1. Background
Breast cancer is one of the most common cancers in women worldwide, representing the most frequently diagnosed malignancy. In the female population, it has the highest mortality rate among neoplasms (1, 2). In Peru, breast cancer was the second most prevalent type of cancer in both genders in 2022 (2). Evidence shows that the burden of disease, economic impact, and mortality of breast cancer are intensifying in middle- and low-income countries (3).
Numerous studies have demonstrated the effectiveness of medicinal plants in treating various illnesses, including hypertension, atherosclerosis, arrhythmia, high cholesterol, and heart disease prevention (4). For instance, Lavandula angustifolia, commonly referred to as "lavender," exhibits stress-reducing properties that may help avert the onset of cardiovascular diseases (5). Additionally, research indicates that extract from Withania somnifera, known as "Ashwagandha," may improve sleep quality in adults (6). Furthermore, studies suggest that combining phytotherapy with Western medicine is more effective in treating coronary lesions in children with Kawasaki disease than relying solely on Western medicine (7). This highlights the numerous benefits of medicinal plants in treating various medical conditions. Medicinal plants have also become increasingly valuable in cancer management. Studies have shown that flavonoids such as genistein, epigallocatechin, and resveratrol can stop the progression and prevent future metastasis of breast cancer (8). Currently, several chemotherapeutic drugs are isolated from natural products, such as vinblastine and vincristine derived from the plant Catharanthus roseus (9).
For breast cancer, multiple treatments such as adjuvant chemotherapy and hormonal therapy may be employed (10). However, these treatments have side effects that affect patients in various ways. Chemotherapy may induce vomiting, nausea, diarrhea, predisposition to infections, and hair loss, while hormonal therapy can lead to hot flashes and night sweats (11). For this reason, complementary therapies that do not involve conventional medicine, such as acupuncture, phytotherapy, and nutritional therapies, are often considered (12).
Phytotherapy is proving to be highly beneficial, making it a relevant complementary or alternative therapy in the treatment of cancer (13). Approximately 50,000 species of medicinal plants constitute 10% of all plant species worldwide (14). Among these, D. spruceanum, formerly known as Dracontium loretense Krause in the Peruvian jungle, holds considerable promise. It is commonly used by rubbing the plant on the skin before entering the jungle or after a bite from the snake Bothrops atrox to neutralize the lethal activity of the poison. The inhabitants of the Peruvian jungle also use it for diseases such as epilepsy, hernia, diarrhea, asthma, herpes, and cancer (15). In addition, D. spruceanum lowers blood glucose levels and has antidiarrheal and antibacterial effects on some gram-positive germs (16, 17). It has also been shown to have antineoplastic functions in Rattus rattus var. albinus with breast cancer (18). However, despite extensive research into its health benefits, the impact of D. spruceanum on human breast cancer cells is still unknown.
2. Objectives
To determine the cytotoxic effect of the chloroform extract and fractions of D. spruceanum on breast cancer cell lines.
3. Methods
3.1. Biological Material
Two breast cancer cell lines were used: MDA-MB-231 (HTB-26) and MCF-7 (HTB-22) (ATCC©, USA). These were stored in liquid nitrogen vapor and provided by the Cell Culture and Immunology Laboratory at Universidad Científica del Sur, Peru. Dracontium spruceanum bulbs were collected from Lamas city in the San Martín Department, at an altitude of 310 to 920 meters above sea level in Peru. A specimen was used for taxonomic identification at the Museo de Historia Natural of the Universidad Nacional Mayor de San Marcos and was collected for research purposes as authorized by the local authority (Servicio Nacional Forestal y de Fauna Silvestre, SERFOR).
3.2. Preparation of Chloroform Extract and Fractions
Chloroform extract and fractions were prepared as previously reported (19). The plant material was washed with tap water, chopped, and dried at 37°C, then crushed into a fine powder. A total of 500.35 grams of this powder was mixed with 800 mL of chloroform (Sigma-Aldrich, USA) and macerated for 24 hours, followed by three hours of sonication in an ultrasound bath (3510, Branson, USA) at 40°C. The solvent was then evaporated in a rotary evaporator (RV10, IKA, China) at 40°C and 340 mbar, and the resulting chloroform extract (eDSBCl) was left to rest in a desiccator for 72 hours.
