1. Background
Multiple sclerosis (MS) is a prevalent chronic disorder of the central nervous system (CNS) that primarily affects younger adults and significantly contributes to long-term neurological impairment. Multiple sclerosis is a debilitating condition characterized by immune-mediated demyelination, inflammation, and neurodegenerative tissue destruction in the CNS (1). It is the most common chronic demyelinating disease in young adults and the leading nontraumatic cause of impairment, affecting over 400,000 Americans and 2.5 million people worldwide. Although MS can be contracted at any age, it is most often diagnosed between 20 and 40 years old. The prevalence in women is 2:1 compared to men, and northern Europeans are more likely to have the condition (2, 3).
Multiple sclerosis has four classifications based on the disease's development pattern: Relapsing-remitting MS (RRMS), secondary-progressive MS (SPMS), primary-progressive MS (PPMS), and progressive-relapsing MS (PRMS), as per the National MS Society (3). Approximately 10% to 15% of patients experience a gradual and continuous deterioration of symptoms from the initial stages without identifiable periods of exacerbation, categorizing them as having a progressive main course (4). Unfortunately, there is no cure for MS; however, 12 disease-modifying therapies (DMTs) are available to treat RRMS. Some of these drugs target the disease's immunological pathophysiology. Glatiramer acetate (GA) and beta-interferons are the primary treatments for RRMS (5). The medication can be administered either subcutaneously or intramuscularly. Although these medications are generally deemed safe, their effectiveness is relatively low (6).
glatiramer acetate's chemical structure mimics myelin basic protein (MBP), misleading antibodies under certain conditions. This decoy mechanism helps reduce inflammation. The inflammatory response makes the blood-brain barrier more susceptible to demyelination and axonal damage (7, 8). Glatiramer acetate, which creates antigens that mimic MBP, is investigated as an autoantigen in MS and has been shown to cause experimental autoimmune encephalomyelitis (EAE), a common MS animal model (8). Glatiramer acetate undergoes hydrolysis at the injection site, enabling its interaction with both antigen-presenting cells (APCs) and lymphocytes (9). Some substances can enter the lymphatic system, be released into lymph nodes, or enter the bloodstream. Animal models administered radiolabeled GA doses showed that the stomach and thyroid exhibited the highest concentrations of GA, whereas the CNS displayed the lowest levels. The hydrophilic nature of GA and its metabolites may hinder their ability to pass through the blood-brain barrier, thus restricting their therapeutic effects to peripheral regions (10). Glatiramer acetate medication is associated with several frequently occurring adverse effects, with an incidence of 10% or more, including injection-site reactions, chest discomfort, rash, dyspnea, and vasodilation (3).
This research aims to develop a nanoliposomal formulation of GA that can cross biological barriers and improve therapeutic efficacy. Liposomes offer many advantages as drug carriers, such as targeted localization, prolonged or controlled release, protection against degradation and elimination, improved therapeutic efficacy, and reduced toxicities compared to conventional drug delivery systems (11). By employing this new approach, the number of drug administrations and related side effects are reduced, leading to increased patient satisfaction and decreased treatment costs.
Liposomes are drug vesicles composed of self-assembled phospholipids arranged into either a single-layered bilayer (unilamellar) or multiple-layered bilayers (multilamellar), enclosing a core compartment filled with aqueous solutions (12). They vary in size from 30 nm to the micrometer scale, with a phospholipid bilayer thickness of approximately 4 - 5 nm (13). As carriers for drugs, liposomes possess remarkable attributes, including the ability to safeguard the enclosed molecules from physiological breakdown (14). The goal is to extend the medicine's effectiveness by increasing its half-life and controlling the release of drug molecules (15-18). Furthermore, liposomes exhibit exceptional biocompatibility and a high level of safety. They can also transport their cargo in a targeted manner to the specific site of disease, using passive and/or active targeting mechanisms. This focused distribution minimizes widespread negative impacts, increases the highest acceptable dose, and improves treatment effectiveness (19, 20). An extensive analysis of the drug database from the official websites of the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) revealed that 14 distinct liposomal products have obtained official authorization (Table 1).
