1. Background
Neuroinflammation and demyelination are key characteristics of multiple sclerosis (MS), a common chronic neurodegenerative disorder that particularly affects the central nervous system (CNS) of young adults (1). Depressive disorders, with heterogeneous symptoms, are highly prevalent among MS patients (2). Depression, which may begin before the clinical diagnosis of MS, can significantly affect the patient’s quality of life (3). Studies indicate that demyelination in the inner prefrontal cortex (PFC) and diffuse white matter (WM), such as the corpus callosum (CC), is associated with increased depressive-like behaviors (4, 5). Additionally, the activation of neuroinflammatory agents and the increase of free radicals, such as nitric oxide (NO), which in turn induces further inflammatory responses, occur in patients with depression (6).
Nitric oxide, a gaseous neurotransmitter, is synthesized from L-arginine through different isoforms of nitric oxide synthase (NOS): Neuronal (nNOS), endothelial (eNOS), and inducible (iNOS) (7). With an unpaired electron, NO can induce neurotoxic effects at high concentrations (8). Research suggests that NO may have a crucial influence on the etiopathogenesis of depression (9). Nitric oxide has a dual role and can modulate various monoamine neurotransmitters, such as serotonin, which is mainly involved in depression (10).
Among various factors that could induce depression, low energy in the brain plays a pivotal role in developing depressive behaviors (11). Studies reveal that energy metabolism disturbances occur in the demyelination areas of MS (12). On the other hand, depression is characterized by a significantly decreased antioxidant status that potentially leads to impaired protection against oxidative stress (7). Thus, applying an exogenous antioxidant that facilitates the maintenance of energy balance by enhancing brain energy metabolism is a therapeutic strategy that can be targeted for ameliorating the depressive-like phenotype in MS patients.
The antioxidant acetyl-L-carnitine (ALC), a substantial molecule involved in the oxidation process of fatty acids, facilitates the absorption of acetyl CoA and its entry into the mitochondria. Acetyl-L-carnitine can cross the blood-brain barrier and plays a critical role in brain energy metabolism and ATP production (13). Evidence shows that depressive symptoms could manifest following a decrease in ALC levels and energy in the brain (14).
By targeting the oligodendrocytes' mitochondria, the copper chelator neurotoxicant cuprizone disrupts the brain's energy metabolism (15). Cuprizone intoxication is a suitable animal model for MS that results in demyelination and emotional impairments (16).
2. Objectives
The current work aimed to investigate the impact of ALC on myelin loss and depressive-like behaviors in the cuprizone mouse model of MS.
3. Methods
3.1. Animals and Grouping
Adult male C57BL/6 mice were purchased from the Center of Pasteur Institute (Tehran, Iran) and housed in the Tarbiat Modares University animal laboratory. The mice were randomly divided into three groups as described below: The control (CTL) mice received regular chow; the cuprizone (CPZ) mice were given 0.2% cuprizone w/w for 12 weeks; the CPZ plus ALC (CPZ+ALC) mice received the cuprizone diet and 300 mg/kg/day ALC by oral gavage for the last three weeks of the study (13).
3.2. Assessment of Depressive-like Behaviors
The despair behavioral tests were conducted to assess depressive-like behaviors and the antidepressant efficacy of ALC in the 12th week. In the forced swim test (FST), each animal swam for 6 minutes, and the immobility time was recorded during the last 4 minutes of the session. In the tail suspension test (TST), the animals were hung on a bar for 6 minutes, and the immobility period, determined as the time displaying no motion, was recorded during the last 4 minutes.
3.2.1. Histopathological Evaluation
First, cervical dislocation and intracardial perfusion were performed. Following brain fixation, tissue processing, and paraffin embedding, coronal sections at levels -1.28 mm and 1.96 mm bregma were obtained for staining using the luxol fast blue-periodic acid Schiff (LFB-PAS) reaction. The severity of demyelination in the CC and PFC was evaluated using Digimizer software.
3.3. Griess Assay
Briefly, the PFC and CC regions were homogenized with ice-cold 20 mM Tris–HCl buffer and centrifuged. To deproteinate the sample, zinc sulfate was added to the supernatant. Then, the supernatants were transferred to tubes containing 0.5 g cadmium. To convert nitrate to nitrite, the microcentrifuge tubes were incubated overnight with agitation. NOx was then assessed using a modified Griess reagent, and absorbance values were read at a wavelength of 540 nm.