To separate the chromatographic fractions, eDSBCl was dissolved in 10 mL of chloroform and eluted in a 50 × 4 cm glass chromatographic column with silica gel G-60 (Supelco, USA). Hexane (Sigma-Aldrich, USA), chloroform (Sigma-Aldrich, USA), chloroform-acetone (Sigma-Aldrich, USA), acetone-methanol (Sigma-Aldrich, USA), methanol, and water were used as solvents and added according to their polarity. Eluates were collected and grouped into 11 chromatographic fractions labeled as F1, F8, F21, F37, F39, F40, F43, F46, F49, F50, and F52. The solvent was then completely removed through evaporation, and the resulting dried fractions were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, USA). The fractions were aliquoted and stored at -80°C until needed for further use.
3.3. Cell Line Culture and Storage
MDA-MB-231 and MCF-7 cell lines were cultured according to the manufacturer's protocol with some modifications. The cell lines were cultured in Dulbecco's Modified Eagle Medium (DMEM) with nutrient mixture F-12 (DMEM/F-12) (Sigma-Aldrich, USA), 10% fetal bovine serum (FBS) (Biowest, France), and 1X antibiotic-antimycotic solution (Biowest, France). The MDA-MB-231 and MCF-7 cell lines were incubated at 37°C with 5% CO2 (Series 8000 DH, Thermo Scientific™, USA), and splitting was done when confluency reached 60 - 80%.
3.4. Cytotoxicity Screening of DsBCl Fractions
Cell lines were counted using a Neubauer chamber, and 7.5 × 103 MDA-MB-231 cells and 5 × 103 MCF-7 cells per well were seeded in 96-well plates (Corning, USA). After 24 hours, cells were treated with 100 μg/mL of the eleven chromatographic fractions and incubated for 24 and 48 hours. Since the fractions were dissolved in DMSO, the final DMSO concentration was 0.5%. A negative control group containing 0.5% DMSO (used as the vehicle) was included, along with a positive control for cytotoxicity consisting of 10% DMSO.
For cell viability analysis, an MTT assay (20) was performed with some modifications. After 24 and 48 hours of incubation, 20 µL of MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] (Sigma-Aldrich, USA) was added to each well at a final concentration of 0.5 mg/mL and incubated for 3 hours. Metabolically active cells reduced MTT to formazan crystals, which were then dissolved with 100 µL of DMSO. Finally, optical density (OD) was read at 570 nm and 600 nm wavelengths in a multimodal plate reader (Synergy LX, Biotek, USA). Each concentration was tested three times in separate experiments, with each experiment being replicated three times.
3.5. Determination of Half-maximal Inhibitory Concentration (IC50)
To determine the half-maximal inhibitory concentration of cell growth (IC50), the chromatographic fractions exhibiting greater cytotoxicity were used. First, MDA-MB-231 and MCF-7 cells were seeded in 96-well plates at a density of 7.5 × 103 and 5 × 103 cells per well, respectively, and incubated at 37°C with 5% CO2. After 24 hours, the cell culture medium was replaced with medium containing fractions F43 and F50 at decreasing concentrations (100, 50, 25, 12.5, 6.25, 3.125, and 1.5625 μg/mL) and incubated for 24 and 48 hours, as described in (21). Cell viability analysis was conducted using the MTT technique, following the previously described protocol. Each concentration was tested three times in separate experiments, with each experiment being replicated three times.
3.6. Morphological Changes in MDA-MB-231 and MCF-7 Cells
Cell morphology was observed during each experiment using an inverted phase-contrast microscope (Nikon, Eclipse TI, Japan). Photographs were taken for each concentration, and untreated cells (vehicle) were used as the control.
3.7. Data Analysis
To determine the cell viability percentage, OD values were subtracted from the blank average. This result was then divided by the average of the negative control and multiplied by 100%. Cells treated with the vehicle (0.5% DMSO) were used as the negative control.
Comparisons of experimental and control groups were performed using one-way Analysis of Variance (ANOVA) and Tukey’s post-hoc test. For IC50 calculation, a nonlinear regression analysis was conducted using cell viability percentage and the logarithm of the evaluated concentrations. Each experiment was conducted in triplicate, and a total of three independent experiments were performed. Furthermore, we employed the Student's t-test to compare the IC50 values between the two cell lines. All statistical analyses were performed using GraphPad Prism Software version 8.02.