| API | Approved Year | Indication |
|---|---|---|
| Doxorubicin hydrochloride (DOX-HCL) | 1995 | Ovarian cancer, Kaposi's sarcoma, and myeloid melanoma |
| Daunorubicin (DOX) | 1996 | Kaposi's sarcoma |
| Amphotericin B | 1997 | Fungal infection affecting the entire body |
| Cytarabine | 1999 | Lymphomatous meningitis |
| DOX.HCI | 2000 | Breast cancer |
| Verteporfin | 2000 | Wet AMD |
| Morphine | 2004 | Postoperative pain |
| MTP-PE | 2009 | Osteosarcoma |
| Bupivacaine | 2011 in USA; 2020 in Europe | Postoperative analgesia |
| Vincristine Sulfate | 2012 | Leukemia |
| Irinotecan hydrochloride trihydrate | 2015; 2016 | Pancreatic adenocarcinoma |
| Daunorubicin, cytarabine | 2017 in USA; 2018 in Europe | Leukemia |
| Recombinant varicella-zoster virus glycoprotein E | 2018 | For protection against shingles and post-herpetic neuralgia |
| Amikacin sulfate | 2018 in USA; 2020 in Europe | Lung disease |
Camargo-Mascarenhas et al. developed and characterized GA-encapsulated liposomes for MS treatment in 2016. Their study showed that liposomes prepared from DPPG have oval, meticulously structured vesicles with an appropriate diameter and high encapsulation efficiency. Their findings suggest further research on this drug using liposomes (21). Molavi et al. investigated the feasibility of transitioning from a daily injection regimen of GA to a monthly long-acting formulation. This was achieved by employing a design strategy centered around the use of polyester-based polymeric microspheres. Developing parenteral sustained-release microspheres (MPSs) for this immune modulator represents a viable strategy to enhance patient adherence by ensuring improved and prolonged effectiveness (22). The research conducted by Hadidi and Pazukion the preparation, characterization, and in vivo efficacy of GA-hydrogel microparticles demonstrated their potential as an innovative drug delivery system for GA in the treatment of RRMS (23).
2. Objectives
This study aims to prepare a nanoliposomal formulation of GA to improve its physicochemical characteristics, such as drug stability and bioavailability. This new formulation seeks to enhance drug efficacy, reduce side effects, and minimize drug-related toxicity by decreasing the frequency of drug administration.
3. Methods
3.1. Chemicals
The GA powder was procured from Tofigh Daru Research and Engineering Company in Iran. Soybean hydrogenated phosphatidylcholine and 1,2-distearoyl-sn-glycero-3-phosphatidylglycerol sodium salt (DSPG) were purchased from Larodan AB Company in Sweden. Cholesterol was obtained from BioBasic Company in Canada. Chloroform, methanol, acetonitrile, and trifluoroacetic acid (TFA), all of HPLC grade, were supplied by Sigma-Aldrich Company. A quantity of 10 milligrams of the substance was measured, combined with 10 milliliters of distilled water with a pH value of 7.4, and stored at 4°C.
3.2. Preparation of Glatiramer Acetate-Loaded Nanoliposomes
Thin film hydration method was used for the preparation of liposomes. To do this, hydrogenated soybean phosphatidylcholine (HSPC), 1,2-Distearoyl-sn-Glycero-3-Phosphatidylglycerol (DSPG), and cholesterol were mixed in a solvent composed of chloroform and methanol at a 2:1 volumetric ratio (9, 24). After the lipids were completely dissolved, the organic solvent was removed at 50°C and 100 rpm for 60 min using a Rotary evaporator (WB Eco Laborota 4000 Model, Heidolph Instruments, GmbH). The trace remaining solvents were then eliminated by blowing nitrogen gas for 10 min. The dried lipidic phase was completely dissolved in 10 ml of distilled water containing 10 mg of GA (60°C, 100 rpm, 30 min). The final solution was probe-sonicated (BANDELIN SONOPULS HD 3100, GmbH) under controlled temperature for 10 min (30 sec on/30 sec off) at 30% amplitude. The homogeneous solution was placed at 4°C to equilibrate for further experiments.
