1. Background
Iran is home to fourteen species of Satureja, with eleven of these being endemic to mountainous regions in the western and northern areas (1, 2). The Lamiaceae family includes many aromatic plants, with Satureja L.—particularly savory—being among the most widely distributed (3). The Satureja genus has been reported to have diverse therapeutic applications in folk medicine (4). Traditionally, it has been used to treat cramps, nausea, indigestion, diarrhea, and other stomach and intestinal disorders (5).
Documented studies have also shown that Satureja L. possesses a wide range of biological properties, including antioxidant, antibacterial, antifungal, and cytotoxic effects (3, 6-8). The acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activities of essential oils from some Satureja species have been examined, revealing potential anti-Alzheimer’s disease effects. These enzymes are crucial in the progression of Alzheimer’s disease; their inhibition can help control the disease's progression (9).
2. Objectives
The wide occurrence of valuable phytochemicals in various plants from the Satureja genus encouraged us to investigate the chemical compositions of their volatile essential oils and their potential medical benefits, specifically regarding cholinesterase (ChE) inhibitory effects and cytotoxic activity. Given that the plant contains a wealth of terpene compounds, including α-eudesmol, which has been shown to have anti-Alzheimer's effects (10), Satureja isophylla was also evaluated for its ChE inhibitory effects.
3. Methods
3.1. Phytochemicals
Various parts of Satureja isophylla, including flowers, leaves, stems, and roots, were collected during the plant's blooming period (August 2022) from the Alborz Mountains in the Chalous area (Mazandaran Province, Iran) at an elevation of 2700 meters. The collected plant parts were dried individually in the shade, stored in well-sealed containers, and placed in the refrigerator. The plant was identified and authenticated at the Tehran University of Medical Sciences Herbarium, Faculty of Pharmacy, Tehran, Iran (Herbarium code: 7129-TEH).
3.2. Volatile Components Isolation
An amount of 100 grams of different parts (flowers, leaves, stems, and roots) of Satureja isophylla was subjected to hydrodistillation using a Clevenger apparatus for 4.5 hours. Anhydrous sodium sulfate was used to dry the samples. The materials obtained during the experiments were stored in amber vials at a low temperature (4°C) for later analysis (11).
3.3. GC Analysis
Essential oils extracted from the plant were analyzed using an Agilent HP-6890 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) equipped with an HP-5MS 5% phenylmethylsiloxane capillary column (30 m × 0.25 mm, 0.25 μm film thickness; A Y type 1: 10 post-column splitter) (Agilent part No: 0101-0595). The column outlet was connected to an Agilent HP-5973 mass selective detector (MSD) operating in electron impact mode (ionization energy: 70 eV) and a flame ionization detector (FID). The initial temperature was maintained at 60°C for 3 minutes, then increased to 246°C at a rate of 3°C /min. The temperatures of the injector and the detectors (MSD and FID) were set at 220ºC and 240ºC, respectively (12). The carrier, fuel, and make-up gases were helium (1.2 mL/min), hydrogen (40 mL/min), and nitrogen (50 mL/min), respectively, with compressed air used at a flow rate of 450 mL/min. n-Alkanes were injected under identical conditions as the samples to calculate retention indices (RIs). Triple injections were performed, and based on the RIs relative to n-alkanes, the mass spectra of the samples were matched with the Wiley 275.L and Wiley 7n.L libraries, and the fragmentation patterns were compared with literature (13).
3.4. In Vitro Cholinesterase Inhibitory Activity
To achieve acceptable enzyme inhibitory activity (20 - 80%), stock solutions of the samples (10 mg/mL) were prepared in methanol and diluted to obtain four different final concentrations: 62.5, 125, 250, and 500 µg/mL. Each well contained 50 µL of potassium phosphate buffer (KH2PO4/K2HPO4, 0.1 M, pH 8), 25 µL of the sample solution, and 25 µL of AChE enzyme at 0.22 units/mL in buffer. After pre-incubation for 15 minutes at room temperature, 125 µL of 5,5′-dithiobis-(2-nitrobenzoic acid) (3 mM in buffer) was added to the mixture. Changes in absorbance at 405 nm were measured using a spectrophotometer after adding 25 µL of the substrate (acetylthiocholine iodide, 3 mM in water). An enzyme-free blank containing all the components was used to account for non-enzymatic reactions. Donepezil was used as a positive control under the same conditions without an inhibitor. IC50 values were calculated graphically based on the log concentration versus % inhibition curves. For the BChE inhibition assay, butyrylthiocholine iodide was used as the substrate. The experiments were conducted in triplicate (14).