4. Results
4.1. Dracontium spruceanum Bulb Fractions Induced Morphological Changes in MDA-MB-231 and MCF-7 cell Lines
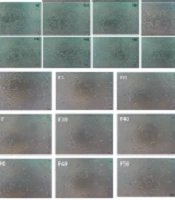
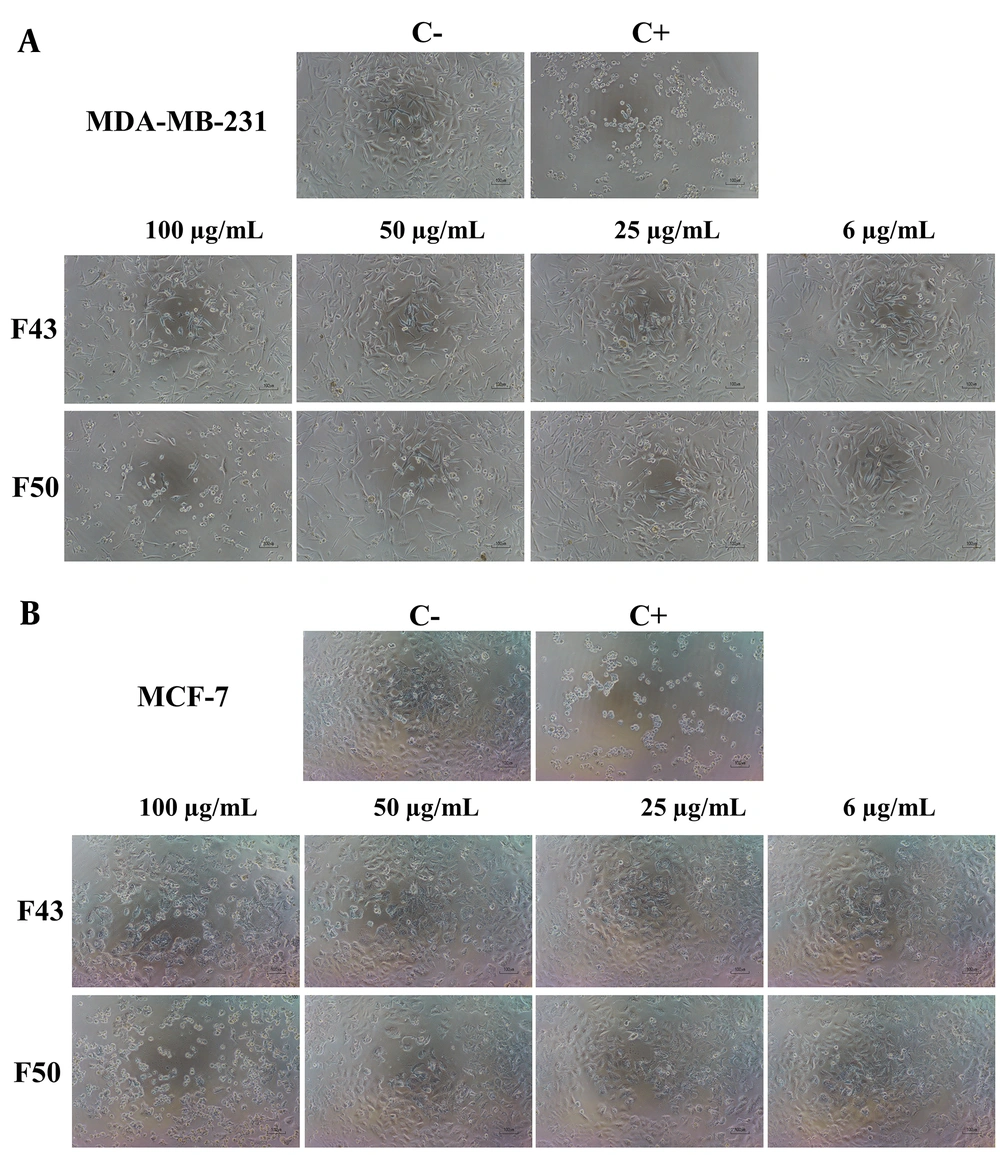
Microscopic examinations revealed noticeable changes in both MDA-MB-231 and MCF-7 cell lines following exposure to the eleven chromatographic fractions derived from eDSBCl. Compared to the control group, both cell lines exhibited an increased presence of cells in suspension, cell fragments, and a reduced number of attached cells. These changes were particularly evident in cells treated with F43 and F50, as depicted in Figures 1A and 1B for MDA-MB-231 and Figures 1C and 1D for MCF-7. Similar effects were also noted in cells exposed to other chromatographic fractions.