3.3. Analysis of Particle Size, Zeta Potential, and Morphology
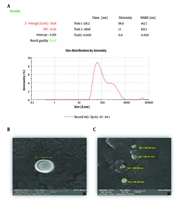
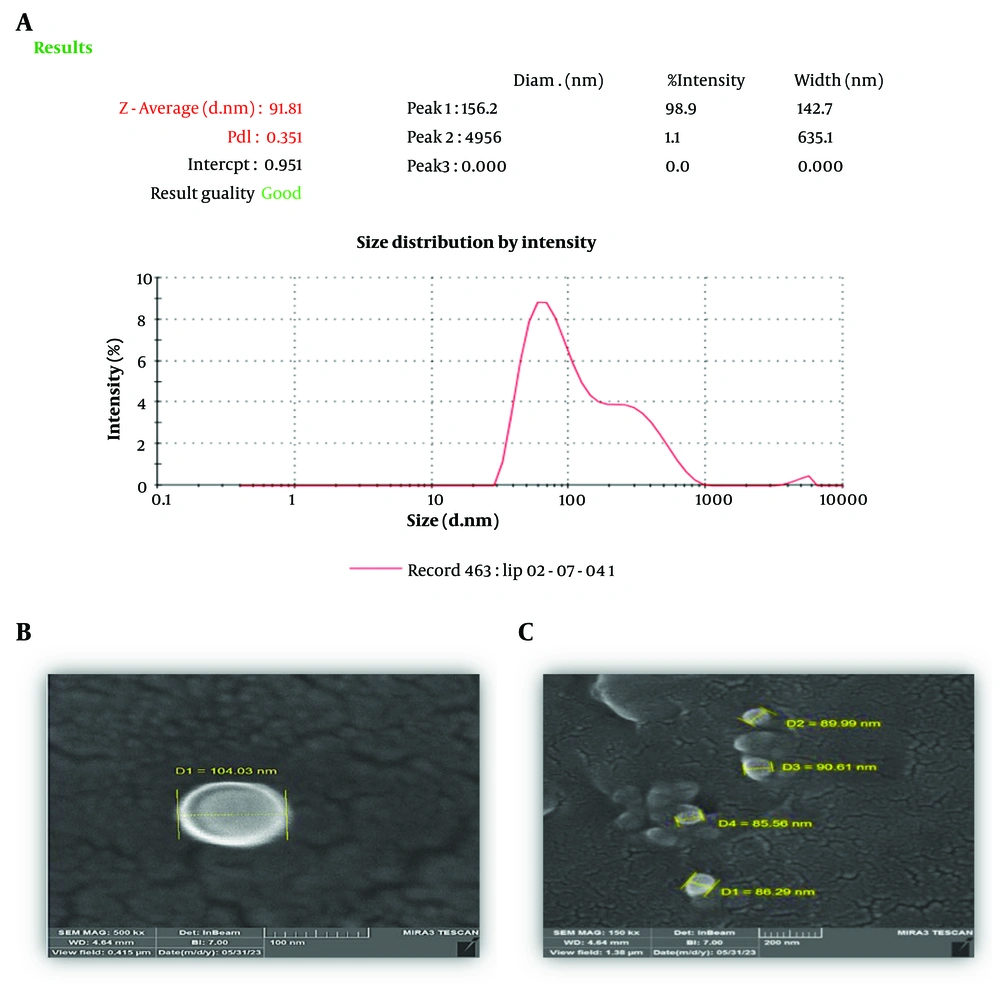
The particle size, Polydispersity Index (PDI), and zeta potential of all formulations were measured using a zeta sizer instrument (Nano-ZS, Malvern Instrument Ltd., UK). The samples were diluted to 1:10 with filtered distilled water and (CA 0.22 µm, Membrane Solution). The hydrodynamic diameter of particles was measured by dynamic light scattering, and the zeta potential of liposomes was evaluated by electrophoretic light scattering. The experiment was conducted at a temperature of 25°C and a scattering angle of 173°. For morphology analysis, a small quantity of liposomal suspension was placed on a gold grid, and the excess liquid was removed using filter paper. The sample was then subjected to vacuum drying for one hour at room temperature (25°C). Liposome morphology was inspected by field emission scanning electron microscopy (FE-SEM). The particle size distribution was confirmed using ImageJ software to analyze FESEM images.
