1. Background
In recent years, much focus has been placed on developing efficient sunscreen products to prevent irradiation-induced skin damage. There are two types of sunscreens: Physical and chemical. Physical sunscreens, like zinc oxide, can cover a wide UV spectrum; however, they require frequent reapplication due to limitations. Chemical sunscreens work by absorbing light and releasing heat from the skin surface but may have side effects due to their chemical origin. Recently, manufacturers have shifted their focus from chemical agents to natural resources (1). Among the extensively researched natural sunscreens are mycosporine-like amino acids (MAAs) molecules. Mycosporine-like amino acids and gadusols are molecules produced by organisms to prevent sunlight damage, with microalgae and cyanobacteria being the main sources. Numerous researchers have evaluated these molecules for efficient sunscreens with antioxidant, repairing, and anti-aging properties (2).
Mycosporine-like amino acids are low molecular weight, water-soluble, nitrogen-rich molecules with maximum UV absorption. They make good sunscreens due to their high optical and thermal stability, strong UV radiation absorption, energy dispersion as heat, and excitation state in short wavelengths, preventing adverse photochemical reactions, including photoproduct formation. Mycosporine-like amino acids have been identified in cyanobacteria, microalgae, macroalgae (mainly in Rhodophyta), and marine animals. Their UV radiation absorption, antioxidant capacity, and physical and chemical properties enable their use in preventing and treating human diseases related to free radicals and UV radiation (3).
Various studies have reported the antioxidant effect of MAAs. For instance, a study in South Korea reported the antioxidant effect of MAAs extracted from Chlamydomonas hedleyi (4). In a review article, Kageyama and Waditee-Sirisattha documented the antioxidant effect of different MAAs investigated to date (5).
Culture conditions can significantly impact the yield of MAAs production in microalgae and cyanobacteria. Sharma investigated the effect of carbon content, salt, and pH on the production of UV-absorbing compounds by Spirulina. They found that using 0.4 M sodium chloride salt at pH 7 and the absence of carbon sources could lead to the production of compounds such as Phycocyanin and Allophycocyanin, while also increasing Phycoerythrin in Spirulina plantesis (6). Additionally, we reported that high salinity in Desmodesmus sp. culture induced the production of MAAs-related compounds (7). Furthermore, optimizing nitrogen and phosphate concentrations in the culture medium of Chlorella vulgaris could affect the type and yield of MAAs production (8). In our previous research, we identified two types of Fischerella sp. as sources for MAAs production. One of them produced at least two different types of MAAs, while another produced one main compound with characteristics similar to shinorine (9).
Moreover, factors influencing the extraction process, such as time, temperature, and type of solvent, can affect the extraction and purification yield. Studies by Karsten et al. demonstrated that pure distilled water was the most efficient solvent for extracting MMAs from Peperomia crispa and Porphyra umbilicalis (10). Similarly, Nishida's studies showed that pure distilled water yielded the best results for extracting MAAs from a red alga Palmaria palmata during a 6-hour extraction process (11). Additionally, Chaves-Pena et al. suggested that MAAs extraction with pure methanol from Rhodophyta could result in the detection of six types of MMAs (12).
2. Objectives
The primary aim of this study was to optimize the extraction process of MAAs from Fischerella sp. F5, a strain previously investigated in our laboratory, and to evaluate its antioxidant effect. Various factors have been identified to influence the extraction yield of MAAs from cyanobacteria and other natural sources, including solvent polarity and volume, mechanical enhancers for cell disruption such as ultrasonic waves, thermal treatment, and extraction duration. Hence, we considered five quantitative factors—solvent type and volume, sonication time, extraction temperature, and duration—for the optimization of MAAs extraction from Fischerella sp. F5 using experimental design. Utilizing experimental design enables the simultaneous assessment of various factors on process outcomes, thereby reducing the number of experiments required to optimize processes. Therefore, the current study was designed employing experimental design methods.