3.4. RNA Extraction, Reverse Transcription, and Quantitative RT-PCR
The total RNA from the CC and PFC of the brain tissue was extracted according to the cDNA synthesis kit protocol. Then, using the Quantscript Reverse Transcription (RT) Kit, the total mRNA was reverse-transcribed. In brief, RNA samples were mixed with dNTPs, 10 × RT mix, random primers, reverse transcriptase, Quant Reverse Transcriptase, and RNase-free water, and then incubated at 37°C for an hour. To quantify mRNA, following cDNA synthesis, the quantitative RT-PCR (qRT-PCR) analysis was carried out on an Applied Rotor-Gene 3000 with the following conditions: GAPDH and target genes were amplified for 40 cycles at 94°C for 30 seconds, 63°C for 60 seconds, and 72°C for 90 seconds. Measurements of relative quantification of target gene expression levels were conducted using the ΔΔCt method, with Ct representing the threshold concentration. Table 1 shows the primer sequences used in the qRT-PCR.
| Gene Symbol and Primer Sequence | Tm (°C) |
|---|---|
| nNOS | 60 |
| 5′-ACGGCAAACTGCACAAAGC-3′′ | |
| 5′-CGTTCTCTGAATACGGGTTGTTG-3 | |
| iNOS | 60 |
| 5′-GTTCTCAGCCCAACAATACAAGA-3′ | |
| 5′-GTGGACGGGTCGATGTCAC-3′ | |
| eNOS | 59 |
| 5′-GGCTGGGTTTAGGGCTGTG-3′ | |
| 5′-CTGAGGGTGTCGTAGGTGATG-3′ | |
| GAPDH | 58 |
| 5′-GTGGAAGGGCTCATGACCACAGTCCATGCC-3′ | |
| 5′-TCTTACTCCTTGGAGGCCATGTAGGCCATG-3′ |
List of Sequences of Primers
3.5. Statistical Analysis
All data from the current study are shown as means ± standard deviation (SD), except for behavioral tests, which are presented as means ± standard error of the mean (SEM). The normality distribution of the results was assessed with the Kolmogorov–Smirnov test. Statistical differences between groups for all evaluations, except for FST, which was assessed using the Kruskal-wallis test, were analyzed using one-way analysis of variance (ANOVA). SPSS and GraphPad Prism were used for statistical analysis and graph drawing, respectively. A P-value of < 0.05 indicates a statistically significant difference.
4. Results
4.1. Effects of ALC Depressive-Like Behaviors
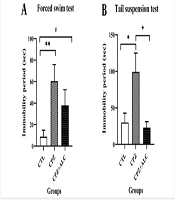
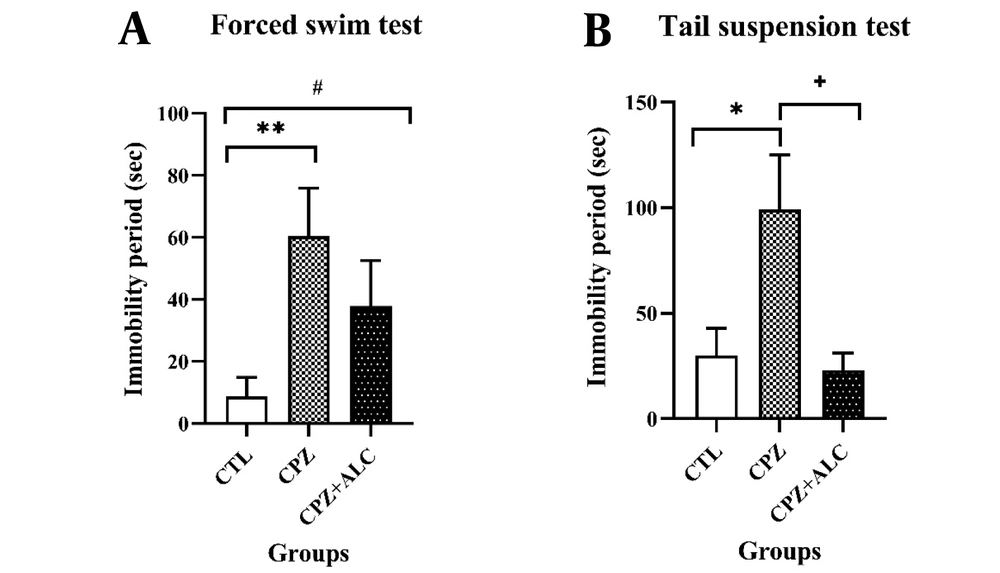
In the FST, the CPZ group revealed a statistically significant increase in the immobility period compared to the CTL group (P < 0.01). Acetyl-L-carnitine treatment showed a lower significant difference in the immobility times compared to the CTL group (P < 0.05). In the TST, the CPZ group showed a statistically significant rise in the immobility period compared to the CTL group (P < 0.05). Following ALC co-administration with CPZ, the immobility time declined significantly in comparison to the CPZ group (P < 0.05) (Figure 1).