MDA-MB-231 cell line treated with 100µg/mL of eleven chromatographic fractions of the chloroform extract of Dracontium spruceanum bulb evaluated at A,24 hours; and B, 48 hours. MCF-7 cells treated with 100µg/mL of eleven chromatographic fractions of the chloroform extract of D. spruceanum bulb evaluated at C, 24 hours; and D, 48 hours.
4.2. Cell Viability After Cytotoxicity Screening with the Fractions
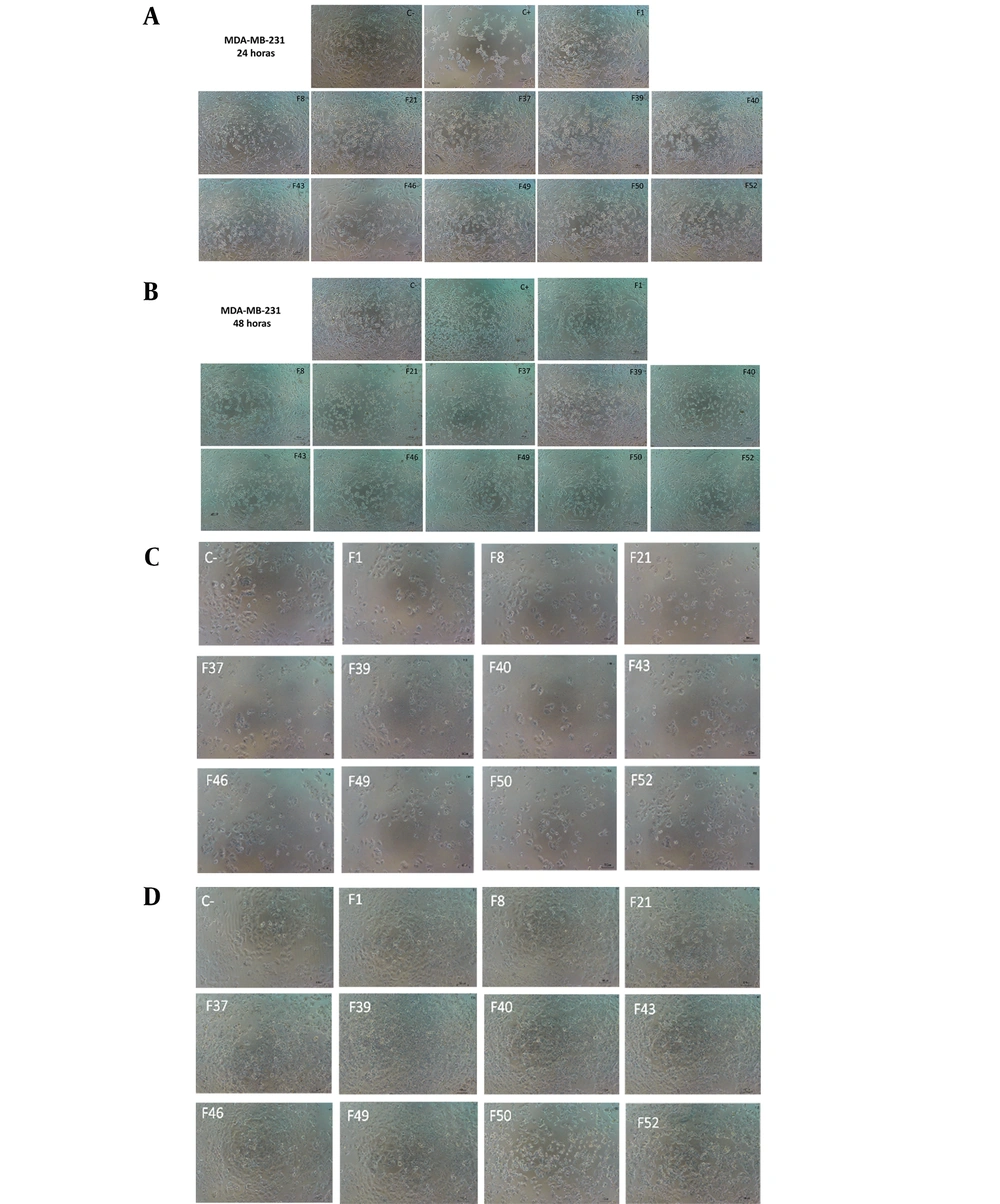
To quantify the cytotoxic effect of the eDSBCl fractions, the cell viability percentage was calculated and is presented as mean values ± standard deviation in Table 1. Notably, F43 and F50 significantly reduced the cell viability of MDA-MB-231 cells at 24 hours and MCF-7 cells at 48 hours, exhibiting the highest cytotoxic effect on cell viability among all the fractions, as illustrated in Figure 2. These findings indicate a marked decrease in viability in both cell lines when treated with these fractions compared to the control group.