3. Methods
3.1. Fischerella sp. Cultivation
Forty milliliters of Fischerella sp. F5 (accession No. OR228690) 25-day culture was cultivated in 200 mL of BG-11 culture medium (consisting of NaNO3, K2HPO4•H2O, MgSO4•7H2O, CaCl2•2H2O, citric acid, ferric ammonium citrate, EDTA, Na2CO3, H3BO3, MnCl2•4H2O, ZnSO4•7H2O, Na2MnO4•2H2O, CuSO4•5H2O, and Co (NO3)2•6H2O from Merck Chemicals, Germany). The cultures were harvested after 28 - 30 days at ambient temperature.
3.2. Mycosporine-Like Amino Acids Extraction and Partial Purification
The biomass was separated by centrifugation (2-16KL, Sigma, Germany) at 9200 RPM for 10 minutes, followed by lyophilization. Subsequently, 10 mg of biomass was extracted for each run at varying concentrations of methanol (HPLC grade, Merck Chemical, Germany). The solvent type and volume, sonication time, extraction temperature, and duration were designed using DesignExpert® software. All sample mixtures were sonicated in an ultrasound bath (S 60 H, Elma, Germany). Extraction was carried out for 24, 48, and 72 hours according to the experimental design. The supernatant was separated by centrifugation at 12 500 RPM for 20 minutes, after which the samples were dried at room temperature. To dissolve the dried samples, 1 mL of deionized water (Millipore, Germany) was added, and each sample solution was transferred to a new microtube separately. For each sample, 0.5 mL of chloroform was added, followed by vigorous shaking and vortexing to remove non-polar content, including pigments and lipids. Samples were then passed through a 0.22 μm syringe filter (MS® MES Syringe Filter) before characterization by high-performance liquid chromatography (HPLC).
3.3. Characterization of Mycosporine-Like Amino Acids
High Performance Liquid Chromatography was performed using a Shimadzu HPLC system equipped with a C18 HPLC column (5 μm, 25 × 4.6 mm) and an isocratic mobile phase consisting of 12.5% methanol with 0.02% trifluoroacetic acid (TFA) aqueous solution plus 10% acetonitrile, with a flow rate of one mL.min-1. Using a Hamilton syringe, 100 µL of each sample was injected into the injection loop. The absorbance spectrum was scanned in the wavelength range of 200 to 800 nm, and the chromatograms of samples were recorded at 254 and 330 nm. The best extract was further analyzed by direct mass spectrometry (MS) using an Agilent triple QQQ (USA) instrument.
3.4. Experimental Design to Optimize Mycosporine-Like Amino Acids Extraction
A 1/2 factorial plan was designed using DesignExpert® 12 software to optimize five factors: Solvent type and volume, sonication time, extraction temperature, and duration (all quantitative). The features of the Experimental Design model are shown in Table 1. Applying the central composite design model, 32 runs in two blocks were designed to optimize these five factors. The total area under the curve (AUC) and its relative percentage, extracted from samples' HPLC chromatograms, were used for the optimization of the MAAs extraction process. For optimization, the central composite design (CCD) was utilized. The software applied an ANOVA test to verify the significance level of the models, considering a P-value less than 0.05 as significant.
| Factor | Name | Minimum | Maximum |
|---|---|---|---|
| A | Time (h) | 24.00 | 72.00 |
| B | Temperature (°C) | 4.00 | 50.00 |
| C | Sonication time (min) | 30.00 | 120.00 |
| D | Methanol ratio (%) | 10.00 | 100.00 |
| E | Solvent volume (mL) | 1.00 | 3.00 |
Characteristics of the Experimental Design Model
3.5. Determination of the Antioxidant Effect
The free radical scavenging of 2, 2-diphenyl-1-picrylhydrazyl (DPPH) was used to determine the antioxidant activity. Briefly, 100 µL of samples, positive controls (ascorbic acid 0.1%), and negative controls (methanol) were added to the 100 µL DPPH reagent. For each sample, a blank was considered, which had methanol instead of DPPH reagents. Then, the absorption of each sample was measured at 517 nm after a 30-minute incubation. Ascorbic acid at a concentration of 1 mg/mL and a DPPH methanolic solution at a concentration of 0.1 mM were used as positive control and DPPH reagents, respectively. The antioxidant activity was calculated based on the DPPH scavenging activity using Equation 1.
Where A0 and A1 represent control and sample absorptions, respectively.