3.4. FTIR Analyze
FTIR analysis was used to investigate possible chemical interactions between GA and the carrier system. Free GA, GA-loaded liposomes, and non-loaded liposomes were individually analyzed by FTIR spectroscopy (AVATAR, THERMO, USA). For this, the lyophilized samples were mixed separately with KBr, and the pellets were formed by placing the samples in a hydraulic press. FTIR analyses were performed in the scanning range of 4000 to 400 cm⁻¹ with a constant resolution of 4 cm⁻¹ at ambient temperature (25°C).
3.5. XRD Analysis
Surface characteristics of the samples were inspected by XRD. This examination aimed to provide insights into the crystalline or amorphous nature of the samples. For this purpose, the samples underwent a drying process using a freeze dryer (Alpha Christ 4 - 1 model, Germany) operating at a temperature of -5°C and a pressure of 0.1 millibar. A small quantity of material (0.2 g) was then finely ground into a powder, uniformly distributed onto the testing plate, and analyzed by the XRD method (PHILIPS instrument, model PW1730, Netherlands).
3.6. Drug Encapsulation Efficiency
The encapsulation efficiency of GA in nanoliposomal vesicles was estimated after separating unentrapped drug from GA-loaded nanoliposomes using Amicon® Ultra Centrifugal Filter Devices (Amicon Ultra-0.5 mL centrifugal filter unit, 30 kDa MWCO, Merck Millipore) (25). The amount of unentrapped drug was measured by reverse phase high-performance liquid chromatography (RP-HPLC). The HPLC system (Knauer Apparatus, SmartLine, Berlin, Germany) was equipped with a reversed-phase column (C18 PerfectSil Target HD column, 4 mm, 25 cm, 5 µm), a SmartLine 2500 pump, and an evaporative light scattering detection (ELSD) system. The mobile phase was a mixture of water, acetonitrile, and TFA (70:30:0.1), which was degassed by sonication. Twenty microliters of filtered (0.22 µm, CA membrane filter) samples were injected into the column under a flow rate of 1 mL/min of the mobile phase. Detection of samples was carried out at 214 nm. The area under the curve (AUC) of the samples was determined, and the amount of GA was estimated from the standard curve equation. The calibration curve was constructed with concentrations of 0.003125, 0.0625, 0.125, 0.25, 0.5, and 1 mg/mL of GA in deionized water. The percent of encapsulation efficiency was then calculated using the following equation:
3.7. In Vitro Release Study
To evaluate the release of drug from nanoliposomes, one milliliter of GA-loaded nanoliposome and one milliliter of drug-free nanoliposome (control) were placed into a dialysis bag with a molecular weight cut-off of 12,400 Da (dialysis tubing cellulose membrane, SIGMA). The dialysis bag was then immersed in 100 mL of the release medium (distilled water), subjected to magnetic agitation, and left undisturbed for 72 hours at a temperature of 37°C. At predetermined time intervals, 1 mL of the release medium was taken and replaced with an equal volume of fresh medium. The amount of drug released into the distilled water was subsequently determined by Bradford assay.
For the Bradford assay, a serial dilution (1, 0.5, 0.25, 0.125, 0.0625, and 0.003125 mg/mL) of standard GA was prepared in distilled water to construct the calibration curve. The mean optical density of each sample (in triplicate) was obtained using a plate reader (Metertech M965, AccuReader instrument, Taiwan) at 595 nm. Finally, the release profile of GA from the carrier was depicted.
3.8. Stability Study
The stability of nanoliposomes containing GA was studied under storage conditions at 4°C for approximately 1 month. For this purpose, changes in parameters such as particle size, homogeneity or PDI, surface charge of nanoparticles (zeta potential), and encapsulation efficiency were inspected.