| Fractions | Cell Viability (%) | |||
|---|---|---|---|---|
| MDA-MB-231 | MCF7 | |||
| 24 h | 48 h | 24 h | 48 h | |
| F1 | 111.99 ± 6.47 | 102.32 ± 5.31 | 99.54 ± 26.95 | 101.33 ± 18.68 |
| F8 | 113.23 ± 5.06 | 103.05 ± 5.94 | 96.23 ± 19.08 | 94.62 ± 11.59 |
| F21 | 114.8 ± 8.61 | 95.56 ± 7.54 | 94.09 ± 22.18 | 87.79 ± 17.22 |
| F37 | 93.01 ± 14.91 | 85.59 ± 3.25 | 86.89 ± 21.44 | 79.28 ± 14.02 |
| F39 | 82.75 ± 8.12 | 79.2 ± 8.10 | 80.65 ± 17.19 | 90.36 ± 8.67 |
| F40 | 82.62 ± 7.10 | 75.94 ± 7.70 | 74.4 ± 17.75 | 87.93 ± 11.61 |
| F43 | 65.89 ± 7.47 | 62.26 ± 5.45 | 71.45 ± 20.06 | 67.36 ± 12.28 |
| F46 | 92.28 ± 5.59 | 90.87 ± 10.24 | 77.81 ± 15.23 | 80.95 ± 15.93 |
| F49 | 105.55 ± 5.01 | 102.66 ± 3.73 | 86.65 ± 15.39 | 85.1 ± 7.27 |
| F50 | 69.77 ± 17.77 | 84.05 ± 11.41 | 66.03 ± 13.09 | 59.09 ± 5.53 |
| F52 | 70.62 ± 10.28 | 81.24 ± 5.16 | 78.44 ± 10.32 | 70.13 ± 12.28 |
In order to assess the cytotoxic impact of the eDSBCl fractions, we computed the cell viability percentage and reported it as mean values ± standard deviation in Table 1. Remarkably, F43 and F50 demonstrated significant reductions in cell viability for MDA-MB-231 cells after 24 hours and for MCF-7 cells after 48 hours, displaying the most pronounced cytotoxic effects on cell viability among all the fractions, as depicted in Figure 2. These results suggest a substantial decrease in viability in both cell lines when exposed to these particular fractions compared to the control group.
4.3. Cell Morphology Treated with Different Concentrations of F43 and F50
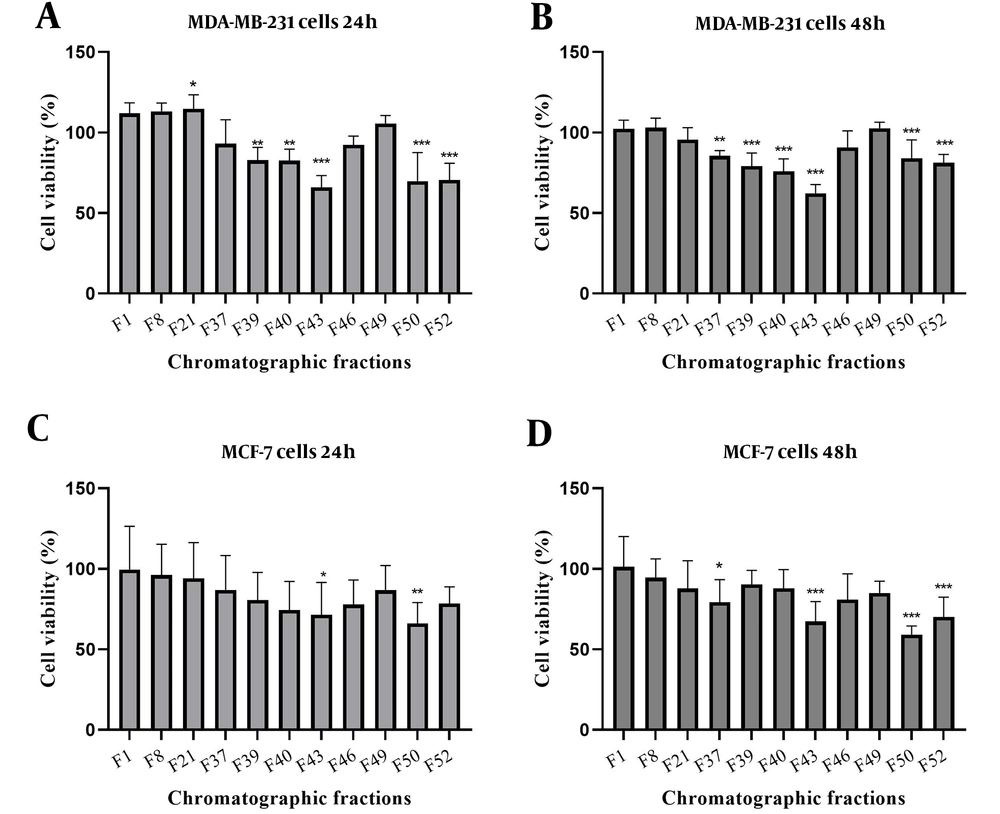