4. Results
4.1. Characterization of Fischerella sp. F5 Extracts by High-performance Liquid Chromatography
The HPLC chromatograms of samples extracted from Fischerella sp. F5 were analyzed to discover peaks showing similar features to MAAs. Most peaks with maximum absorbance between 330 - 340 nm exhibited a retention time of about 3 minutes. The sum of the AUCs of these peaks and their relative percentage compared to the sum of all peaks’ AUCs were reported as MAA content and MAAs%, respectively, in Table 2. Runs 18 and 32 exhibited the highest value for MAA content, and run 32 had the highest value for MAA%. Accordingly, it seems that increasing the temperature for a short time and using a low percentage of methanol resulted in increased MAAs extraction efficiency. However, at room temperature, the extraction process with 55% methanol could result in promising extraction efficiency.
| Runs | Time | Temperature | Sonication Time | Methanol % | Solvent/Biomass | MAA Content | MAA% | Runs | Time | Temperature | Sonication Time | Methanol % | Solvent/Biomass | MAA Content | MAA% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | 4 | 120 | 100 | 3 | 141 733 | 54.998 | 17 | 24 | 4 | 30 | 10 | 3 | 240 635 | 67.058 |
| 2 | 24 | 4 | 120 | 10 | 1 | 160 578 | 44.867 | 18 | 24 | 50 | 120 | 10 | 3 | 4 612 592 | 67.887 |
| 3 | 48 | 27 | 75 | 55 | 2 | 226 182 | 59.142 | 19 | 24 | 50 | 30 | 10 | 1 | 302 597 | 54.977 |
| 4 | 24 | 50 | 120 | 100 | 1 | 231 576 | 62.844 | 20 | 48 | 27 | 75 | 55 | 2 | 226 182 | 59.142 |
| 5 | 72 | 4 | 120 | 10 | 3 | 190 063 | 52.898 | 21 | 48 | 27 | 120 | 55 | 2 | 306 473 | 41.585 |
| 6 | 24 | 4 | 30 | 100 | 1 | 151 182 | 63.272 | 22 | 48 | 27 | 75 | 55 | 2 | 226 182 | 59.142 |
| 7 | 24 | 50 | 30 | 100 | 3 | 195 858 | 64.796 | 23 | 48 | 27 | 75 | 55 | 2 | 226 182 | 59.142 |
| 8 | 72 | 4 | 30 | 100 | 3 | 230746 | 59.288 | 24 | 48 | 27 | 75 | 55 | 1 | 198 758 | 52.553 |
