1. Background
The hydatid cyst is the larval stage of Echinococcus granulosus sensu lato, which accounts for an important zoonotic infection worldwide (1). Definitive hosts, including dogs and other carnivores, pass the parasite eggs through defecation into the environment. Subsequently, the eggs are ingested by intermediate hosts, which include domestic livestock such as camels, horses, pigs, cattle, sheep, and humans (1). After ingesting infective eggs, hexacanth embryos develop in intermediate hosts, penetrate the intestinal mucosa, enter the systemic bloodstream, and migrate to major organs where they form hydatid cysts within a few months (1, 2). Hydatid cysts can affect various organs, mainly the liver (50% - 70%) and the lungs (20% - 30%), and occasionally the central nervous system, kidneys, spleen, muscles, and bones (3, 4).
Surgery is commonly used as the primary treatment for hydatid cysts, but there is a risk of the infestation spreading during surgery, which can lead to the formation of new cysts (5). To mitigate this risk, it is recommended to use scolicidal agents to deactivate the cyst contents [protoscoleces (PSCs)], thereby reducing the chances of relapse. Various scolicidal agents, such as cetrimide, 10% povidone-iodine, 95% ethyl alcohol, 20% hypertonic saline, hydrogen peroxide, and 20% silver nitrate, have demonstrated effectiveness in deactivating cyst contents (6-9). Implementing appropriate drug therapy before and after surgery, as well as during cyst aspirations, can help minimize relapse, secondary hydatidosis, the need for re-operative surgery, and ultimately eradicate the infection (10-13).
Given the necessity for alternative therapeutic approaches to address parasitic infections and counteract drug resistance, there is a growing interest in exploring the potential of herbal medicines in treating various parasitic diseases (14).
Plant metabolites have emerged as a promising therapeutic resource for controlling hydatid cysts due to their long-standing use as anti-parasitic agents, coupled with advantages such as cost-effectiveness, safety, and widespread availability (14, 15). Thymbra spicata L., commonly known as Mediterranean thyme (black thyme), is a well-known medicinal plant classified within the Lamiaceae family. It is predominantly found in the Eastern Mediterranean region, Iraq, and Iran (16). Studies have shown that this plant exhibits potent antibacterial, antifungal, antioxidant, and anti-inflammatory effects in modern medicine (17-20). Although different studies have revealed the presence of compounds such as carvacrol, thymol, γ-terpinene, α-pinene, and p-cymene in the essential oil of this plant, the presence of these compounds can be influenced by factors such as the time of harvesting, the part of the plant, and the extraction method (21). The above-ground components of T. spicata are often used to make tea or as an ingredient in seasonings for various culinary dishes. Prior research has highlighted the antibacterial, antioxidant, and anticancer attributes of T. spicata essential oil (TSEO).
2. Objectives
This study aimed to evaluate the in vitro and in vivo efficacy of the essential oil derived from T. spicata against PSCs and hydatid cysts of E. granulosus.
3. Methods
3.1. Plant Material
The aerial parts of T. spicata were collected from the Rica and Zaruni regions in Khorramabad city, Lorestan, Iran. The herbarium code (10 485) was officially sanctioned by the Agricultural Research, Education, and Extension Organization of Lorestan. Samples were collected in August 2022, and the aerial parts of the T. spicata plant were used for preparing essential oil. The plants underwent a washing process with distilled water and were subsequently air-dried at a temperature of 45°C for 48 hours. Following this, the plants were finely ground using an electric grinder (HR3770, Philips, Netherlands), and the resulting powder was used for extraction purposes.
3.2. Preparation of TSEO
The essential oil was obtained by extracting 30 grams of dried T. spicata powder through hydro-distillation using a Clevenger apparatus (Avizhe, Tehran, Iran). The essential oil was obtained using the water distillation method for 3 hours using a Clevenger-type device. Subsequently, the extracted oil underwent drying with anhydrous sodium sulfate, filtration using a sterilized Whatman No. 1 (Sigma-Aldrich, Germany) filter, and was stored at 4°C for further testing (22).
