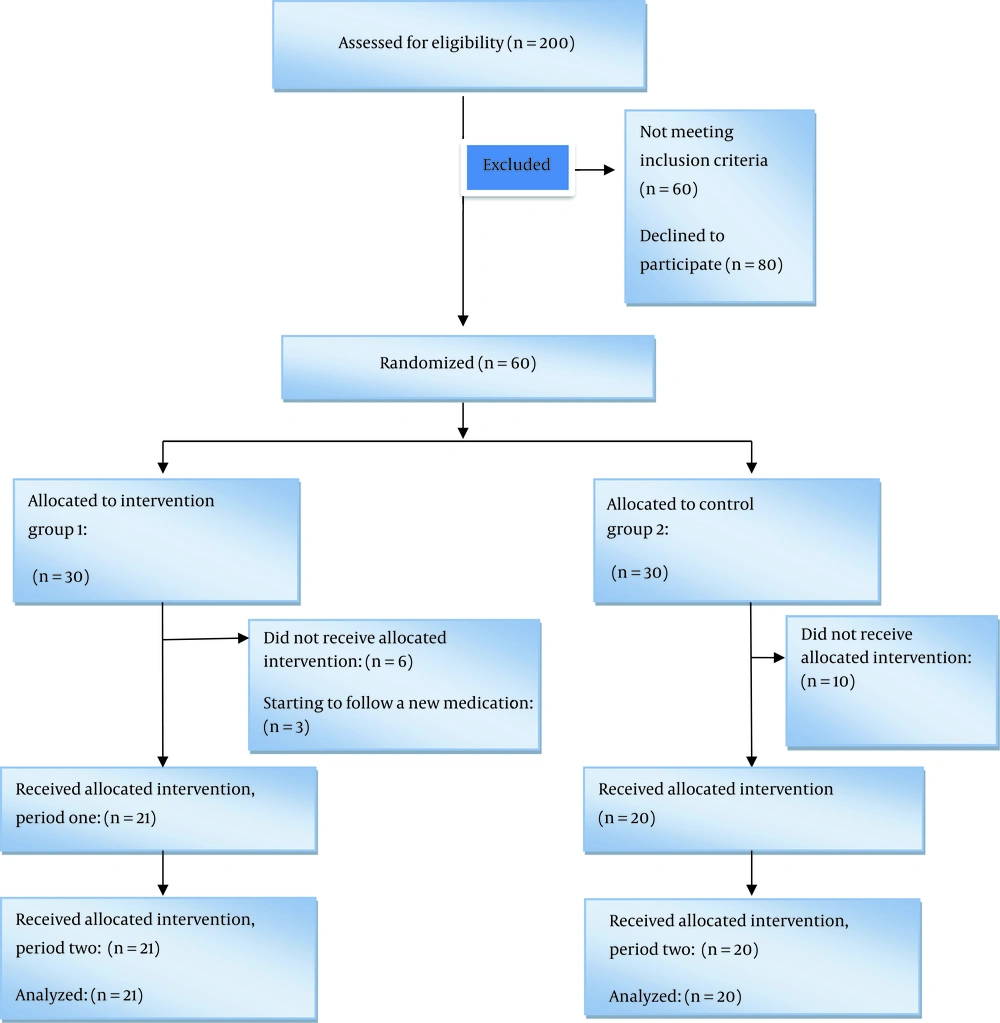
Participants were randomly allocated to two groups by using a computer-based randomization program (n = 30 in each group), which is illustrated in Figure 1. This was a double-blind study in which participants and the operator of intervention were blinded.
3.4. Intervention
Four capsules of cinnamon were taken every day for 8 weeks by each participant in the intervention group (500 mg in each capsule and 2 grams in total (8)) and 4 capsules of wheat flour by the control group as the placebo (500 mg in each capsule). Cinnamon capsules and placebo capsules were prepared by the pharmacology laboratory at the Traditional Medicine Faculty of Tehran University of Medical Sciences. Cinnamon capsules were prepared by macerating cinnamon bark.
The characteristics of the real drug and placebo were identical in color, appearance, smell, and taste. Moreover, the quality of placebo capsules was identical to the real drug in physical form, sensory perception, packaging, and labeling, and these characteristics were checked by a pharmacist. The research team checked the participants every week by calling them to be sure about taking the capsules, and they had to go to the MS center every 2 weeks to get their capsules.