| 9 | 72 | 4 | 120 | 100 | 1 | 182 871 | 69.024 | 25 | 48 | 4 | 75 | 55 | 2 | 250 591 | 54.111 |
| 10 | 72 | 4 | 30 | 10 | 1 | 149 577 | 60.511 | 26 | 72 | 27 | 75 | 55 | 2 | 247 931 | 59.323 |
| 11 | 72 | 50 | 120 | 10 | 1 | 344 045 | 63.335 | 27 | 24 | 27 | 75 | 55 | 2 | 254 364 | 67.532 |
| 12 | 48 | 27 | 75 | 55 | 2 | 226 182 | 59.142 | 28 | 48 | 27 | 75 | 55 | 3 | 161 552 | 60.536 |
| 13 | 48 | 27 | 75 | 55 | 2 | 226 182 | 59.142 | 29 | 48 | 50 | 75 | 55 | 2 | 150 254 | 54.786 |
| 14 | 72 | 50 | 120 | 100 | 3 | 172 193 | 52.104 | 30 | 48 | 27 | 75 | 100 | 2 | 212 992 | 60.145 |
| 15 | 72 | 50 | 30 | 10 | 3 | 218 731 | 47.053 | 31 | 48 | 27 | 75 | 10 | 2 | 371 487 | 59.059 |
| 16 | 72 | 50 | 30 | 100 | 1 | 7 3745 | 27.73 | 32 | 48 | 27 | 30 | 55 | 2 | 119 1720 | 89.608 |
Data of the Experimental Design and Extraction Process
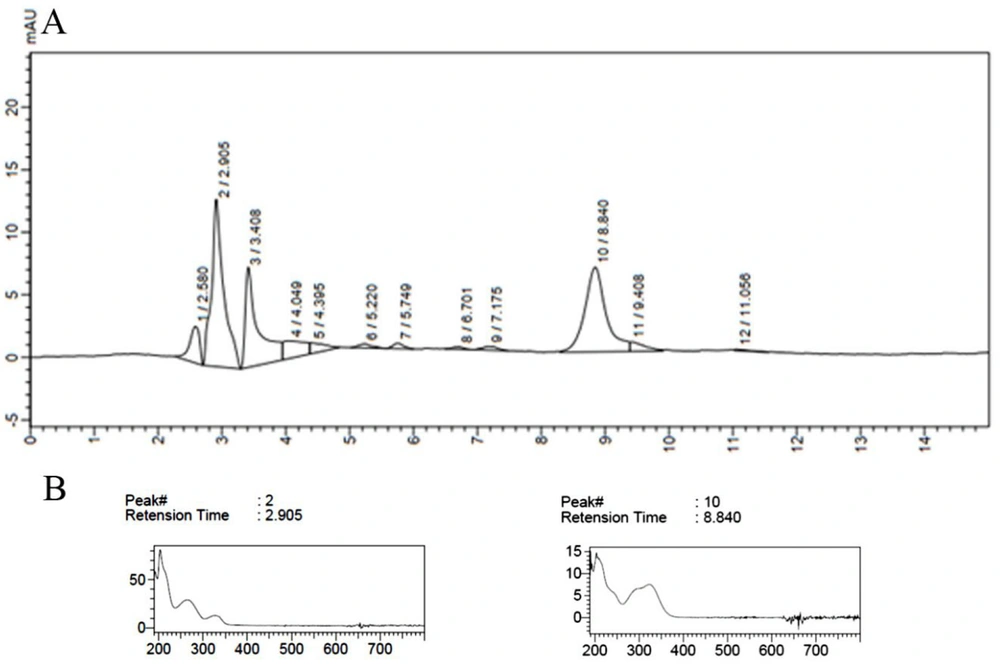
Some samples contained more than one peak with maximum absorption between 300 to 400 nm, as recorded for run 11 (Figure 1). As seen in the HPLC chromatogram, a peak with a retention time (Rt) of 2.905 and another at 8.8 min were recorded. This run had the highest value for total extraction time, sonication time, and temperature at low concentrations of methanol, suggesting that increasing these three factors resulted in more efficient extraction of compounds with less polar characteristics. Previously, three peaks with retention times around 3.5 - 5 min had been observed in our study on this species of Fischerella sp.
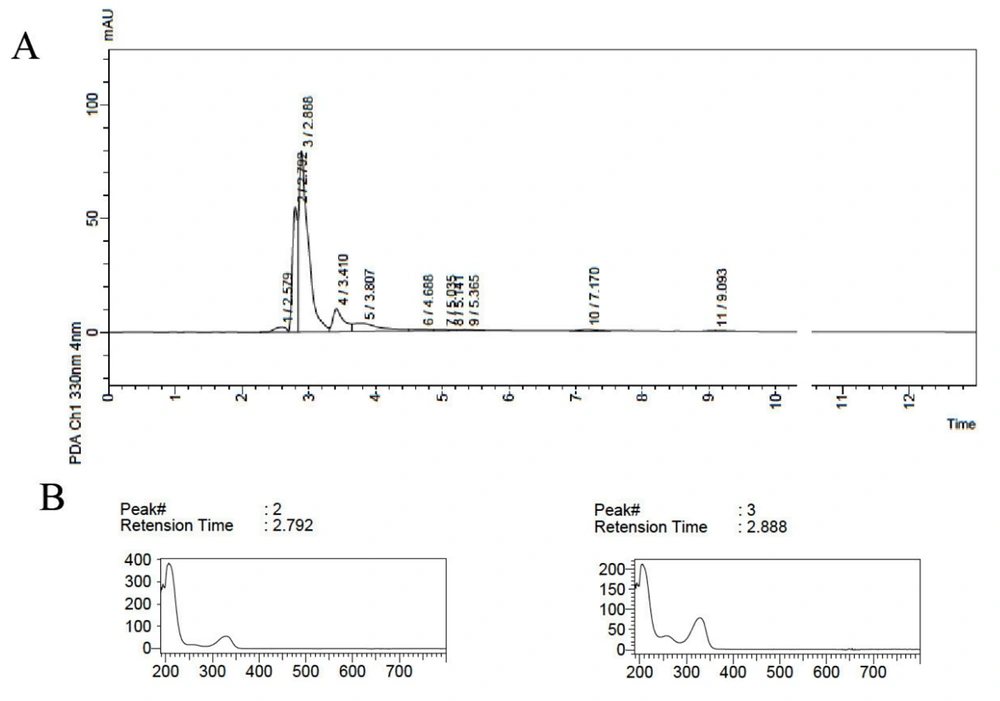
For run 2, in which the extraction process was performed at high temperature and an extended time in the presence of 10% methanol, similar to run 11, two closely recorded peaks with maximum absorptions at 300 - 400 nm were observed, and the peak with Rt = 8.8 min was not recorded (Figure 2). This indicates that time and temperature also influenced the chemical type of extracted compounds.