3.9. Biocompatibility Assay
The 1321N1 (human astrocytoma) cell lines were obtained from the Pasteur Institute of Iran. Cell culture experiments were conducted according to ATCC guidelines, using approved cell culture media and conditions. To do this, 1×104 cells were seeded in each well in Dulbecco's Modified Eagle Medium (DMEM), supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin stock solutions, and incubated in a humidified environment with 5% carbon dioxide (CO2) at 37°C for 24 hours.
Afterward, the cells were treated with various doses of samples (1, 0.5, 0.25, 0.125, 0.062, and 0.003 µg/mL) and subsequently incubated for 48 and 72 hours. At each time point, the proportion of live cells was estimated by adding 20 µl of MTT solution (5 mg/mL) to each well and incubating the plate for 4 hours. After this time, 150 µL of dimethyl sulfoxide (DMSO) was added to each well, and the plate was agitated in the dark for at least 2 hours at room temperature to completely dissolve the purple sedimented formazan. The optical density of the cells was measured using a microplate reader (Metertech M965, AccuReader instrument, Taiwan) at a wavelength of 570 nm. Cell viability was estimated using the following formula:
4. Results
4.1. Analysis of Particle Size, Zeta Potential, and Morphology
Particle size, zeta potential, and polydispersity of the nanoliposomes were 91.2 ± 1.3 nm, -27.3 ± 1.2 mV, and 0.34 ± 0.03, respectively (Figure 1A). The morphology of the vesicles under FESEM demonstrated that the nanoliposomes were uniform in size and spherical in shape (Figure 1B, C).
4.2. Drug Encapsulation Efficiency
The amount of drug encapsulated in the nanoliposome was determined by HPLC as mentioned previously. The encapsulation efficiency (DEE%) was estimated to be approximately 70.2 ± 1.7%.
4.3. FTIR Analysis
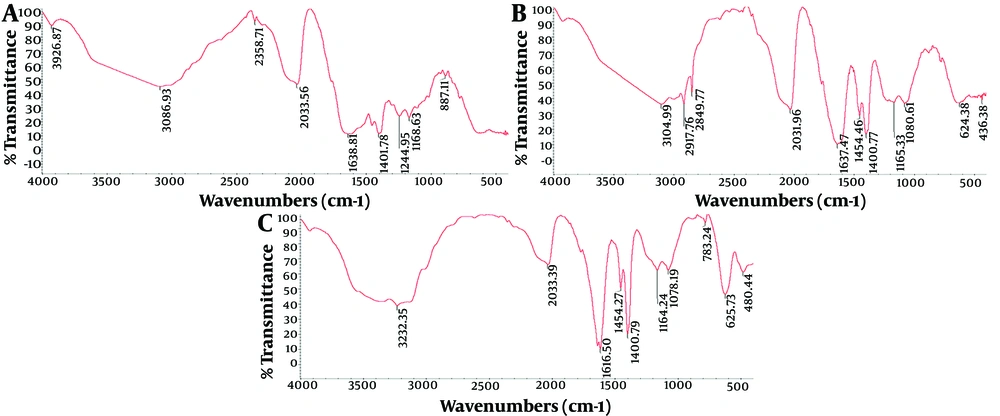
The results of the FTIR analysis are presented in Figure 2. Figure 2A corresponds to standard GA. The spectrum of GA shows a peak at 3086.93 cm⁻¹, which is related to the hydroxyl group (-OH) and the amino group (NH₂) present in the phenol ring of the amino acid tyrosine. Additionally, peaks at 2357.71 cm⁻¹, 2033.56 cm⁻¹, 1638.81 cm⁻¹, and 1401.95 cm⁻¹ correspond to the C-H bond, C=O bond, amide group (CONH₂), and COO-symmetric stretching bond, respectively.
The graphs presented above depict a comparative analysis of the three FTIR patterns associated with the glatiramer acetate (GA) drug, nanoliposomes encapsulating GA, and empty nanoliposomes (control). The graphs are labeled as follows: Graph A represents the FTIR pattern of GA; graph B represents the FTIR pattern of the liposome containing the drug; and graph C represents the FTIR pattern of the liposome without the drug.