4.2. Effects of Acetyl-L-Carnitine on Demyelination
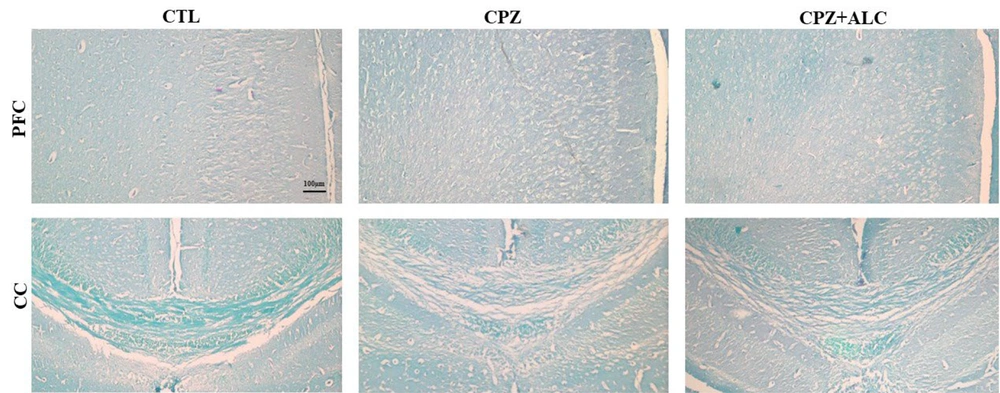
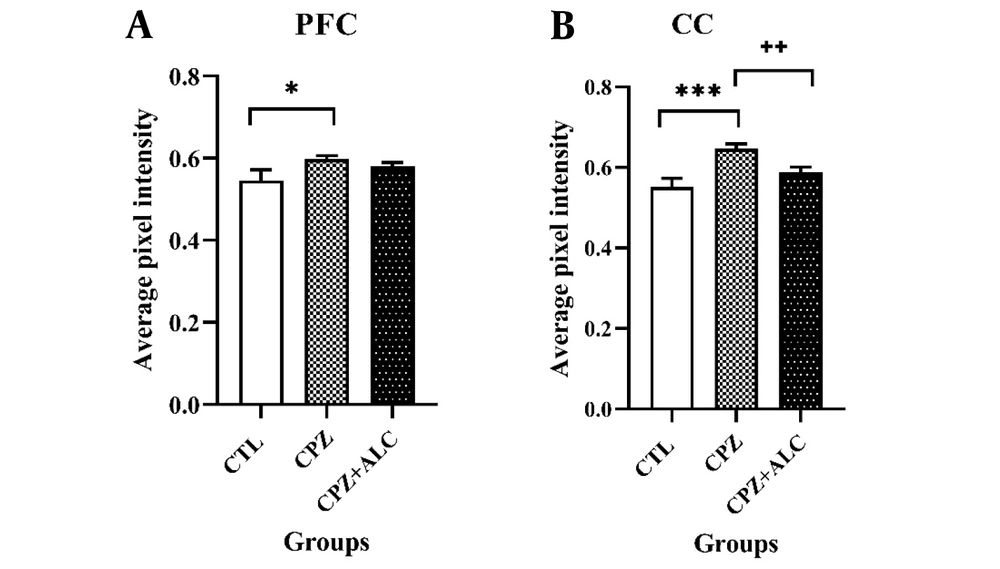
Cuprizone treatment resulted in extensive demyelination in the brain tissue. Histopathological evaluations revealed a significantly higher amount of demyelination events in the PFC (P < 0.05) and CC (P < 0.001) in the CPZ group compared to the CTL group. Acetyl-L-carnitine reduced cortical demyelination to the extent that there was no statistical difference between the demyelination scores of the CTL and CPZ+ALC groups. Acetyl-L-carnitine significantly alleviated CPZ-induced demyelination in the CC, resulting in a significantly lower demyelination score in the CPZ+ALC group (P < 0.01) (Figures 2 and 3).