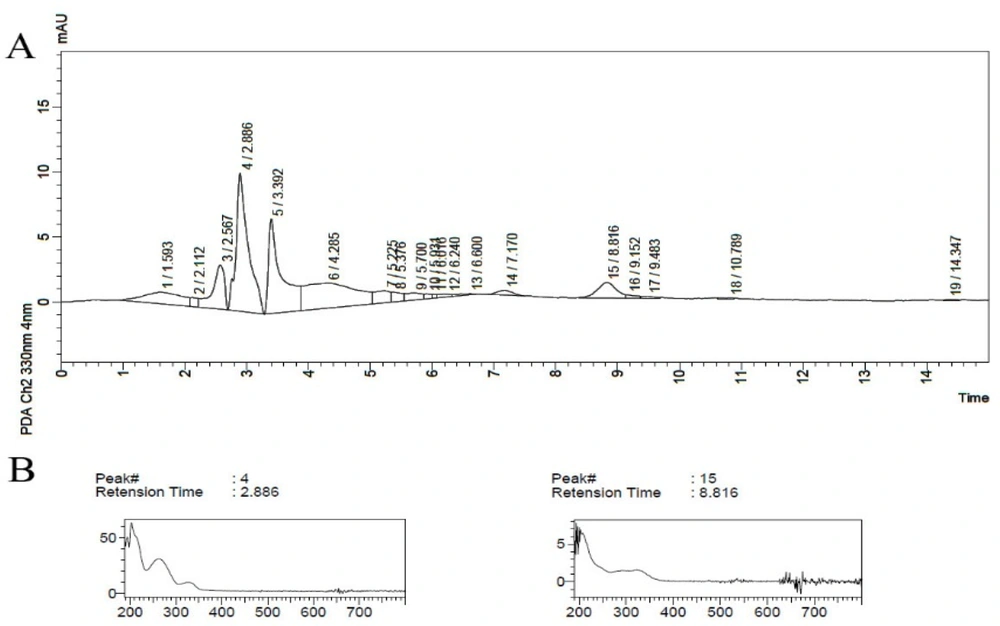
It was observed that at higher temperatures with a decrease in sonication time (run 15), the peak of the less polar compound with Rt = 8.8 min reappeared (Figure 3).
4.2. Modeling the Extraction Process and Optimized Model for Mycosporine-Like Amino Acids Extraction
The data were analyzed using DesignExpert software, and a two-level five-factor method CCD was applied for optimization based on the interaction between different factors.
Moreover, the 2FI model was applied to extract the coefficient numbers of each factor influencing the MAA content of the extracted sample. The final formula was presented in the form of Equation 2, with the adjusted R2 equal to 0.77. This means that the model is roughly suitable for predicting the extraction yield by modulating these five factors.
As seen in the proposed model for predicting MAA content from extraction factors, all single factors had a positive effect on the extraction yield; nevertheless, the interactions between these factors (combined factors) did not essentially have a positive effect on the extraction yield. Additionally, regarding the P-values reported for each component of the final model, the combined factors had non-significant values on the model designed for extracted MAA content, except for the interactions of temperature with time, sonication time, and methanol ratio. Finally, the optimized factors were predicted based on this model (Table 3).
| Run | Time | Temperature | Sonication Time | Methanol Ratio | Solvent volume | MAA Content Predicted | %MAA Predicted | Desirability | |
|---|---|---|---|---|---|---|---|---|---|
| 18 | 24 | 50 | 120.0 | 10.0 | 3.0 | 4301865.16 | 67.73 | 0.93 | Selected |
The Optimized Model for for Mycosporine-Like Amino Acids Extraction
The optimization for MAA% and antioxidant activity was also considered using CCD. The statistical data showed that none of these models could present a satisfactory adjusted R2, and the coefficients calculated for each factor did not have significant value (data not presented).