In Figure 2B, the peak at 3104.99 cm⁻¹ corresponds to the presence of the hydroxyl functional group (OH). Peaks at 2917.76 cm⁻¹ and 2849.77 cm⁻¹ indicate the stretching vibrations of the CH₂ and CH₃ groups, respectively, in the fatty acid portions of phosphatidylcholine, cholesterol, and phosphoglycerol. The stretch band at 2033.56 cm⁻¹ signifies the presence of the carbonyl group (C=O). Additionally, peaks at 1637.47 cm⁻¹ suggest the presence of a double bond (C=C) in the second ring of cholesterol and the amide group (CONH₂) in the amino acid. The wavelength at 1454.46 cm⁻¹ corresponds to the asymmetric stretching vibrations of the CH₂ and CH₃ groups in cholesterol. Lastly, the stretching bond at 1080.81 cm⁻¹ is attributed to the deformation of the cholesterol ring.
Based on the FTIR spectrum presented in Figure 2C, the peak at 3232.35 cm⁻¹ corresponds to the hydroxyl functional group (OH). The stretching bond at 2033.39 cm⁻¹ indicates the presence of the carbonyl group (C=O). The wavelength at 1616.50 cm⁻¹ signifies the existence of a double bond (C=C) in the second ring of cholesterol. The wavelength at 1454.27 cm⁻¹ is associated with the asymmetric stretching vibrations of the CH₂ and CH₃ groups in cholesterol. The wave number of 1078.19 cm⁻¹ is attributed to the deformation of the cholesterol ring, while peaks ranging from 480 to 900 cm⁻¹ are attributed to the out-of-plane bending of C-H bonds in cholesterol. According to the FTIR results, no chemical interaction occurred between GA and the carrier components, and the stability of the drug is maintained during the production process of nanoliposomes.
4.4. XRD Analysis
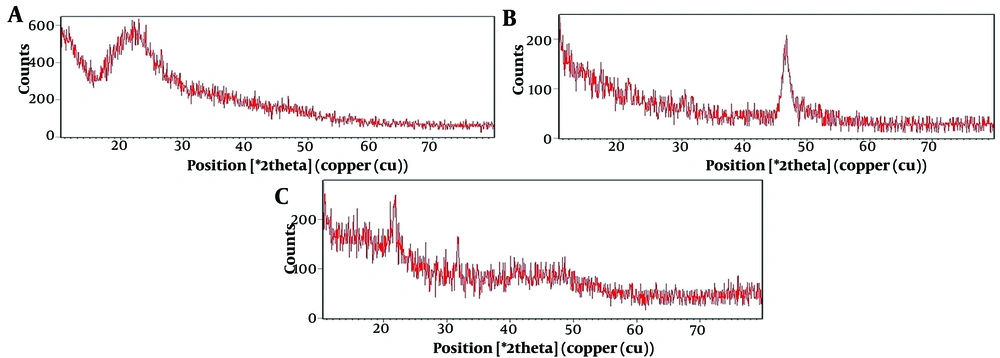
The chemical structure of GA, GA-loaded, and non-loaded nanoliposomes was evaluated by XRD (Figure 3). This figure shows the X-ray radiation angle on the horizontal axis and the X-ray radiation intensity on the vertical axis. The XRD examination confirmed that the liposomes had an amorphous structure, indicating a lack of crystallinity. Figure 3B also confirms the presence of GA in the nanoliposomes.