3.5. Cell Viability Assay
MRC-5 (human fetal lung fibroblast), MCF-7 (human breast adenocarcinoma), ACHN (human renal adenocarcinoma), PC-12 (adrenal pheochromocytoma), and AsPC-1 (human pancreatic cancer) cell lines were provided by the National Cell Bank of Iran, Pasteur Institute of Tehran, Iran. For culture conditions, the media was supplemented with 10% fetal calf serum (w/v), glutamine, penicillin (100 U/mL), and streptomycin (10 µg/mL). The cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
Cytotoxicity was assessed using the MTT assay, which measures the reduction of tetrazolium dye by mitochondrial enzymes in living cells (15). A 96-well plate was seeded with MRC-5, ACHN, PC-12, AsPC-1, and MCF-7 cells at densities of 105 cells/well and 104 cells/well, respectively, and incubated in a humidified atmosphere at 37°C with 5% CO2. Control wells contained no essential oil, and blank wells contained only growth medium to account for background absorbance.
Essential oils were dissolved in dimethyl sulfoxide (DMSO) and added to the culture medium after overnight incubation at 37°C to allow cell attachment. Each well was limited to 0.5% DMSO. Essential oil dilutions (50 - 500 µg/mL) were added to triplicate wells and incubated for 24 and 48 hours. After incubation, the medium was removed, and MTT was added to each well at a final concentration of 0.5 mg/mL. The plates were incubated for an additional 4 hours at 37°C. To dissolve the formazan crystals, formed by mitochondrial dehydrogenase enzymes in viable cells, 200 µL of DMSO was added. Optical density was measured using a Bio-Rad microplate reader (Model 680) at 570 nm, with background correction at 655 nm. The percentage of cell viability was calculated based on the background absorbance correction.
4. Results
4.1. Chemical Compositions of Volatile Oils
The yields of essential oils from different parts of the plant were 0.7% for flowers, 0.6% for leaves, 0.3% for stems, and 0.2% for roots. A total of 51 components were identified in the essential oils from these different parts. In the study, 96% of the compounds in the flower essential oil were identified, with major components being camphor (18.28%), α-eudesmol (11.45%), and elemol (4.30%). In the leaves, 95.13% of the essential oil components were identified, with the primary compounds being camphor (19.29%), α-eudesmol (17.68%), and elemol (4.83%). For the stems, 92.98% of the essential oil components were identified, with major compounds being α-eudesmol (28.06%), camphor (14.74%), and elemol (7.77%). In the roots, 89.78% of the essential oil components were identified, with the primary compounds being α-eudesmol (34.68%), elemol (9.87%), and γ-eudesmol (8.45%) (Table 1).
| No. | Component | RIa | Flowers | Leaves | Stems | Roots |
|---|---|---|---|---|---|---|
| 1 | α-Thujene | 930 | 0.88 | 1.1 | 0.15 | - |
| 2 | α-Pinene | 939 | 3.81 | 4.97 | 0.88 | - |
| 3 | Camphene | 954 | 5.27 | 6.98 | 1.44 | - |
| 4 | β-Pinene | 979 | 1.63 | 2.16 | 0.52 | - |
| 5 | Mesitylene | 995 | 2.2 | - | - | - |
| 6 | α-Terpinene | 1017 | - | 0.62 | 0.25 | - |
| 7 | ο-Cymene | 1026 | - | 3.52 | 1.08 | - |
| 8 | Limonene | 1029 | 4.46 | 3.11 | 0.72 | - |
| 9 | γ-Terpinene | 1060 | 1.44 | 1.32 | 0.61 | - |