4.3. LC/MS Analysis of Extract Using the Optimized Method
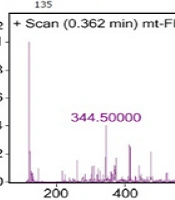
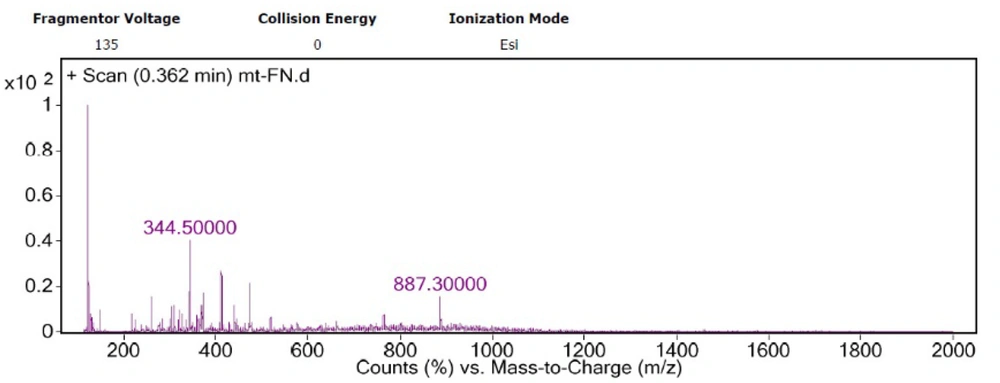
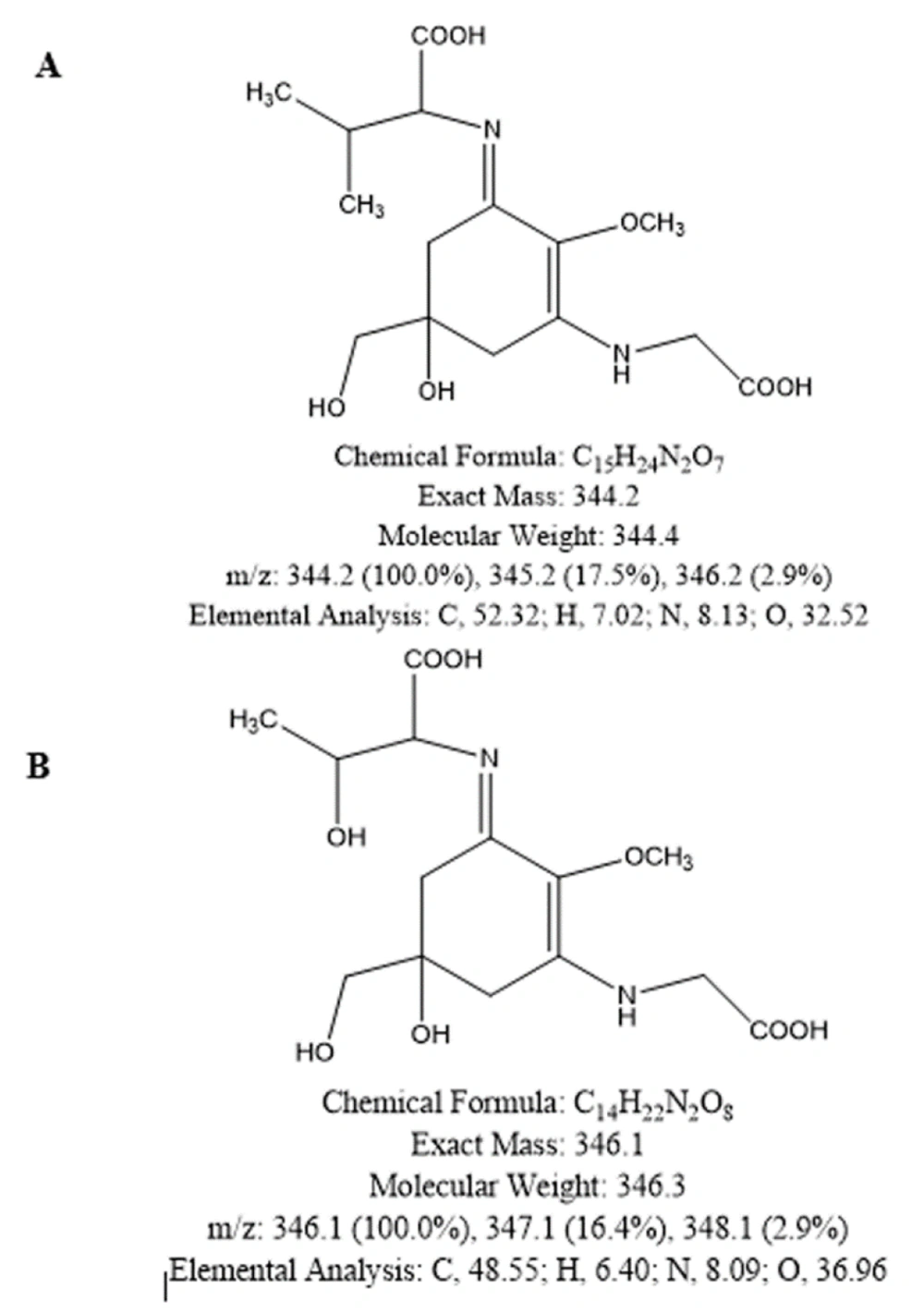
The optimized factors were considered for a new run of MAA extraction from 10 mg dried biomass of Fischerella sp. F5, and then, the extracted sample was analyzed by HPLC once more. The extracted sample of the optimized model was further analyzed by LC/MS. The main peak with λmax = 335 nm has m/z of 344 (Figure 4), which showed the most similarity with the features corresponding to mycosporine-glycine-valine (Figure 5A) or porphyra (Figure 5B). The peak of 887, which was seen in the MS spectrum, may be related to MAA derivatives, such as glycosylated MAAs.
4.4. Antioxidant Effect of Selected Mycosporine-Like Amino Acids Extracts
The results of the antioxidant investigation of the selected extracts were reported in Table 4.
| Runs | Antioxidant(%) | Runs | Antioxidant(%) |
| 1 | 59.38 ± 2.8 | 17 | 21.58 ± 8.5 |
| 2 | 32.84 ± 9.7 | 18 | 21.98 ± 0.57 |
| 3 | 25.40 ± 1.4 | 19 | 27.81 ± 1.99 |
| 4 | 47.92 ± 1.99 | 20 | 25.60 ± 1.4 |
| 5 | 39.88 ± 7.1 | 21 | 31.03 ± 8.25 |
| 6 | 35.45 ± 5.97 | 22 | 25.80 ± 1.4 |
| 7 | 39.88 ± 1.4 | 23 | 25.10 ± 1.4 |
| 8 | 41.29 ± 0.57 | 24 | 20.78 ± 3.4 |
| 9 | 31.63 ± 3.41 | 25 | 14.95 ± 1.4 |
| 10 | 47.92 ± 1.42 | 26 | 32.44 ± 18.2 |
| 11 | 46.71 ± 1.99 | 27 | 39.48 ± 19.1 |
| 12 | 26.10 ± 1.4 | 28 | 36.66 ± 2.6 |
| 13 | 25.50 ± 1.4 | 29 | 34.85 ± 15.3 |
