1. Background
The use of medicinal plants, a practice deeply rooted in human history, has served as a cornerstone in treating various health conditions. In recent times, natural products have emerged as a vital resource for discovering life-saving medications (1). In contrast to synthetic modern medicines, medicinal herbs offer a reliable source of natural remedies, having been used as both food and medicine for centuries (2).
Many of these plants contain valuable components that exhibit various pharmacological effects, such as antimicrobial, anti-inflammatory, hepatoprotective, anticancer, and antioxidant properties (3-6). Although there have been remarkable advancements in cancer treatments like chemotherapy, immunotherapy, and targeted therapy, these treatments typically come with several side effects and consequences that may be challenging to manage (7).
Despite the limitations of current cancer treatments, efforts are being made to develop more effective medications from natural sources (8). For example, anticancer drugs like vincristine and vinblastine, derived from medicinal plants, have shown promising results (9).
Arbutus L. is a captivating genus that belongs to the Ericaceae family, which includes approximately 50 species distributed across America, the Mediterranean, and the Canary Islands. In Jordan, Arbutus spp. has been recorded in Irbid, Ajloun, Jerash, Salt, and Amman (10). It has been traditionally used in indigenous medicine for various purposes, including alleviating gastrointestinal discomfort such as indigestion and stomachaches, treating coughs, bronchitis, and sore throats, and treating skin infections and wounds (10, 11).
A. andrachne L. is a small tree used for woodwork, and its fruits, which are pleasant to taste, are used to make jam (11). Arbutus species are utilized in traditional medicine for various purposes. For instance, the leaves of Arbutus unedo are traditionally used for their diuretic, urinary antiseptic, antidiarrheal, astringent, depurative, and antihypertensive properties (12).
In addition to these traditional uses, recent studies have revealed that Arbutus spp. also exhibit both cytotoxic and antioxidant properties, suggesting potential therapeutic benefits. For example, Alexandre et al. found that supercritical fluid extracts of A. unedo had stronger antiproliferative effects on HeLa cells compared to MCF-7 cells (13). Methanol extracts of A. andrachne showed antioxidant and cytotoxic effects on HepG2 and Huh7 liver cancer cells (14). A dichloromethane extract from A. pavarii leaves was cytotoxic to PC3 prostate cancer cells (15). A. unedo honey protected human lymphocytes from irinotecan-induced damage (16), and computational models revealed significant antioxidant activity of Arbutus flavonoids (17). Additionally, A. unedo fruits were found to contain bioactive compounds with antioxidant properties (18).
Our investigation into natural compounds to combat life-threatening diseases is a compelling and pioneering endeavor.
2. Objectives
This study aims to explore the antioxidant properties and potential antiproliferative effects of extracts derived from the fruits of A. andrachne on various cancer cell lines, including breast cancer (T47D and MCF-7), colorectal cancer (Caco-2 and HCT-116), and normal skin fibroblast (MRC-5) lines. Unlike previous studies that primarily focused on the leaves and other parts of Arbutus spp., our research uniquely examines the fruits of A. andrachne, providing new insights into their therapeutic potential for cancer treatment. Additionally, this study evaluates their potential as natural antioxidants for various industries, expanding the existing knowledge base.
3. Methods
3.1. Chemicals and Instruments
The study employed a variety of chemicals and laboratory equipment from reputable suppliers.
Cell Lines: Human breast adenocarcinoma cells (MCF-7), breast cancer cells (T47D), colorectal adenocarcinoma cells (Caco-2), colon cancer cells (HCT116), and human skin fibroblast cell lines (MRC-5) were obtained from the American Type Culture Collection® (ATCC).
Chemicals: Dulbecco's modified eagle medium (DMEM), L-glutamine, penicillin-streptomycin, trypsin-Ethylenediaminetetraacetic acid (trypsin-EDTA), and Ethylenediaminetetraacetic acid (EDTA) were sourced from Euro-clone, Italy. Trypan blue stain, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and ascorbic acid were obtained from Sigma, USA. Absolute ethanol, methanol, dimethyl sulfoxide (DMSO), and phosphate buffer were purchased from Sigma-Aldrich, USA. Potassium ferricyanide (K3[Fe (CN)6]), trichloroacetic acid (TCAA), and ferric chloride (FeCl3(H2O)x) were obtained from Fisher Scientific, USA. Anhydrous sodium sulfate (Na₂SO₄) was sourced from Analar, England. n-hexane/Gas Chromatography (GC) grade was purchased from Tedia, USA. A mixture of n-alkanes (C8–C20) hydrocarbons was procured from Fluka, Switzerland.
Laboratory Equipment: The hydro-distillation process for essential oil extraction utilized a Clevenger-type apparatus from JSGW, India. Chemical analysis of the oil sample was performed using a Varian Chrompack CP-3800 gas chromatography coupled with a mass spectrometry detector (GC/MS/MS-200) made by Saturn, Netherlands.
3.2. Plant Materials Collection
During the autumn of 2019, the fruits of A. andrachne L. were procured from Debbien in Jerash City, Jordan (32.24440613327969, 35.82941730410599). Prof. Sawsan Oran, a skilled plant taxonomist, identified and authenticated the plant, and a voucher specimen (AA-2019-05-05) was obtained and preserved at the herbarium of the Department of Biological Sciences, Faculty of Sciences, at the University of Jordan in Amman.