| 10 | Cis-Sabinene hydrate | 1070 | 4.12 | 2.72 | 1.68 | 1.3 |
| 11 | Terpinolene | 1089 | - | - | 0.19 | - |
| 12 | Linalool | 1097 | - | 0.6 | 0.38 | 1 |
| 13 | Trans-Sabinene hydrate | 1098 | - | - | 0.83 | 0.58 |
| 14 | exo-Fenchol | 1121 | 2.75 | 1.1 | 0.68 | - |
| 15 | Trans-2-Pinanol | 1135 | 3.24 | - | 1.88 | - |
| 16 | Camphor | 1146 | 18.28 | 19.29 | 14.74 | 4.32 |
| 17 | Borneol | 1165 | 2.48 | 0.91 | 1.02 | 1.02 |
| 18 | Terpinen-4-ol | 1177 | 5.19 | 4.72 | 3.91 | 2.08 |
| 19 | α-Terpineol | 1188 | 2.34 | 1.95 | 1.77 | 1.2 |
| 20 | Cis-Dihydro carvone | 1193 | 0.83 | 0.61 | 0.25 | 0.33 |
| 21 | Trans-Carveol | 1216 | - | 1.08 | 1.95 | - |
| 22 | Nerol | 1229 | 2.61 | - | - | 0.95 |
| 23 | Neral | 1238 | 1.41 | 1.06 | 0.63 | 0.66 |
| 24 | Carvotanacetone | 1247 | 1.28 | 0.85 | 0.99 | 0.75 |
| 25 | Geranial | 1267 | 1.71 | 1.13 | 0.79 | 1 |
| 26 | Isobornyl acetate | 1285 | 1.15 | 0.6 | 0.53 | 0.62 |
| 27 | Thymol | 1290 | 0.45 | - | - | 0.51 |
| 28 | α-Ylangene | 1375 | - | - | 0.20 | - |
| 29 | β-Bourbonene | 1388 | 1.21 | 1.1 | 2.04 | 0.7 |
| 30 | β-Elemene | 1390 | - | - | 0.37 | - |
| 31 | (E)-Caryophyllene | 1419 | 2.82 | 2.86 | 2.82 | 1.91 |
| 32 | α-Humulene | 1454 | - | - | 0.14 | - |
| 33 | Germacrene D | 1481 | 1.68 | 1.41 | 1.10 | 1.5 |
| 34 | Elixene | 1511 | - | - | - | 0.86 |
| 35 | γ-Cadinene | 1513 | 0.22 | - | 0.31 | - |
| 36 | δ-Cadinene | 1523 | 0.36 | - | 0.56 | 0.51 |
| 37 | Elemol | 1549 | 4.30 | 4.83 | 7.77 | 9.87 |
| 38 | Germacrene B | 1561 | 0.79 | - | - | - |
| 39 | Spathulenol | 1578 | 0.88 | 1.22 | 1.31 | 2.4 |
| 40 | Caryophyllene oxide | 1583 | 0.82 | 1.18 | 1.28 | 2.28 |
| 41 | γ-Eudesmol | 1632 | 2.93 | 4.03 | 5.99 | 8.45 |
| 42 | Agarospirol | 1641 | 1.01 | 0.42 | 1.75 | 3.66 |
| 43 | α-Eudesmol | 1653 | 11.45 | 17.68 | 28.06 | 34.68 |
| 44 | epi-α-Bisabolol | 1684 | - | - | 0.38 | |
| 45 | Hexahydrofarnesyl acetone | 1847 | - | - | - | 0.35 |
| 46 | Diisobutyl phthalate | 1867 | - | - | - | 0.37 |
| 47 | Ethyl hexadecanoate | 1993 | - | - | - | 1 |
| 48 | Linoleic acid ethyl ester | 2139 | - | - | - | 1.53 |
| 49 | Ethyl Oleate | 2168 | - | - | - | 1.3 |
| 50 | Pimara-7,15-dien-3-ol | 2253 | - | - | - | 0.41 |
| 51 | Methyl isopimarate | 2298 | - | - | - | 1.30 |
| Total | - | 96.00 | 95.13 | 92.98 | 89.78 |
aValues are presented as No. (%).
b Retention index of the literature (13).
Few studies have evaluated the chemical components in Satureja isophylla essential oil. Ebadollahi et al. identified thymol (41.5%), p-cymene (25.9%), γ-terpinene (16.9%), β-myrcene (2.1%), and α-terpinene (1.6%) in the essential oil of Satureja isophylla (16). Sefidkon and Jamzad reported that the major constituents in the essential oil were α-eudesmol (11.3%), β-eudesmol (9.6%), camphor (7.1%), β-caryophyllene (6.1%), γ-eudesmol (5.8%), and geranial (5.5%) (2), which are highly consistent with the essential oil identified in this study. It was also reported that α-eudesmol (47.8%), β-eudesmol (9.2%), γ-terpinene (3.8%), and cymene (1.3%) were present in high amounts, while thymol constituted only 0.1% of the total essential oil from Satureja isophylla. This work represents the first analysis and identification of essential oils from different parts of Satureja isophylla, including flowers, leaves, stems, and roots.