| 14 | 35.45 ± 5.4 | 30 | 42.49 ± 0.1 |
| 15 | 28.82 ± 5.7 | 31 | 54.15 ± 8.5 |
| 16 | 34.25 ± 6.5 | 32 | 54.96 ± 9.7 |
Antioxidant Activity of Extracted Samples a
Accordingly, it seems that the samples which were extracted with 100% methanol have a higher antioxidant effect. The highest value of antioxidant activity was calculated for runs 31 and 32, in which the highest amount of MAAs was extracted. However, it gives the impression that the antioxidant activity was not directly related to the amount of MAAs content, as seen in the sample of run 18, which showed 21% antioxidant activity compared to the 54.9% of run 32, despite both having the highest amount of MAAs content. Mycosporine-like amino acids extracts% in the extract of run 18 was about 67% compared to 89% for run 32; therefore, the antioxidant activity might be negatively influenced by impurities rather than MAAs, which were present in the extract. According to the experimental design conditions applied for MAA extractions, it might be concluded that a low concentration of methanol, either in low or high temperature of the extraction process, resulted in similar antioxidant activities, as seen in samples of run 17 and 18, respectively. However, statistical analysis could not find any significant relationship between the amount or percentage of MAAs and antioxidant activity. It might be concluded that a change in extraction conditions might change the type of chemical compound in the final extract.