4.3. Effects of Acetyl-L-Carnitine on Nitric Oxide Level
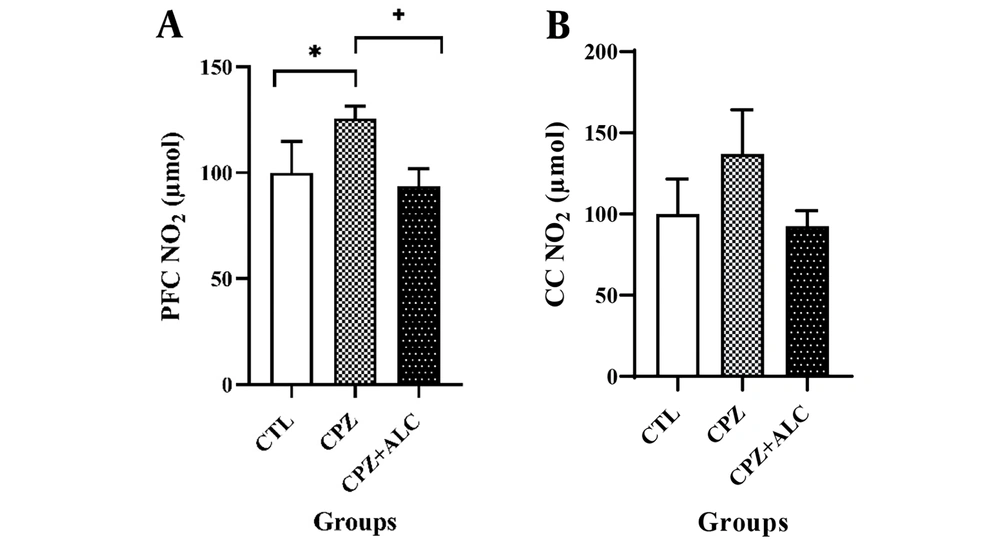
The biochemical results for NO in the PFC show that the NO level in the PFC was significantly increased in the CPZ group compared to the CTL group (P < 0.05). However, the CPZ+ALC group demonstrated a considerably reduced NO level in the PFC compared to the CPZ group (P < 0.05). Surprisingly, there were no statistical differences in NO levels between groups in the CC (Figure 4).
4.4. Effects of Acetyl-L-Carnitine on the Nitric Oxide Synthase Genes
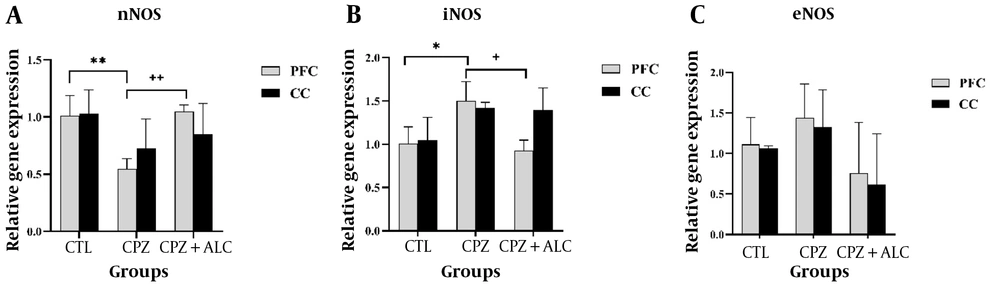
In their PFC, mice exposed to CPZ had significantly reduced nNOS gene expression compared to the CTL group (P < 0.01). However, the CPZ+ALC group demonstrated a significantly increased nNOS gene expression in the PFC compared to the CPZ group (P < 0.01). The data showed a significantly increased iNOS gene expression in the PFC of the CPZ group compared to the CTL group (P < 0.05). Additionally, there was a statistically significant reduction in iNOS gene expression in the PFC of the CPZ+ALC group compared to the CPZ group (P < 0.05). Interestingly, the same trend was also present in the CC but was not statistically significant. There were no statistical differences in eNOS gene expression between groups. Moreover, nNOS, iNOS, and eNOS showed no significant differences in the CC among the experimental groups (Figure 5).
Expression of nitric oxide (NO) synthase genes, including nNOS, iNOS, and eNOS in the brain tissue. ** and ++ display the P < 0.01 between CPZ group vs the CTL group; and CPZ+ALC group vs CPZ group, in order. * and + shows the P < 0.05 between CPZ group vs the CTL group; and CPZ+ALC group vs CPZ group, in order
5. Discussion
Due to psychological symptoms such as depression, the quality of life of MS patients is significantly affected (5). One aspect of MS pathogenesis is a disruption in brain energy metabolism, which potentially could result in the development of depression (17). On the other hand, the energy level in the brain directly correlates with its ALC level, so a decrease in ALC could potentially lead to depression (18). Since previous studies have revealed that ALC resembles antidepressant agents with fewer side effects and works faster than traditional antidepressant drugs (14), we applied the antioxidant ALC to alleviate depressive-like behaviors in the cuprizone-intoxicated mouse model of MS.