To randomize the study population and eliminate dietary confounding factors, the estimated energy requirement was used individually for each participant, and they followed their balanced diet one week before the intervention program and stayed on it until the end of the study. Macronutrient composition in this diet was as follows: 55% carbohydrates, 18% protein, and 27% fat.
3.5. Measurements
Data collection was performed by a trained interviewer (nutritionist). The information obtained included sociodemographic data, physical activity level, anthropometric indices, pain level and food recalls. A valid and reliable form of a 24-h food recall questionnaire (including two working days and one weekend or holiday) was completed by interviewing the participants at the beginning and the end of the study. Physical activity was measured through the short form of the International Physical Activity questionnaire (IPAQ), which was previously validated to use in Iran (9-11). A visual analog scale (VAS) was used to evaluate the pain levels of patients (12).
Before the intervention session, eligible participants underwent anthropometric measurements including body weight (with digital personal scale (Seca 761, Germany)), height (measuring tape (Seca 206, Germany), and waist and hip circumference with ergonomic circumference measuring tape (Seca 201, Germany), and scales were calibrated at the beginning of every day. Moreover, body mass index (BMI) was calculated using a person's height and weight. The formula is BMI = kg/m2, where kg is a person's weight in kilograms and m2 is their height in meters squared.
Venous blood samples (5 mL) were taken by a nurse at the beginning and end of the study to measure hs-CRP and IL-6 by ELISA. Blood samples were stored in a cooler and analyzed in the Nutrition Department Laboratory of Shiraz University of Medical Sciences.
3.6. Statistical Analysis
All statistical analyses were performed using SPSS version 22. All main outcomes are presented as mean (SD) and frequency (%) for continuous and categorical variables, respectively. The statistical significance level was set at a two-tailed type I error of 0.05, and deviation from normal distribution was examined by calculating skewness and by the Kolmogorov-Smirnov test.
The chi-square test, paired t-test, and independent t-test were used to evaluate categorical variables, comparison within groups and between groups (or non-parametric tests), respectively. The Nutritionist-4 software was used for analyzing the food and energy intake of participants.
4. Results
Nineteen participants withdrew from the study. Reasons for withdrawal are listed in Figure 1. Thirty-six (87.80%) participants were female, and twenty-six patients were married (63.41%).
Table 1 shows the sociodemographic characteristics of the participants in the intervention and control groups.
| Variable | Intervention (N = 21) | Control (N = 20) | P-Value |
|---|---|---|---|
| Age, y | 44.22 ± 11.80 | 42.94 ± 9.55 | 0.61 |
| Gender | 0.59 | ||
| Woman | 19 (90.47) | 17 (85) | |
| Man | 2 (9.52) | 3 (15) | |
| Marital status | 0.65 | ||
| Single | 7 (33) | 8 (40) | |
| Married | 14 (67) | 12 (60) | |
| Education | 0.86 | ||
| Uneducated | 2 (9) | 1 (5) | |
| Primary and secondary education | 4 (19) | 3 (15) | |
| High school | 5 (24) | 4 (20) | |
| Diploma | 10 (37) | 8 | |
| University education; bachelor and master | 6 (22.2) | 4 |
Sociodemographic Characteristics of Participants in Two Groups of Intervention and Controla
The results of the physical activity questionnaire and anthropometric measurements of the participants in the intervention and control groups are listed in Table 2. The results revealed that there was no significant difference between the two groups at baseline. There was no significant difference in hs-CRP, IL-6, and pain levels between the intervention and control groups at baseline.
| Intervention (N = 21) | Within Group P-Value | Control (N = 20) | Within Group | P-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Between Groups (Week 0) | Between Groups (Week 8) | |||
| Physical activity, MET | 684.70 ± 791.10 | 670.95 ± 824.01 | 0.70 | 687.03 ± 619.50 | 792.89 ± 732.05 | 0.33 | 0.19 | 0.60 |
| BMI, kg/m2 | 26.03 ± 4.16 | 25.37 ± 3.91 | 0.000 | 24.54 ± 4.55 | 24.60 ± 4.67 | 0.194 | 0.28 | 0.569 |
| Waist, cm | 91.04 ± 11.46 | 87.33 ± 9.73 | 0.001 | 90.65 ± 11.54 | 90.85 ± 11.93 | 0.163 | 0.91 | 0.307 |
| Weight, kg | 68.19 ± 13.75 | 66.38 ± 13.08 | 0.001 | 65.00 ± 14.43 | 65.15 ± 14.60 | 0.186 | 0.47 | 0.777 |
| Height, cm | 162.19 (6.36) | - | - | 162.25 (7.07) | - | - | 0.97 | - |
Physical Activity Levels and Anthropometric Measures of Participants in Two Groups of Intervention and Controla
As shown in Table 2, weight changes in 8 weeks of intervention were significant in the intervention group but not in the control group (P = 0.001), but this variable did not significantly differ between the two groups.