A dose-dependent effect on cell morphology was observed in both cell lines. Cells in suspension were considered dead, while attached cells were deemed viable. Notably, cell detachment began at a concentration of 50 µg/mL for both cell lines. In contrast, most cells in the control group remained attached, highlighting the specific impact of the treatment on cell morphology (Figure 3).
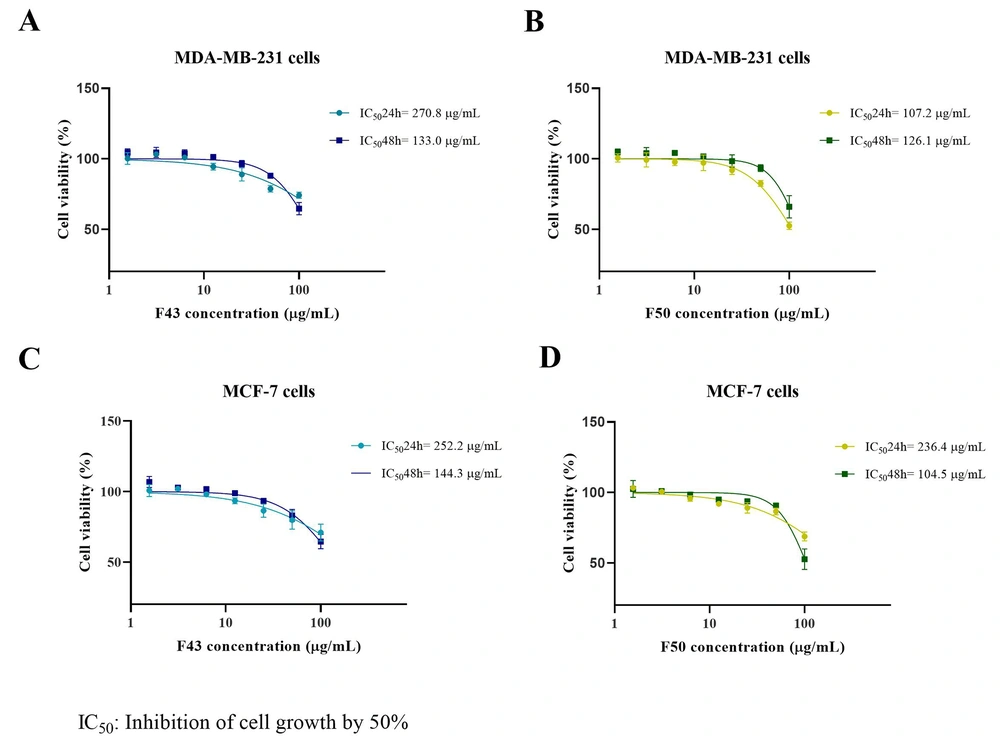
4.4. Half-maximal Inhibitory Concentration (IC50) of eDSBCl Chromatographic Fractions
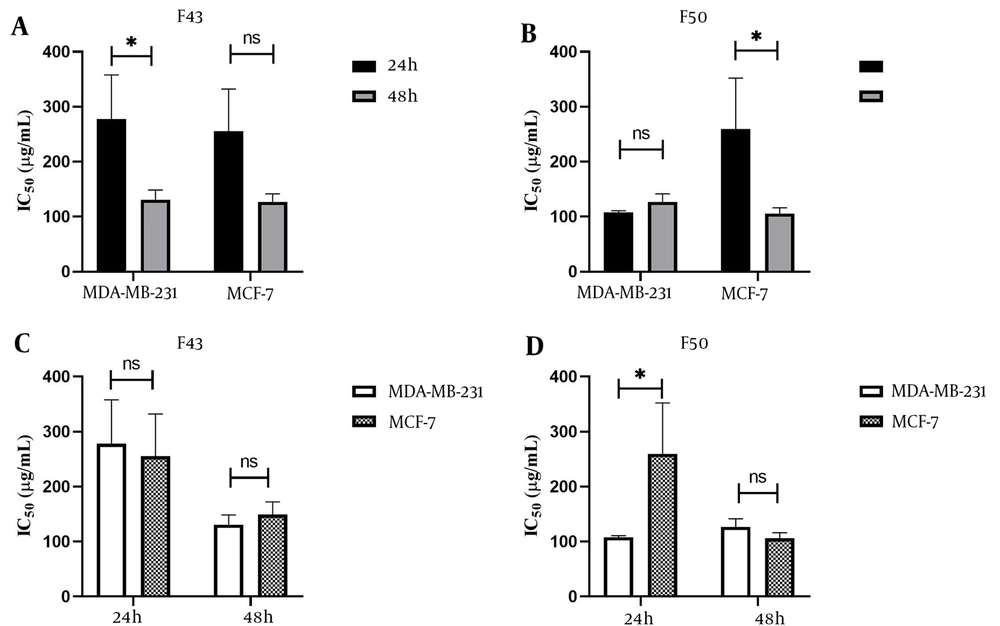
The IC50 values for F43 and F50 were determined in both MDA-MB-231 and MCF-7 cells at 24 and 48 hours of exposure. For F43, the IC50 values were 270.8 µg/mL in MDA-MB-231 and 252.2 µg/mL in MCF-7 cells at 24 hours, decreasing to 133.0 µg/mL and 144.3 µg/mL, respectively, at 48 hours. For F50, the IC50 values were 107.8 µg/mL in MDA-MB-231 and 206.8 µg/mL in MCF-7 cells at 24 hours, reducing to 126.7 µg/mL and 108.1 µg/mL, respectively, at 48 hours. t-tests showed no significant difference between 24-hour and 48-hour IC50 values, except for F43 in MDA-MB-231 cells (P < 0.05) and F50 in MCF-7 cells (P < 0.05) (Figure 4). Additionally, the comparison between IC50 values of F43 and F50 among both cell lines showed no significant differences, except for F50 at 24 hours, as illustrated in Figure 5.