3.3. Chromatography-Mass Spectrometry Analysis of TSEO
The phytochemical components of TSEO were examined through the utilization of a Gas chromatography-mass spectrometry (GC-MS) system, which included an Agilent 6890 Gas Chromatograph coupled with an Agilent 5973N Mass Spectrometer (Palo Alto, CA, USA). The system operated in electron ionization (EI) mode at 70 eV and was equipped with an HP-5 column (30 m × 0.25 mm, i.d., 0.25 mm film thickness) and an SPB-1 column (30 m × 0.25 mm, i.d., 0.25 mm thickness). The column temperature ranged from 40 to 280°C with a scanning rate of 10°C/min, followed by continuous maintenance at 200°C for 30 minutes, and subsequently an incremental rate of 5°C/min up to 280°C. The injector and interface temperatures were set at 250°C. Helium (99.9%) was employed as the carrier gas at a 1 mL/min flow rate. Identification of phytochemical compounds was achieved by comparing mass spectra with established mass patterns in the Wiley 7/NIST05 mass spectral libraries database available at https://webbook.nist.gov/chemistry/ (23, 24).
3.4. Collection of Protoscoleces
The PSCs were obtained from hydatid cysts extracted from the livers of naturally infected goats and sheep. The hydatid fluid was extracted using a sterile Pasteur pipette and transferred into a sterile 50 mL Falcon tube. The PSCs were allowed to sediment at the base of the tube for a duration of 30 minutes. Subsequently, the supernatant was removed, and the PSCs were rinsed thrice with sterile normal saline (25).
3.5. Viability Test
To assess their potential for survival, PSCs were treated with a 0.1% eosin solution, applied onto a glass slide, overlaid with a cover slip, and examined using a light microscope. Deceased PSCs exhibit a red coloration as a result of absorbing the eosin dye (Sigma-Aldrich, Germany), whereas viable PSCs maintain their colorless appearance due to their impermeable nature (25).
3.6. In Vitro Effects of TSEO on Protoscoleces
Different concentrations (10, 30, and 50 µg/mL) of TSEO (the selection of these concentrations was based on the primary experiments which showed the high efficacy and low toxicity of these concentrations) were prepared for the treatment of PSCs, selected based on preliminary experiments. A PSCs suspension with a density of 2×103/mL and viability of up to 90% was created, and 0.5 mL of this suspension was exposed to 0.5 mL of each concentration of TSEO. The tubes were gently mixed and then placed in an incubator at 37 °C for durations of 10, 20, 30, and 60 minutes. Subsequently, the upper phase of the samples was removed, and the settled PSCs were stained with 0.1% eosin dye solution for a period of 5 minutes (26). The 50% inhibitory concentration (IC50) values were ascertained utilizing GraphPad Prism version 8.0.
3.7. Exterior Ultra-Structural Effects of TSEO on Protoscoleces
The treated PSCs were initially fixed in 2.5% glutaraldehyde for 4 hours at room temperature, followed by three washes with PBS, each lasting 10 minutes. Subsequently, a dehydration process was carried out using increasing concentrations of ethanol (50%, 70%, 80%, 90%, and 100% v/v) for 10 minutes each. Critical point drying was then conducted using a critical point dryer (S4160, Hitachi, Japan) for 30 minutes. The cells were affixed onto a metal scanning electron microscopy (SEM) stub and coated with gold using an SEM coating unit (E5100 Polaron, UK). The coated sample was examined using scanning electron microscopy (JEOL 64000, Japan) at an acceleration voltage ranging from 15 to 25 kV.
3.8. Expression Gene Level of Caspase-3 by Real-time PCR
After exposing PSCs to various concentrations of TSEO and treating them, the caspase-3 gene expression was assessed using real-time PCR (ABI StepOnePlus, applied biosystems, Foster City, CA, USA). RNA extraction was carried out using Biofact's commercial RNA extraction kit (Biofact, South Korea), and the purity and integrity of the extracted RNA were checked using a NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA) and 1% agarose gel electrophoresis. Complementary DNA was produced with a cDNA synthesis kit (Biofact, Korea) using 1 μg of total RNA and random hexamer primers. The thermal cycling conditions, based on previous studies, involved an initial denaturation step at 96°C for 4 minutes, followed by 35 cycles at 95°C for 30 seconds, 58°C for 40 seconds, and 73°C for 40 seconds. Real-time qPCR was conducted to analyze caspase-3 gene expression using the SYBR Green master mix kit (BioFact, South Korea), with the β-actin gene serving as an internal control to standardize mRNA levels in the test samples. All samples were analyzed in triplicate. The relative gene expression was determined using the 2−ΔΔCt method. The primer sequences used were as follows: Caspase-3 forward: 5′-TTCATTATTCAGGCCTGCCGAGG-3′, Caspase-3 reverse: 5′-TTCTGACAGGCCATGTCATCCTCA-3′. β-actin forward: GTGACGTTGACATCCGTAAAGA, β-actin reverse: GCCGGACTCATCGTACTCC.