4.2. In Vitro ChE Inhibitory Activity
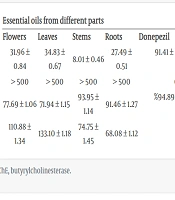
The results of AChE inhibitory activities of essential oils from different parts of Satureja isophylla are presented in Table 2. Compared to other parts of the plant, the essential oils from roots, flowers, and leaves exhibited moderate AChE inhibitory activity. In terms of BChE activity, Satureja isophylla showed significant inhibition of BChE compared to AChE (Table 2). It was found that the essential oils from Satureja isophylla had below-average inhibitory activity (IC50 > 500 µg/mL) for AChE. On the other hand, essential oils extracted from different parts of Satureja isophylla exhibited high inhibitory activities against BChE. Donepezil, as a reference inhibitor, demonstrated IC50 values of 0.02 µg/mL against AChE and 0.22 µg/mL against BChE. According to the in vitro ChE inhibitory test, Satureja isophylla displayed higher levels of selective activity against BChE than against AChE. Moreover, the high α-eudesmol content in these essential oils suggests a good response to ChE inhibition, contributing to potential anti-Alzheimer and antispasmodic properties (10). Based on Table 1, which summarizes the components and concentrations of the essential oils from different parts of the plant, it can be concluded that a higher amount of α-eudesmol correlates with a stronger ChE inhibitory effect. This trend is evident as the concentration of α-eudesmol increases from flowers to roots. It is noteworthy that the ChE inhibitory effects of essential oils from different parts of Satureja isophylla have not been reported before, making this the first report on their ChE inhibitory activity.
| Variables | Essential Oils from Different Parts | ||||
|---|---|---|---|---|---|
| Flowers | Leaves | Stems | Roots | Donepezil | |
| AChE (%Inhibition) at 500 µg/mL of sample | 31.96 ± 0.84 | 34.83 ± 0.67 | 8.01 ± 0.46 | 27.49 ± 0.51 | 91.41 ± 0.07 (0.1 µg/mL of donepezil) |
| AChE; IC50 (μg/mL) | > 500 | > 500 | > 500 | > 500 | 0.02 ± 0.00 |
| BChE (% inhibition) at 500 µg/mL of sample | 77.69 ± 1.06 | 71.94 ± 1.15 | 93.95 ± 1.14 | 91.46 ± 1.27 | %94.89 ± 0.10 (5 µg/mL of donepezil) |
| BChE; IC50 (μg/mL) | 110.88 ± 1.34 | 133.10 ± 1.18 | 74.75 ± 1.45 | 68.08 ± 1.12 | 0.22 ± 0.03 |
Abbreviations: AChE, acetylcholinesterase; BChE, butyrylcholinesterase.
4.3. In Vitro Cytotoxicity Test
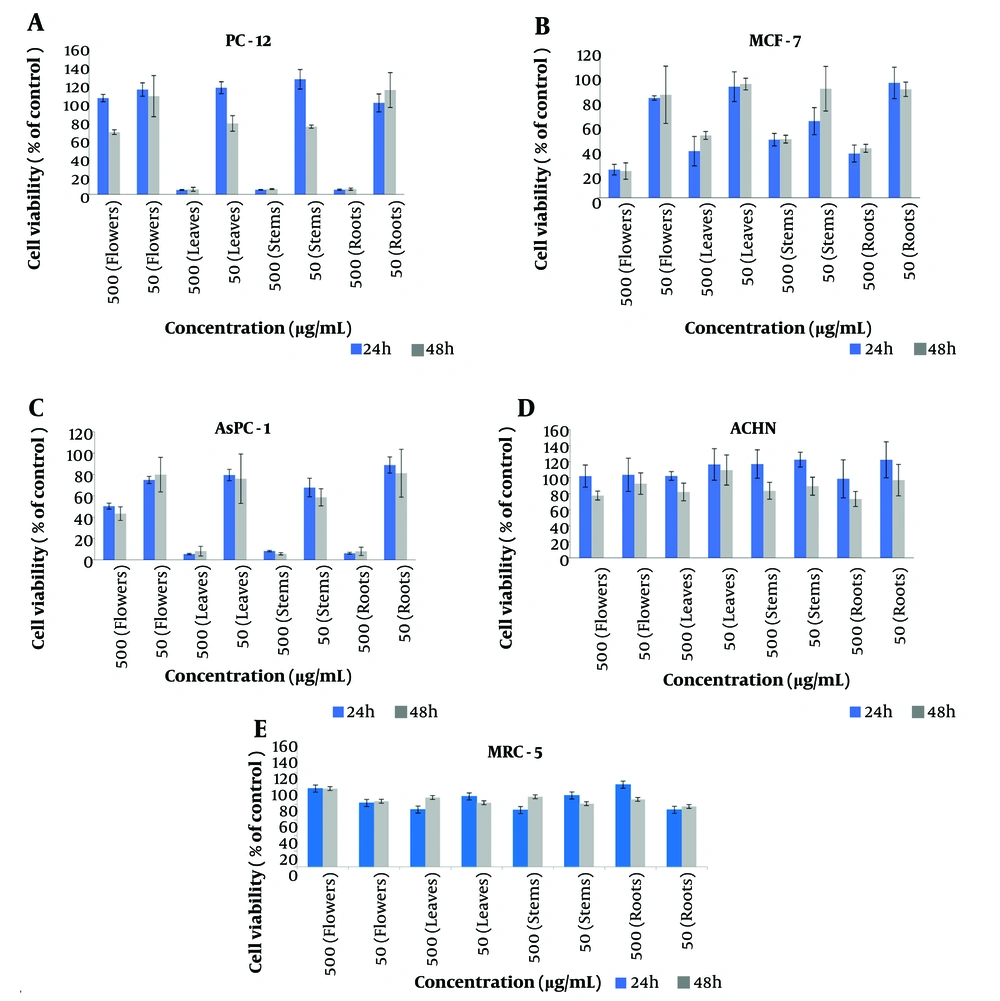
Numerous studies have demonstrated that essential oils are generally cytotoxic but not mutagenic (17, 18). To evaluate the cytotoxicity of the essential oils, the growth inhibitory effects of the extracts were assessed against four cancer cell lines—MCF-7 (human breast adenocarcinoma), ACHN (human renal adenocarcinoma), AsPC-1 (human pancreatic cancer), and PC-12 (adrenal phaeochromocytoma)—as well as MRC-5 (a normal cell line) using the MTT assay (19, 20). Cell viability was determined based on the relative absorbance of essential oils in treated cells compared to control cells (Figure 1).