Half-maximal Inhibitory Concentration (IC50) of the cytotoxic effect on the cell line MDA-MB-231 with the chromatographic fractions of the chloroform extract of Dracontium spruceanum bulb A, F43; and B, F50. Half-maximal Inhibitory Concentration of the cytotoxic effect on the MCF-7 cell line with the chromatographic fractions of the chloroform extract of D. spruceanum bulb C, F43; and D, F50. The IC50 values at 24 and 48 h for fraction 43 and 50, respectively, are shown in the upper right of each graph.
5. Discussion
The aim of this study was to investigate the cytotoxic effect of chromatographic fractions derived from the chloroform extract of D. spruceanum on breast cancer cell lines MDA-MB-231 and MCF-7. Among the eleven fractions evaluated, two fractions, specifically F43 and F50, demonstrated a moderate cytotoxic effect on these cell lines. This finding highlights the potential of specific metabolites within D. spruceanum chloroform extract as effective agents against breast cancer.
The IC50 values for F43 on MDA-MB-231 cells were 270.8 ± 18.07 µg/mL and 133.0 ± 17.99 µg/mL at 24 and 48 hours, respectively, and for F50, the IC50 values were 107.2 ± 2.97 µg/mL and 126.1 ± 14.77 µg/mL at 24 and 48 hours, respectively. These results are significantly lower than those reported by Isbilen and Volkan, who showed that Allium willeanum H. bulb (AWB) extract had cytotoxic activity with IC50 values of 1207.3 ± 189 µg/mL at 24 hours and 862.6 ± 112 µg/mL at 48 hours in the same cell line (22). Other studies found an IC50 value of 347.44 ± 98.78 μg/mL for the MDA-MB-231 cell line using an aqueous extract of Ardisia crispa (23) and an IC50 value of 315.22 µg/mL at 48 hours using lipophilic extracts of leaves of Cynara cardunculus L. var altilis with antiproliferative effects (24).