3.9. In Vivo Effect of TSEO on Hydatidosis in Mice
3.9.1. Animals
Fifty male BALB/c mice, aged between 40 and 60 days and weighing 25 - 30 grams, were selected for the study. Mice were maintained under standard conditions (24 ± 1°C, 12-hour light/dark cycle, and humidity of 40 – 70%) with access to water and food ad libitum.
3.9.2. Induction of Hydatidosis in Mice
All mice were intraperitoneally administered 0.5 mL of sterile PBS containing 2 × 103 live PSCs. After twelve weeks, one mouse from each group was randomly selected, deeply anesthetized via i.p. injection of ketamine (15 mg/kg) + xylazine (100 mg/kg) (Bremer Pharma, Germany), and examined for the presence of hydatid cysts (27).
3.9.3. Treatment of Infected Mice
The mice were randomly divided into four groups, each consisting of 10 individuals. The groups were then assessed based on their respective treatment regimens, which were administered for 28 days. The infected mice were subjected to different treatments, including albendazole at a dosage of 200 mg/kg/day, TSEO at doses of 20, 30, and 40 mg/kg/day, as well as normal saline. Subsequently, the internal organs of the mice were analyzed to determine the presence, quantity, size, and weight of hydatid cysts across the various treatment groups (28).
3.9.4. Toxicity of TSEO in Healthy Mice
The toxicity of TSEO was determined by assessing liver and kidney function in healthy mice that received TSEO at doses of 20, 30, and 40 mg/kg/day for 28 days. After 28 days of treatment, the mice were euthanized using ketamine-xylazine (100 mg/kg), and upon opening the abdomen, blood samples were collected from their hearts. After centrifugation of the blood samples, the obtained serum samples were examined using diagnostic biochemical kits from Pars Azmoon, Iran, to evaluate serum levels of kidney function indicators [blood urea nitrogen (BUN) and creatinine (Cr)] and liver function indicators [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] (29).
3.10. Statistical Analysis
The mortality rates of PSCs were quantified and presented as mean ± SD for statistical evaluation. Group distinctions were assessed through one-way analysis of variance (ANOVA). Statistical significance was defined as a P-value less than 0.05.
4. Results
4.1. Chromatography-Mass Spectrometry
Based on the hydro-distillation approach, the essential oil yield [percent weight to dry weight (w/w)] based on three repetitions was 0.78 ± 0.12% w/w. A total of 25 compounds (97.09%) were identified. As indicated in Table 1, the monoterpene compounds carvacrol (65.88%), γ-terpinene (9.71%), and p-cymene (7.82%) were the most abundant compounds in TSEO, respectively.
| Compounds | Percentage | KI a |
|---|---|---|
| Hexanal | 0.01 | 805 |
| 2-Hexenal | 0.05 | 860 |
| Hexanol | 0.02 | 869 |
| α-Thujene | 1.11 | 930 |
| α-pinene | 0.69 | 942 |
| Camphene | 0.12 | 962 |
| 1-Octen-3-ol | 0.36 | 983 |
| Myrcene | 2.09 | 992 |
| 3-Octanol | 0.13 | 1 001 |
| α-phellandrene | 0.32 | 1 016 |
| α-terpinene | 2.04 | 1 025 |
| P-cymene | 7.82 | 1 036 |
| β-phellandrene | 0.31 | 1 042 |
| Ocimene | 0.05 | 1 046 |
| γ-terpinene | 9.71 | 1 069 |
| Terpinolene | 0.17 | 1 097 |
| Linalool | 0.19 | 1 105 |
| Nonanal | 0.03 | 1 111 |
| Terpinen-4-ol | 1.28 | 1 198 |
| α-terpineol | 0.14 | 1 220 |
| Thymol | 0.3 | 1 292 |
| Carvacrol | 65.88 | 1 328 |
| (E)-Caryophyllene | 2.51 | 1 435 |
| Spathulenol | 0.31 | 1 596 |
| Caryophyllene oxide | 1.45 | 1 603 |
Abbreviation: KI, Kovats Retention Index.
aComparison of the retention time of the investigated volatile molecules of TSEO with the retention time of n-alkanes under the same condition.