4.5. In Vitro Release Study
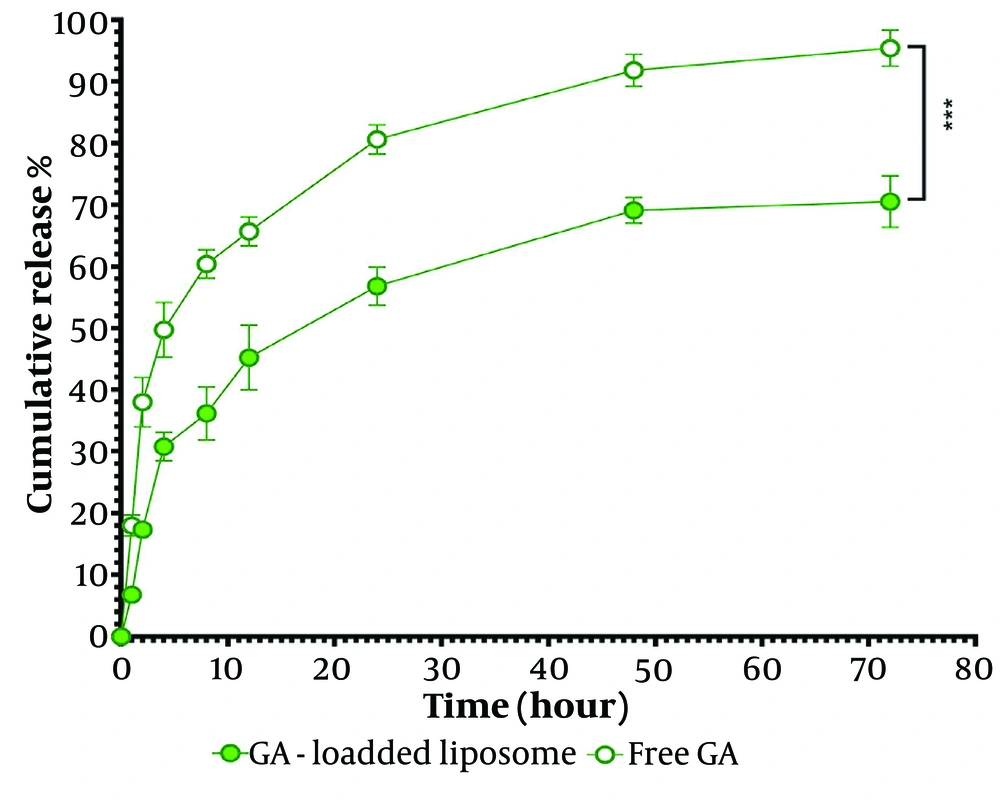
Figure 4 illustrates the release profile of GA from the nanoliposomal system. The experiment showed that unencapsulated GA (free GA) was released very quickly, while the release of GA from the carrier indicated a slow-release pattern. Approximately 50% of GA was released from the unencapsulated GA, and 30% was released from the encapsulated GA formulations after 4 hours. Initially, a rapid release of the drug (burst effect) from the liposomes was observed, followed by a sustained release phase. The total release of GA from free GA and encapsulated GA was 95.4 ± 2.6% and 70.5 ± 3.4%, respectively.
4.6. Stability Study
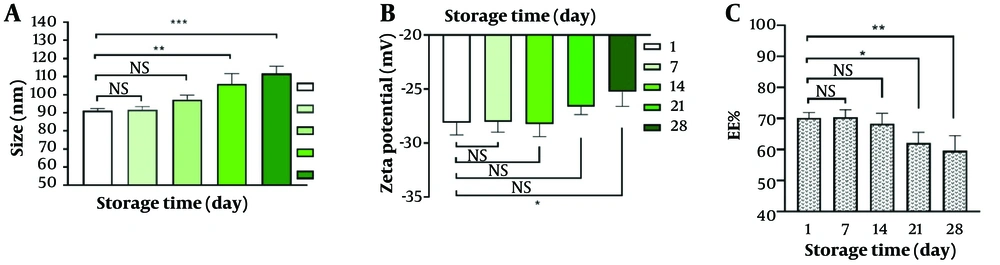
The stability of the nanoliposome was assessed during storage at 4°C for approximately one month. Changes in encapsulation efficiency, particle size, and zeta potential were evaluated as indicators of stability (Figure 5). The results showed that the nanoliposomal formulation of GA remained stable for at least two weeks. After one month from the production of GA-nanoliposome, the reduction in encapsulation efficiency (EE%) was about 10%, the particle size increased to 112 nm, and the zeta potential decreased by 2 units (from -27 mV to -25 mV).