The results of the 24-h food recall are listed in Table 3. As shown in Table 4, IL-6 and hs-CRP levels decreased significantly in the intervention group compared to the control group (P < 0.05). Comparison of pain levels in the intervention and control groups at baseline and week 8 showed that in the intervention group, they decreased significantly (Table 4).
| Intervention (N = 21) | Within Group P-Value | Control (N = 20) | Within Group | P-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Between Groups (Week 0) | Between Groups (Week 8) | |||
| Energy, kcal | 824.22 ± 110.5 | 712.55 ± 90.58 | 0.33 | 801.01 ± 107.22 | 895.5 ± 123.01 | 0.58 | 0.53 | 0.86 |
| Carbohydrate, g | 128.01 ± 20.60 | 94.98 ± 15.24 | 0.32 | 109.50 ± 17.80 | 146.00 ± 26.00 | 0.54 | 0.94 | 0.30 |
| Fat, g | 32.60 ± 5.75 | 45.25 ± 8 | 0.94 | 29.60 ± 5.20 | 24.00 ± 4.30 | 0.92 | 0.29 | 0.28 |
| Protein, g | 40.90 ± 7.20 | 35.98 ± 6.40 | 0.51 | 24.12 ± 4.01 | 27.30 ± 5.00 | 0.64 | 0.10 | 0.52 |
| Intervention (N = 21) | Within Group P-Value | Control (N = 20) | Within Group | P-Value | |||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Between Groups | |||
| IL-6 | 2.84 ± 2.06 | 1.86 ± 0.43 | 0.049 | 2.04 ± 0.35 | 2.22 ± 0.40 | 0.991 | 0.008 |
| hs-CRP | 4.15 ± 4.48 | 1.83 ± 2.02 | 0.035 | 3.35 ± 5.25 | 4.05 ± 3.61 | 0.520 | 0.022 |
| Visual Analog Scale | 54.28 ± 19.12 | 24.76 ± 16.31 | 0.000 | 41.50 ± 22.30 | 43.25 ± 21.47 | 0.069 | 0.003 |
Comparison of Immunological Factors and Pain Levels in Intervention and Control Groups in Baseline and Week 8a
5. Discussion
Multiple sclerosis is an inflammatory disease in which demyelination occurs, and it is the second leading cause of disability in youths. Thus, studies in this field can be very helpful. This study was designed to evaluate the effect of cinnamon powder on inflammatory factors, pain, and anthropometric indices in patients with stage IV MS. The homogeneity of patients in this study indicated adequate randomization.
5.1. IL-6 and hs-CRP
The results of this study showed that there was a significant reduction in IL-6 levels in the intervention group compared to the control group. Faikoh et al. (13) investigated liposome-encapsulated cinnamaldehyde (which is extracted from cinnamon) for its in vitro antibacterial activity against aquatic pathogens and effects on in vivo immunity and protection parameters against Vibrio vulnificus and Streptococcus agalactiae. These authors demonstrated that cinnamaldehyde had antimicrobial activity against aquatic pathogens and reduced the levels of IL-1β, IL-6, IL-15, NF-κb, and TNF-α; consequently, it should be recommended as an immunostimulant to protect bacteria-infected fish, which is in line with our results.
Hong et al. (5) found that the administration of cinnamon aqueous extract in mice significantly reduced serum IL-6 levels, which is in line with our results. In a study in mouse RAW264.7 macrophages, aimed at testing the hypothesis that cinnamon polyphenol extract regulates immune function involving genes encoding TTP, proinflammatory cytokines, and glucose transporter (GLUT) families, Cao et al. (4) showed that this extract decreased IL-6 mRNA levels significantly (39 to1868 fold), in agreement with our findings. Askari et al. similarly investigated the effect of cinnamon on the immune system and its insulin-sensitizing effect on patients with nonalcoholic fatty liver disease in a double-blind study, they showed a significant decrease in hs-CRP (P < 0.05) in the intervention group compared to the control group (8).