4.2. In Vitro Protoscolicidal Effects
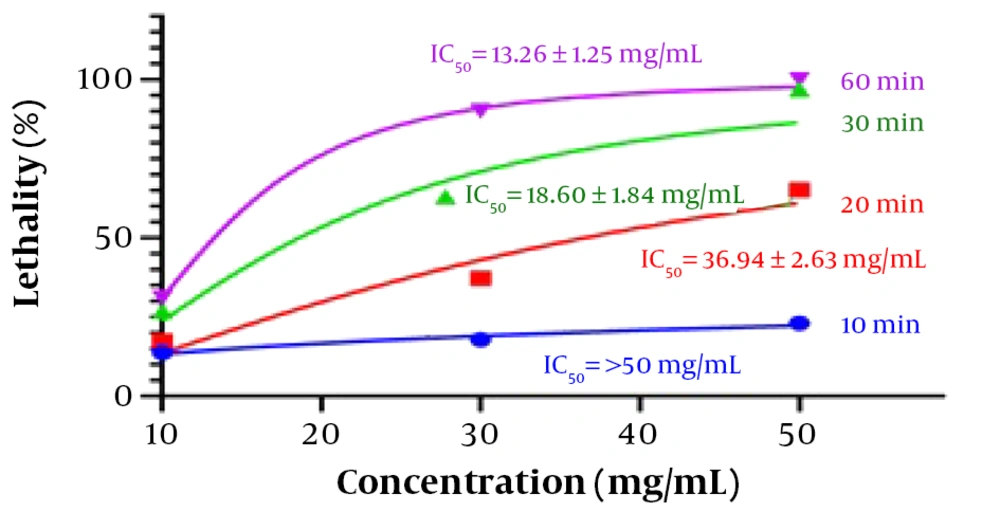
The protoscolicidal experiments were conducted using different TSEO concentrations with incubation times ranging from 10 to 60 min (Table 2). The results showed that the scolicidal activity of TSEO at 10 µg/mL was approximately 31.08 ± 3.52% of maximum efficiency. With an increase in TSEO concentration, the scolicidal effect also increased, reaching approximately 90.15 ± 4.04% lethality at a concentration of 30 µg/mL, and 100.0 ± 0.0% lethality at a concentration of 50 µg/mL after 60 min of exposure. Based on the results, the IC50 values for TSEO at 20, 30, and 60 min were 36.94 ± 2.63, 18.60 ± 1.84, and 13.26 ± 1.25 µg/mL, respectively (Figure 1). Staining of cysts following treatment with increasing concentrations of TSEO demonstrated a significant increase in eosin permeability. Figure 2 illustrates the change in cyst wall permeability to eosin after exposure to TSEO for 10, 20, 30, and 60 min.
| TSEO and Treatment Time (min) | Mortality Rate (%) |
|---|---|
| 10 (µg/mL) | |
| 10 | 13.77 ± 2.80 |
| 20 | 17.40 ± 2.68 |
| 30 | 26.96 ± 2.53 |
| 60 | 31.08 ± 3.52 |
| 30 (µg/mL) | |
| 10 | 17.82 ± 0.29 |
| 20 | 37.29 ± 3.67 |
| 30 | 62.21 ± 2.19 |