4.7. Biocompatibility Assay
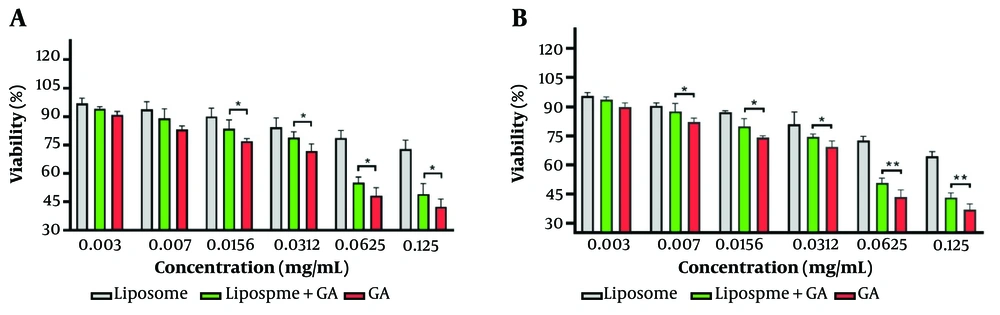
Figure 6 depicts cell viability at three different time points (48h and 72h). The findings reveal that the toxicity of free-drug nanoliposomes was significantly lower than that of GA-nanoliposomes and the pure drug at all incubation times. The toxicity of GA-nanoliposomes was lower than that of the pure drug. However, with increasing incubation time and drug concentration, the toxicity of the empty liposome (control), GA-nanoliposome, and free drug increased. At a concentration of 0.0156 mg/mL after 48 hours of treatment, the cell viability rates for liposomes (free-GA nanoliposomes), GA-nanoliposomes, and free GA were 90%, 84%, and 77%, respectively. At the maximum concentration (0.156 mg/mL), these rates decreased to 73%, 49%, and 42%, respectively. Beyond the concentration of 0.007 mg/mL, a significant difference was observed between GA-nanoliposomes and free GA.
5. Discussion
The initial design of an ideal formulation, aligned with scientific standards, is crucial in the development of nanocarriers, as it enables the achievement of the project’s ultimate objectives. Successful production of a nanocarrier with the desired characteristics requires the implementation of appropriate methodologies. The ongoing investigation focuses on developing an optimal nanoliposome formulation containing GA as the active medicinal ingredient. Glatiramer acetate is used to treat relapsed MS and is a combination of four amino acids that suppress the immune system's attack on nerves in the brain and spinal cord, preventing MS recurrence and reducing the chances of patient disability. The project aims to enhance the drug’s effectiveness by creating a nanoliposome formulation. Additionally, we seek to reduce the drug's toxicity to body cells and address issues related to the need for frequent injections, which increase side effects, treatment costs, and patient dissatisfaction.
Glatiramer acetate-nanoliposomes exhibited a surface charge of approximately -27 mV and a particle size of 91 nm. We successfully encapsulated GA into the nanoliposome system with about 70% efficiency, comparable to results from other researchers (23). FTIR and XRD analyses indicated no chemical interaction between GA and the nanoliposome. We observed a reduced rate of drug release for GA compared to its unbound state, which may enhance GA bioavailability relative to glatiramer intake. By minimizing off-target effects on healthy cells, we can significantly mitigate the adverse effects associated with GA, potentially preventing their occurrence. The biocompatibility study results demonstrated that the toxicity of GA-nanoliposomes is lower than that of free GA, indicating that the carrier positively impacts the reduction of free GA toxicity.
The stability results revealed that after 28 days of storage at 4°C, the size of the nanoliposomes increased slightly but remained within the optimal range for nanoliposomes. Zeta potential, a crucial factor influencing the stability of nanoparticles, showed that a higher zeta potential value provides greater stability by inducing repulsive forces among the nanoparticles. These repulsive forces help reduce the tendency of nanoparticles to aggregate and adhere to each other (26). The current study demonstrated that liposomal nanoparticles with an appropriate negative charge are stable drug delivery carriers. Our results indicated that the incorporation of DSPC into the liposome structure is responsible for the observed negative surface charge in the nanoparticles. DSPC, an anionic emulsifier, plays a crucial role in enhancing the negative charge exhibited by nanoliposomes. The reduction in encapsulation efficiency during storage can be attributed to the hydrophilic nature of the drug, which increases its tendency to release from the carrier system. Overall, our findings suggest that GA-nanoliposomes could be a promising strategy for the treatment of MS patients, pending successful in vivo experiments and clinical trials.