In an 8-week cinnamon powder intervention in diabetic patients, there was no significant change in inflammatory indices such as hs-CRP, where the investigators could find a significant within-group reduction but not a between-group change (14). In the aforementioned study, the participants were diabetic patients, while the main target group of our study was patients with MS, which might have accounted for the difference in results.
In the same study, cinnamon consumption did not cause any significant change in the intervention group compared to the control group in terms of weight, BMI, waist and food intake (24-h food recall questionnaire) (14), similar to what we found. In contrast, in a study by Mashhadi et al. (15), female taekwondo players were recruited to receive dietary ginger and cinnamon, and in the end, their muscle soreness and inflammation were evaluated. In this six-week intervention, athletes took cinnamon, ginger, or placebo, and at the end of the study, there were no significant changes in IL-6 levels in the cinnamon and ginger groups in comparison with the placebo group. The Likert scale, used as an indicator of muscle soreness, showed a reduction in soreness in cinnamon and ginger groups. The difference in results may be due to different levels of inflammation in patients with MS and athletes (15).
5.2. Pain Level
The VAS results showed that pain was significantly decreased in the intervention group (compared to the control group). In an interesting study, Zhang et al. (16) examined the in vivo anti-inflammatory, antinociceptive, and analgesic activities of an essential oil recipe consisting of the supercritical fluid CO2 extract of white pepper, long pepper, cinnamon, saffron, and myrrh. Based on their results, this oil had no significant analgesic activity on the hot plate test in mice, which is not in line with our study because our results showed a significant reduction in pain of patients with MS (P < 0.05). A recent review article showed that cinnamon is extensively used for pain reduction in dysmenorrhea, but the results are drawn from poor clinical trials, so cinnamon effects on dysmenorrhea are still unclear (17). The difference between our results and those of Zhang et al. (16) might have been due to our target group. In a study by Jaafarpour et al. (18), cinnamon and ibuprofen were compared in the treatment of primary dysmenorrhea in a randomized, double-blind clinical trial. In this study, eight weeks after the intervention, mean pain severity in the cinnamon group was significantly lower than that in the placebo group (P < 0.001), but at various times, mean pain severity in the ibuprofen group was significantly less than in the cinnamon and placebo groups (P < 0.001). These results are not in line with our results, and this may be due to the fact that herbal medicine cannot be compared with a conventional drug.
5.3. Anthropometric Measures
In the present study, patients in the intervention group had a significant reduction in weight at week 8 compared with baseline. But comparison of weight in the two study groups did not show any significant difference. A double-blind, randomized, placebo-controlled clinical trial was conducted to investigate the effect of 3 g/day cinnamon (8 weeks) on glycemic status, lipid profile and body composition in type 2 diabetic patients (19). In this study, body weight, body fat mass and body mass index were significantly decreased after 8 weeks of intervention compared to the control group, but anthropometric measurements did not change. Diabetes mellitus and MS are similar regarding the inflammatory condition of patients, but it is obvious that the levels and type of inflammation are different, which may be the reason for the different results. In another study in rats, supplementation with different polyphenolic plant extracts (almond, apple, cinnamon, orange blossom, hamamelis, lime blossom, grapevine, and birch) was investigated regarding anti-obesity effects. Apple and cinnamon extracts caused a decrease in body weight (20). The results of this study are in line with our results.
To the best of our knowledge, there are few studies to date that have investigated pain levels, hs-CRP, and IL-6 levels in patients with MS who were given cinnamon powder or extract.
5.4. Strength and Limitations
To the best of our knowledge, there is no published study investigating the effect of cinnamon on MS complications. The most powerful aspect of this study was its randomized and blinded design, which makes it powerful and effective. Also, unlike other trials, we could decrease the effect of confounding factors, where we demonstrated no significant difference between the intervention and control groups at baseline.
Some limitations of the present study should be acknowledged. The progressive nature of MS in patients could have affected the results of cinnamon consumption, and this could have precluded any conclusions regarding cinnamon’s effect alone. It would be better if we could measure and compare the investigated factors in different MS types.
5.5. Future Studies
Our suggestion for future studies is to choose a larger sample size and investigate the appropriate dose of cinnamon. Also, this study would be more powerful if participants were followed up to determine the long-term effects of cinnamon after discontinuation of its use.
5.6. Conclusions
Multiple sclerosis is a debilitating and progressive inflammatory disease, and cinnamon may be helpful for improving inflammatory markers and pain in patients with MS.