| 60 | 90.15 ± 4.04 |
| 50 (µg/mL) | |
| 10 | 23.02 ± 2.08 |
| 20 | 65.13 ± 3.42 |
| 30 | 97.07 ± 1.12 |
| 60 | 100 ± 0.00 |
| 0.9% normal saline (µg/mL) | |
| 10 | 3.44 ± 0.15 |
| 20 | 5.46 ± 0.18 |
| 30 | 8.09 ± 0.11 |
| 60 | 15.10 ± 1.03 |
| 20% hypertonic saline (µg/mL) | |
| 10 | 95.75 ± 0.34 |
| 20 | 100 ± 0.00 |
| 30 | 100 ± 0.00 |
| 60 | 100 ± 0.00 |
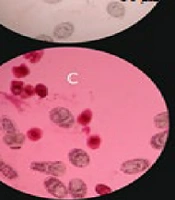
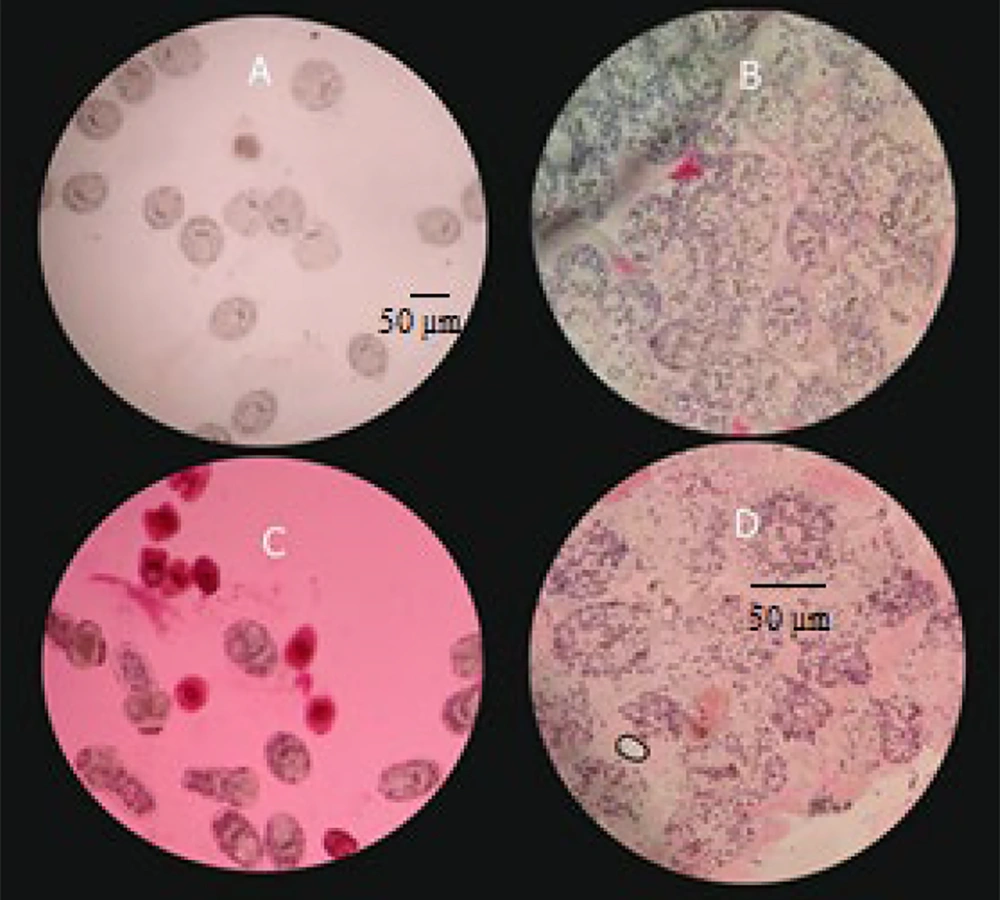
Light microscopy images of protoscoleces treated with 50 mg/mL of Thymbra spicata essential oil. A, untreated and unstained protoscoleces (control); B, untreated protoscoleces stained with eosin; C, T. spicata essential oil-treated protoscoleces after 10 min exposure stained with eosin; D, T. spicata essential oil-treated protoscoleces after 60 min exposure stained with eosin.
4.3. Exterior Ultrastructural Effects of TSEO on Protoscoleces
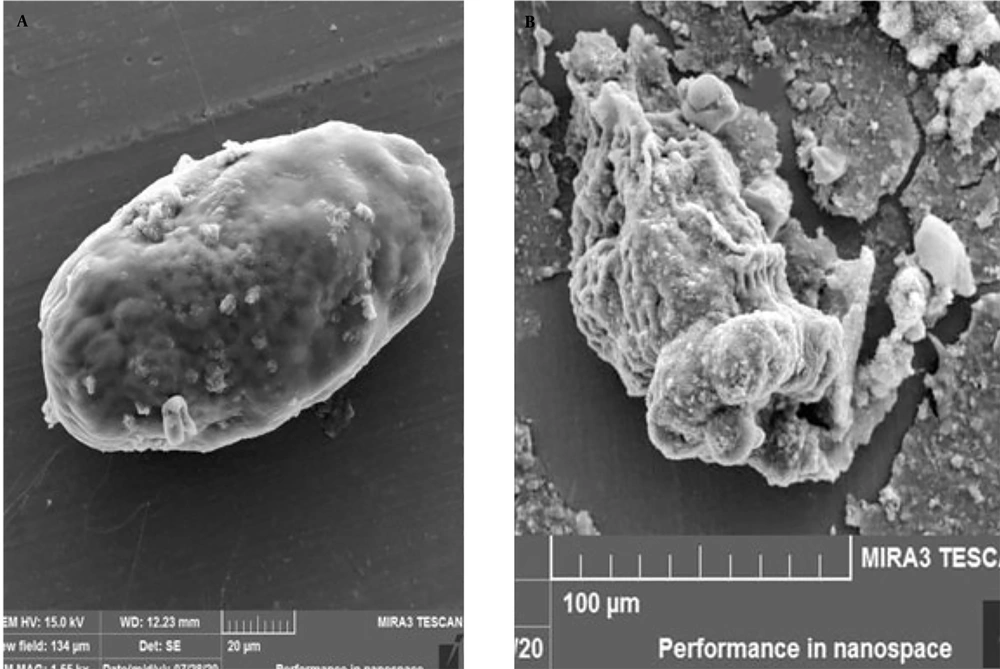
Scanning electron microscopy images of PSCs treated with 50 µL/mL of TSEO for 1 h of incubation showed ultrastructural damage, rostellar disorganization, alterations in the teguments, and deformation of the cyst structure (Figure 3), indicating that TSEO leads to damage of the membrane and cell wall in hydatid cyst PSCs.