Following ALC co-administration, the immobility time was remarkably decreased. These results are consistent with previous studies where cuprizone led to a significantly prolonged immobility time (19). The neuroprotective effects of ALC in ameliorating depressive-like behaviors in the CPZ group may be attributed to increased brain energy levels. Furthermore, ALC may have alleviated depressive-like behaviors in cuprizone-demyelinated mice through its modulation of serotonin secretion.
Studies have proposed that demyelination, a hallmark of MS, is one of the etiologies of depression (20, 21). It is noteworthy that the remyelination process requires a high level of energy consumption (22). The PFC and CC have been identified as principal areas involved in mood disorders (4, 23), and their myelin content could be considered an important indicator of depression (20, 24). The histopathological study of the PFC and CC revealed significant demyelination. These findings align with the cuprizone-induced demyelination shown earlier (25). The significant demyelination in the CC and PFC may play essential roles in developing depressive-like behaviors, and the beneficial effects of ALC in accelerating the remyelination process may be attributed to providing the necessary energy in this demyelinated mouse model of MS.
Studies show that a high concentration of free radicals, including NO, exists within the inflammatory demyelination lesions of MS (26). When NO exceeds its physiological limit, it induces reactive forms of NO, which are very harmful to the brain (27). Nitric oxide in the brain acts as a second messenger, interacts with the serotonergic system (28), and is one of the most substantial systems related to the neurobiology of depression (29). In this study, we focused on evaluating the pro-oxidant process of NO production in the PFC and CC in the brain of neurotoxicant cuprizone mice and the possible effectiveness of antioxidant ALC on their depressive-like behaviors. Nitric oxide levels significantly increased in the PFC but not in the CC of mice that received 12 weeks of a 0.2% cuprizone-enriched diet. In agreement with our results, several studies showed that the amount of NO increased in the PFC in psychiatric conditions like depression (30, 31). On the neuroanatomical level, the PFC is believed to play a pivotal role in developing a depressive-like phenotype (85). Regarding the complex neurobiology of NO in depression, studies reveal that maintaining NO within its physiological range is the most probable therapeutic strategy (10). The potential antidepressant effects of exogenous ALC in alleviating depressive-like behavior may be attributed to the modulation of NO and, consequently, the modulation of serotonin and other neurotransmitters, as well as attenuating oxidative/nitrosative stress, affecting NO production in the PFC.
To determine which sources produced NO in the brain tissue, nNOS, iNOS, and eNOS genes in the PFC and CC were evaluated via qRT-PCR. Although nNOS and eNOS are mainly expressed steadily in neurons and endothelial cells regardless of the cell's metabolic state, iNOS exists in trace amounts under healthy conditions and is synthesized de novo in some immunological cells, such as microglia, via inflammatory triggering (32). In agreement with our results, iNOS protein expression in in vitro demyelinating experiments was significantly raised (33). Our results revealed that nNOS significantly declined and iNOS dramatically elevated in the PFC following cuprizone intoxication. Acetyl-L-carnitine consumption led to the enhancement of nNOS and a decrease in iNOS expression in the PFC. Our data support that the NO level increase in the PFC may be generated due to iNOS overexpression in the presence of the neurotoxicant cuprizone.
Recovery and reconstruction of energy production and maintaining the energy balance by applying exogenous ALC as an energy source to compensate for the reduced energy in the brain of the mouse model of MS could be addressed as a therapeutic strategy. In the current study, we used ALC to ameliorate demyelination-induced depressive-like behaviors in the cuprizone model of MS. The direct and indirect effects of ALC on energy metabolism, NO, and consequently serotonin modulation are probable neuroprotective mechanisms involved in alleviating depressive-like behaviors in the cuprizone mouse model of MS. Due to the unclear and complicated pathobiology of MS-related depression (3, 10), further clinical and experimental investigations are needed to clarify and understand the molecular mechanisms of depression origin in MS patients and provide effective and practical therapeutic approaches. In conclusion, ALC could ameliorate depressive-like behaviors in the cuprizone-intoxicated mouse model of MS. Enhancement of the myelin content and modulation in NO production via influencing the NOS genes in the PFC may be the mechanisms responsible for ALC's beneficial neuroprotective effects.