5. Discussion
One of the important metabolites extracted from microalgae is MAAs. Mycosporine-like amino acids consist of approximately 20 relatively similar water-soluble compounds, and various methods for their extraction and identification have been reported (13). According to previously published work, Fischerella sp. F5 was found to produce UV absorbent compounds. The MAA compounds discovered had a retention time of about 3.5 to 5 minutes with UV absorption at 330 - 340 nm (9). Additionally, the effect of culture optimization on MAA production showed that increasing nitrate and phosphate ion concentrations could enhance the yield of MAAs production (14). We used the experimental design method to optimize five influential factors on extraction efficiency. To minimize the number of tests required, the central composite design was applied. This approach has previously been used for optimizing MAAs production (8) and extracting R-phycoerythrin-enriched extracts from Sarcopeltis skottsbergii (15).
Metabolites extracted from cyanobacteria and microalgae have been widely studied and have shown promising applicability in the fields of agriculture, energy supply, pharmaceuticals, nutrition, and cosmetic-sanitary industries. Cyanobacterial metabolites exhibit diverse and valuable biological activities, including antimicrobial, anti-inflammatory, and UV-absorbing properties (16). The main compound detected in the optimized extraction product showed a maximum absorbance at around 335 nm, with a mass-to-charge ratio of 344. These features are highly similar to the characteristics of Mycosporine-glycine-valine. This MAA was previously reported from Euphausia superba under photosynthetically active radiation (PAR) with additional ultraviolet radiation (UVR) (17), Phaeocystis antarctica (18), and Artemia urmiana under PAR and low salinity conditions (19). Recently, an in silico study claimed that mycosporine–glycine–valine has the potential to effectively inhibit angiotensin-converting enzyme (ACE2), and consequently, it could be explored as a therapeutic agent for coronavirus disease 19 (COVID-19) (20). Moreover, a peak of higher molecular weight was also observed in the MS spectrum; it may be a glycosylated MAA. Glycosylated MAAs have been previously reported in Nostoc commune with m/z of 1051 and 721, which exhibited 27% antioxidant activity (21).
According to the results, it seems that this type of experimental design could not precisely lead to the best condition with the maximum amount of MAA (as an index for extraction) and MAA% (as an index for purification). However, this approach has previously been applied to optimize the extraction process of a biliprotein from a red macroalgae Sarcopeltis skottsbergii (15), as well as to obtain extracts with high antioxidant activity from a red seaweed Gracilaria mammillaris (22).
The effects of solid–liquid ratio, extraction time, and temperature on the yield of MAA extraction from four red macroalgae, including Bangia fusco-purpurea, Gelidium amansii, Gracilaria confervoides, and Gracilaria sp., were evaluated. It was found that increasing these factors could lead to an increase in MAA extraction yields, reaching a maximum level at specific values of these factors (23). Our study also revealed that elevating the extraction time and temperature and increasing the solvent/biomass ratio could enhance the yield of MAA extraction.
5.1. Conclusions
According to our findings, the optimal conditions predicted for the maximum yield of MAA extraction from Fischerella sp. F5 indicate that using a 3-volume ratio (related to biomass) of 10% methanol, 120 minutes of sonication time, and maintaining the temperature of 50°C for 24 hours could result in the best MAA extraction. Verification of the best condition showed that this process could yield MAA content similar to the best run of experiments, with an MAA% of 60 ± 2.2%, falling within the medium range.