4.4. Expression Gene Level of Caspase-3 by Real-time PCR
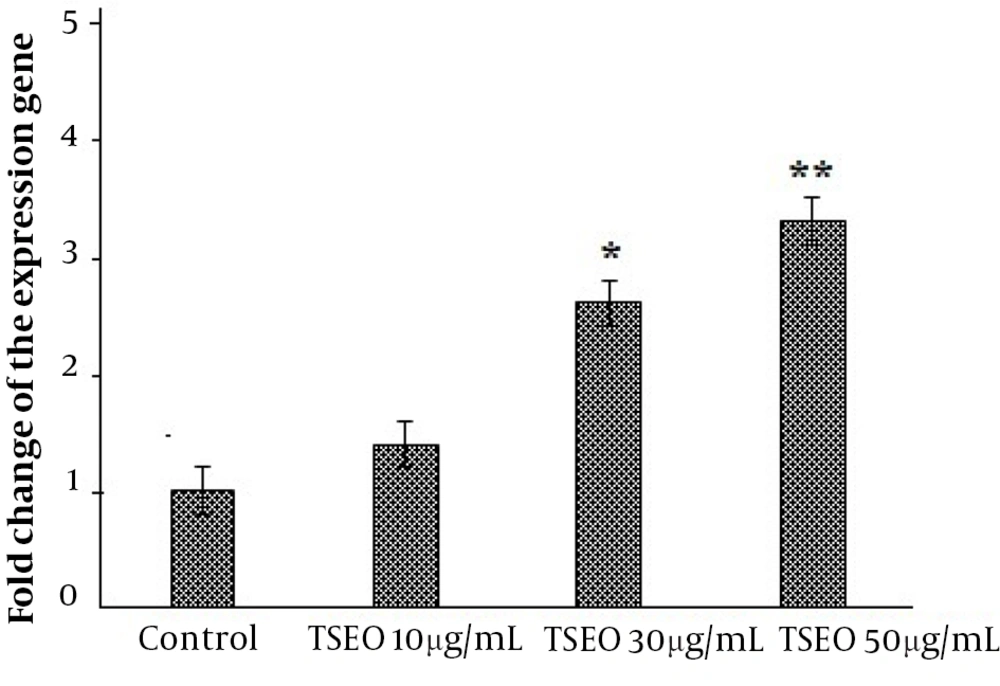
Our real-time PCR results showed that after exposure of PSCs to TSEO, the expression level of the caspase-3 gene increased over time with increasing concentration (P < 0.05) (Figure 4).