This study also examined the effectiveness of F43 and F50 on the MCF-7 cell line. The IC50 values obtained for F43 and F50 are lower than those reported by Isbilen and Volkan for Allium willeanum H. bulb extract, specifically 2972.2 ± 234 µg/mL at 24 hours and 1076.7 ± 118 µg/mL at 48 hours (22). Another investigation found that the aqueous extract of Ardisia crispa had an IC50 value greater than 1000 μg/mL at 72 hours (23). Nasseri et al. evaluated the cytotoxic effect of extracts and fractions of Artemisia haussknechtii on MCF-7 cells, showing IC50 values of 987.98 ± 4.23 µg/mL in the extract after 48 hours. The petroleum ether, dichloromethane, and n-butanol fractions had IC50 values of 377.18 ± 3.36 µg/mL, 297.17 ± 7.99 µg/mL, and 1094.85 ± 9.24 µg/mL, respectively, with dichloromethane having the highest cytotoxic effect (25). Finally, a study on the effects of extracts and fractions of Glochidion velutinum showed IC50 values of 522 µg/mL, 523 µg/mL, and 431 µg/mL for the aqueous fractions, n-hexane, and G. velutinum extract, respectively, on MCF-7 cells (26). Therefore, D. spruceanum could contain diverse metabolites with greater cytotoxic activity than those reported in other plants (22, 23, 25, 26).
Over time, the IC50 values of F43 and F50 decreased in the MCF-7 cell line, indicating a time-dependent effect. This trend was also observed for F43 in the MDA-MB-231 cell line, but for F50, there was a non-significant increase in IC50 value at 48 hours. This may be due to the unique characteristics of each cell line; MDA-MB-231 cells represent triple-negative breast cancer subtypes, which tend to behave more aggressively and are less differentiated than other breast cancer types such as MCF-7 cell lines. MCF-7 cells represent a luminal subtype that is positive for estrogen receptors and retains some epithelial characteristics (27). It is possible that the cancer cells may show signs of recovery after exposure to the extract, as reported in a previous study using the chloroform fraction of Buddleja incana on the gastric cancer cell line AGS (28). However, additional research at the molecular level is necessary to confirm this hypothesis.
In this study, chloroform was used as the initial solvent because it has been reported to extract plant terpenoids, flavonoids, and other metabolites (29), which have potential anticancer activity (30). The chloroform extract of Commelina benghalensis exhibited the best cytotoxic activity among methanol, ethanol, benzene, and hexane extracts in MDA-MB-231 cells, with IC50 values of 134 and 79 µg/mL at 24 and 48 hours, respectively (31). It has been determined that a chloroform concentration below 0.1% shows no cytotoxic effect on cancer cells (32).
Dracontium spruceanum is a medicinal plant that contains various secondary metabolites, including alkaloids, flavonoids, and coumarins (33). These compounds have demonstrated anticancer activity, with flavonoids like epigallocatechin, genistein, and resveratrol being particularly effective at suppressing tumor growth and preventing metastasis in breast cancer (8). Investigations on gastric cancer revealed that the hexane fraction of D. spruceanum leaves and the chloroform fraction of the bulbs exhibited significant cytotoxic effects on the AGS cancer cell line, with IC50 values of 37.87 µg/mL and 82.59 µg/mL, respectively (measured at 24 hours). However, the methanol extract of Dracontium loretense leaves did not show any cytotoxicity on non-cancerous MDCK cells, even at high concentrations (34). Further research is needed to determine the toxicity of D. spruceanum on healthy human cells, but its bioactive compounds suggest its potential as a promising therapeutic option for cancer treatment (15).
5.1. Conclusions
In conclusion, the chromatographic fractions of the chloroform extract of the D. spruceanum bulb have demonstrated efficacy against MCF-7 and MDA-MB-231 breast cancer cell lines. This study reveals that fractions F43 and F50 have the highest cytotoxic effects compared to other fractions. Moreover, F50 showed better cytotoxic activity against MDA-MB-231, a triple-negative breast cancer cell line, making it a promising candidate for development as a therapeutic agent. However, further studies are necessary to fully comprehend the underlying mechanisms of this effect and to assess its effectiveness and safety in preclinical models. Additionally, further chemical, biochemical, and biological studies are needed to elucidate the composition of the metabolites responsible for the cytotoxic activity.