In vitro cytotoxic effects of Satureja isophylla essential oils on PC-12 (A), MCF-7 (B), AsPC-1 (C), ACHN (D), MRC-5 (E) cells, as determined by MTT assay. DMSO was used as a solvent in the control group. Results are presented as a viability ratio. A minimum of three independent experiments were conducted to obtain the mean value.
The essential oils obtained from different parts of Satureja isophylla exhibited varying effects on cancer cells depending on the plant part from which they were extracted. At a concentration of 500 µg/mL, the essential oil extracted from the stems showed significant effects on PC-12, AsPC-1, and MCF-7 cells (5.85%, 5.64%, and 48.64%, respectively) and displayed no cytotoxic effects after 48 hours. Essential oils from the flowers demonstrated notable anticancer properties against MCF-7 cells (22.14% at 500 µg/mL after 48 hours), while the leaf extract had a significant effect on PC-12 (5.58%) and AsPC-1 (8.14%) cell lines at the same concentration and duration. Essential oils from the roots also exhibited a remarkable cytotoxic effect on PC-12 (5.71%) and AsPC-1 (7.94%) at 500 µg/mL after 48 hours. Overall, essential oils from different parts of Satureja isophylla showed effective anticancer and cytotoxic effects. This study is the first to report the cytotoxic activity of Satureja isophylla and may serve as a basis for further pharmacological investigations of these compounds.
5. Discussion
An evaluation of the chemical profile of Satureja isophylla essential oils was conducted, along with assessments of anticholinesterase and anticancer activities. Several studies have demonstrated the anticholinesterase activity of Satureja species. For instance, γ-terpinene and carvacrol are responsible for this activity in S. thymbra and S. spicata, respectively; however, the levels of these compounds in Satureja isophylla were not significant (21, 22). Another study attributed the effects of S. capitata to carvacrol, γ-terpinene, and ρ-cymene (23). Additionally, carvacrol and α-pinene showed higher anticholinesterase inhibitory effects than other terpenoids (above 70%), while menthol and piperitone exhibited higher BChE inhibition (24). However, research by Mihajilov-Krstev et al. indicated that all components in the essential oil of S. montana contributed to both AChE and BChE inhibitory effects (25).
This study demonstrates that Satureja isophylla is a valuable pharmacological source. Notably, high amounts of camphor in the essential oil of Salvia lavandulaefolia, alongside 1,8-cineol, are responsible for its high anticholinesterase activity (26). The ChE assay results suggest that the higher percentages of α-eudesmol and camphor, as well as non-covalent interactions between these compounds and the ChE enzyme, might account for the higher inhibitory activity observed in the essential oils of the roots, stems, and flowers of Satureja isophylla compared to its leaves. Additionally, preliminary in vitro studies revealed that Satureja isophylla essential oils exhibited significant anticancer effects against AsPC-1, MCF-7, and PC-12 cell lines, with various parts of the plant showing effective anticancer and cytotoxic effects.