4.5. In Vivo Effect of TSEO on Hydatidosis in Mice
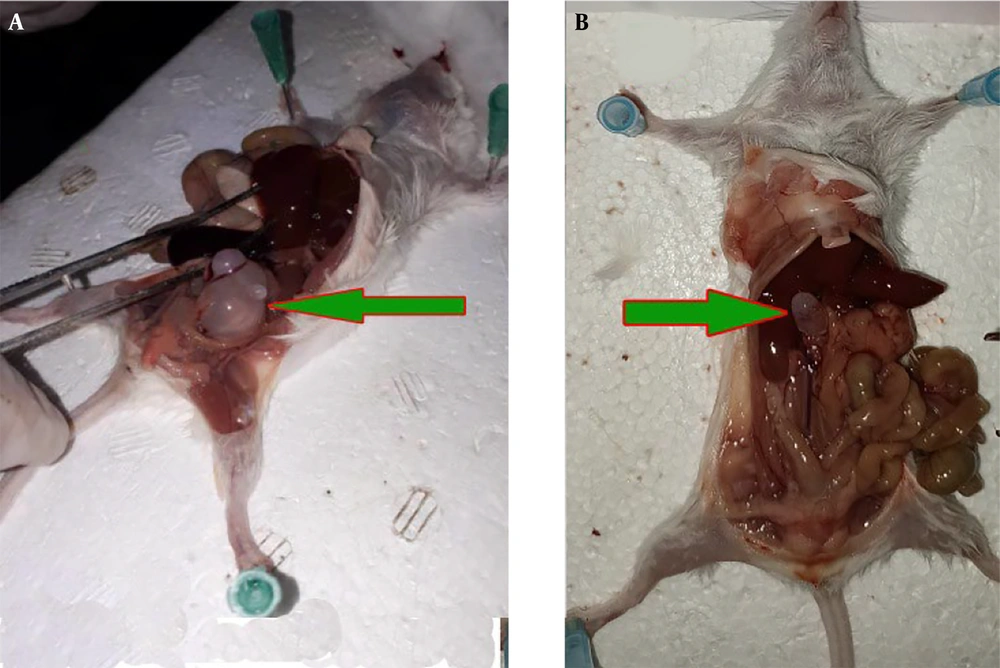
In the in vivo assay, after conducting treatment on the infected mice, it was identified that the hydatid cysts manifested as liver and peritoneal cysts (Figure 5). Analysis of the data revealed that the negative control group of mice, which received normal saline, exhibited the highest quantity, weight, and size of hydatid cysts. Conversely, the groups administered TSEO demonstrated a notable reduction in the average number, size, and weight of hydatid cysts, as evidenced by statistical significance (P < 0.05). Particularly, a significant (P < 0.001) decline in both the size and number of hydatid cysts was observed in infected mice treated with TSEO at a dosage of 40 mg/kg (Table 3).
| Groups | Mean Number of Cysts | Cysts Weight (g) | Mean of Size (mm) |
|---|---|---|---|
| Negative control | 17.3 ± 3.51 | 4.98 ± 0.86 | 0.63 ± 0.89 |
| TSEO 20 mg/mL | 12.4 ± 2.56 b | 3.87 ± 0.56 | 0.31 ± 0.074 b |
| TSEO 30 mg/mL | 10.0 ± 0.0 c | 3.22 ± 0.47 b | 0.27 ± 0.034 c |
| TSEO 40 mg/mL | 9.6 ± 1.51d | 2.64 ± 0.39 c | 0.18 ± 0.022 d |
| Albendazole 200 mg/kg | 8.0 ± 0.0 d | 2.01 ± 0.17 c | 0.069 ± 0.012 d |
Abbreviation: TSEO, Thymbra spicata essential oil.
aValues are expressed as mean ± SD.
b P < 0.05 compared with the control normal saline group.
c P < 0.001 compared with the control normal saline group.
d P < 0.001 compared with the control normal saline group.
4.6. Toxicity of TSEO in Healthy Mice
Addressing the toxicity aspects of TSEO, the results of the analysis of biochemical parameters of liver and kidney function showed that after receiving TSEO at doses of 20, 30, 40 mg/kg/day for 28 days, although there were variations in the parameters examined, there was no notable discrepancy observed in comparison to the control group that was administered normal saline (Figure 6).
The toxicity of Thymbra spicata essential oil on the liver and kidney function of healthy mice through evaluating the serum levels of (A), liver function indicators [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)]; and (B), kidney [blood urea nitrogen (BUN) and creatinine (Cr)]
5. Discussion
Here, we aimed to evaluate the in vitro and in vivo efficacy of TSEO against PSCs and hydatid cysts of E.granulosus. We found that the monoterpenes compounds carvacrol (65.88%), γ-terpinene (9.71%), and p-cymene (7.82%) were the most abundant compounds in TSEO, respectively. Unlu et al. showed that the main compounds in Turkish TSEO were carvacrol (60.39%), γ-terpinene (12.95%), and p-cymene (9.61%), respectively (30). Markovic et al. reported that the main compounds in Greek TSEO were carvacrol (74.5%) and γ-terpinene (11.2%) (31). Mosleh et al. demonstrated that during the pre-flowering period, TSEO contained 7 compounds, with the main constituents being γ-terpinene (15.5%) and carvacrol (79.3%). Conversely, during full flowering, TSEO was composed of 11 compounds, with carvacrol (70.5%) and γ-terpinene (14.9%) identified as the major constituents (32). This change in their composition and percentage can be due to the harvesting season of the plant, the part used, and the extraction methods (32).
We found that increasing the concentration of TSEO enhanced the scolicidal effect. At a concentration of 30 µg/mL, lethality reached approximately 90%, and 100% lethality was achieved at a concentration of 50 µg/mL after 60 minutes of exposure. By conducting an in vivo assay, it was observed that treatment with TSEO led to a significant reduction in the average number, size, and weight of hydatid cysts, as supported by statistical significance. With respect to the protoscolicidal effects of Thymus spp., Hizem et al. demonstrated that T.capitatus essential oil, when concentrated at 2 and 3 mg/mL, led to the complete eradication of PSCs within 5 and 1 minute, respectively. Additionally, analyses conducted using GC/MS techniques revealed that carvacrol constituted the predominant component of the essential oil, accounting for 82.4% of its composition (33). Pensel et al. showed that the essential oil derived from T. vulgaris demonstrated a protoscolicidal effect, resulting in a significant decrease in PSC viability to 35.3% after a 60-day incubation period (34). Fabbri et al. reported that the highest protoscolicidal effect was achieved using a concentration of 10 μg/mL of carvacrol. Upon examination at the ultrastructural level, it was observed that the germinal layer of cysts treated with carvacrol showed a loss of the characteristic multicellular structure. A decrease in cyst weight was observed after administering 40mg/kg of carvacrol over a 20-day period to mice with hydatid cysts (35). Albani et al. demonstrated that the most significant protoscolicidal effect was observed in the combinations of carvacrol and thymol in ratios of 9:1 and 5:5, leading to a notable decrease in viability six days post-incubation, consistent with the observed ultrastructural changes. They also reported that the combination of thymol (40 mg/kg) and carvacrol (40 mg/kg) showed a tendency to reduce the weight of the cysts compared to the control group (36). In addition, reviews have shown the high potency of carvacrol-rich plants such as Zataria multiflora, Satureja khuzistanica, Satureja hortensis, Eucalyptus globulus, Syzygium aromaticum, and Origanum minutiflorum against PSCs and hydatid cysts of E. granulosus (37).
Today, morphological changes in the ultrastructure of microbial cells were observed using SEM (38). Scanning electron microscopy images of the cyst treated with 50 µL/mL of TSEO for 1 hour of incubation showed ultrastructural damage, rostellar disorganization, alterations in the teguments, and deformation of the cyst structure. This indicates that TSEO leads to damage to the membrane and cell wall in hydatid cyst PSCs. It is commonly recognized that apoptosis serves a dual purpose in the interaction between the host and hydatid cysts by facilitating survival and suppression mechanisms (39). Caspase enzymes, notably caspase-3, are essential for the progression of apoptosis, with caspase-3 specifically playing a crucial role in DNA fragmentation and the structural changes associated with cell death (39). Our real-time PCR results showed that after exposing PSCs to TSEO, the expression level of the caspase-3 gene increased over time with increasing concentration. This indicates that apoptosis induction is one of the possible protoscolicidal mechanisms of action of TSEO.
Regarding the antimicrobial mechanisms of action of monoterpenes, studies have reported that these compounds display their efficacy by affecting cell membrane permeability, disrupting DNA synthesis, causing leakage of proteins and nucleic acids, producing reactive oxygen species, inducing oxidative stress, and triggering apoptosis (40). Therefore, it can be suggested that the high efficacy of TSEO is probably due to the presence of monoterpene compounds such as carvacrol.
Addressing the toxicity aspects of TSEO, the analysis of biochemical parameters related to liver and kidney function revealed that after receiving TSEO at doses of 20, 30, and 40 mg/kg/day for 28 days, although there were variations in the parameters examined, no significant differences were observed compared to the control group that received normal saline. These results indicate that the administration of TSEO at these specified doses, even over 28 days, does not exhibit any harmful effects on the functionality of essential liver and kidney tissues.
5.1. Conclusions
The findings of the study confirmed the promising in vitro and in vivo effects of TSEO against hydatid cyst infection. Considering the possible mechanisms, TSEO provoked cell wall damage and induced apoptosis. However, more studies are needed to clarify these findings and accurately elucidate the mechanisms of action.
![The toxicity of <i>Thymbra spicata</i> essential oil on the liver and kidney function of healthy mice through evaluating the serum levels of (A), liver function indicators [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)]; and (B), kidney [blood urea nitrogen (BUN) and creatinine (Cr)] The toxicity of <i>Thymbra spicata</i> essential oil on the liver and kidney function of healthy mice through evaluating the serum levels of (A), liver function indicators [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)]; and (B), kidney [blood urea nitrogen (BUN) and creatinine (Cr)]](https://services.brieflands.com/cdn/serve/3170b/19eaa7d83555ad393cdfae0786859de2cd424745/jjnpp-146063-i003-F6-preview.webp)