3.3. Preparation of Crude Extracts from Fruits of Arbutus andrachne
In this study, air-drying was carried out for three to four weeks at room temperature (23 - 25°C) in the dark, after which the plants were powdered using an electric blender. The powdered plant parts were then soaked in three different solvents—distilled water, absolute ethanol, and absolute methanol—in a ratio of 50 g per 500 mL. A hotplate magnetic stirrer was used to stir the mixture, and the solvents were allowed to stand for three days. After standing, the extracted solution underwent filtration via Whatman No. 1 filter paper. The solvents were then evaporated at 60°C using a rotary evaporator, and the filtered extracts were allowed to dry thoroughly (18). The crude extracts were stored at 4°C for future experiments.
3.4. Arbutus andrachne Essential Oils Extraction
A quantity of 500 grams of dried fruits from A. andrachne underwent hydrodistillation for three hours using a Clevenger-type apparatus to extract essential oils. Once the essential oils had been obtained, they were dried using anhydrous sodium sulfate. The samples were then stored with utmost care in sealed, opaque vials at a constant temperature of 4°C until their analysis was conducted. The percentage of essential oils, expressed in weight by weight,
3.5. GC-MS Analysis
The chemical analysis of oil samples from the fruits of A. andrachne was performed using a Varian Chrompack CP-3800 GC-MS-200 system. One microliter of each oil sample was diluted with GC-grade n-hexane before analysis. The GC system utilized a DB-5 capillary column (30 m length, 0.25 mm width, 0.25 µm film thickness) composed of 5% diphenyl and 95% dimethyl polysiloxane, with helium as the carrier gas at a flow rate of 1 mL/min. The analysis began at 60°C for 1 minute, then increased at 3°C/min to 246°C, which was maintained for 3 minutes. A flame ionization detector (FID) with an electron ionization source (70 eV, 180°C) was used. The mass spectra of the oils were compared with those in the NIST, Wiley, and Adam-2007 libraries, with Adam's library being the primary reference (19). Additionally, the calculated Kovats indices (KI) were matched with literature values for similar polarity columns.
3.6. Ferric Reducing Antioxidant Power Assay (FRAP)
To test the reducing activity of a plant extract, 1 mL of the extract (1 mg/mL in methanol) was mixed with 2.5 mL of phosphate buffer and 2.5 mL of potassium ferricyanide. The mixture was incubated at 50°C for 20 minutes. After adding 2.5 mL of trichloroacetic acid, the mixture was centrifuged at 3000 rpm for 10 minutes. The supernatant was collected and combined with 2.5 mL of distilled water and 0.5 mL of ferric chloride. Absorbance was measured at 700 nm using a spectrophotometer, compared to a blank phosphate buffer, with ascorbic acid used as a positive control. Increased absorbance indicated higher reducing activity (20), and results were expressed as Fe2+ equivalents using a standard curve.
3.7. Cell Culture
The study aimed to uncover the potential of various plant extracts in combating different types of human cancer cells, including colorectal adenocarcinoma cells (CaCo-2), colon cancer cells (HCT116), human breast adenocarcinoma cells (MCF7), breast cancer cells (T47D), and the human skin fibroblast cell line (MRC-5). The cells were grown in DMEM with 1% of 2 mM L-glutamine, 100 μg/mL penicillin-streptomycin, and heat-inactivated fetal bovine serum (FBS) at a concentration of 10%. Following standard culture protocols, the cells were incubated at 37°C, with 5% CO2 and 95% humidity, until they reached 75 - 80% confluence. Subsequently, phosphate-buffered saline (PBS) was used to wash the cells, and trypsin-EDTA was employed to separate them into single-cell suspensions. The viability of the cells was then quantified using the trypan blue dye exclusion assay (21).
3.8. MTT Assay
The cell viability assay was conducted using the MTT assay to evaluate the effect of plant extracts administered at varying concentrations on the cells. Prior to treatment, 1 × 104 cells were meticulously seeded in each well of a 96-well cell culture plate and allowed to adhere for 24 hours in a humidified incubator maintained at 37°C with 5% CO2. Then, increasing concentrations of plant extracts (3.125, 6.25, 12.5, 25, 50, 100, and 200 μg/mL in dimethyl sulfoxide (DMSO)) were introduced into the cells in 100 μL volumes. The assay control wells were supplemented with culture medium and 0.01% DMSO, while the negative control wells contained only fresh media. A positive control of doxorubicin (0.05 - 50 μg/mL) was also included.
Following a 72-hour incubation period, 20 microliters of 5 milligrams per milliliter of MTT dissolved in phosphate-buffered saline were added to each well and incubated for three hours. After removing the MTT, 200 µL of DMSO was added to each well to dissolve the crystals. A microplate reader was then used to measure the absorbance at 570 nm and 630 nm wavelengths; the difference between the two values was subsequently analyzed. The absorbance of the negative control was subtracted to mask it. The viability of the assay control cells was taken as the reference point, with its absorbance value considered to be 100%. To determine the effectiveness of the treatment, the absorbance values of treated cells were calculated as a proportion of the control (21).
The following formula was used to calculate the percentage of surviving cells:
The half-inhibitory concentrations (IC50) were determined by measuring the concentration at which each cell line's growth was inhibited by 50%. Three independent IC50 values were obtained and averaged to ensure accuracy, thereby providing a reliable estimate of IC50 for each cell line.
3.9. Statistical Analysis
The experimental protocol involved three independent replications, and the resulting data were presented as mean ± SEM. The IC50 values for the various assays were determined using linear regression analysis. An ANOVA was performed using GraphPad Prism 5.0 (San Diego, USA) to investigate the relationship between the IC50 of plant extracts and cancer cell lines compared to a normal cell line. Dunnett’s multiple comparison test was subsequently applied to compare the IC50 of the test plant extracts against cancer cell lines with the normal cell line. These results were validated with a significance level of P < 0.05.
4. Results
4.1. Arbutus andrachne Oil Extraction and Phytochemical Investigation
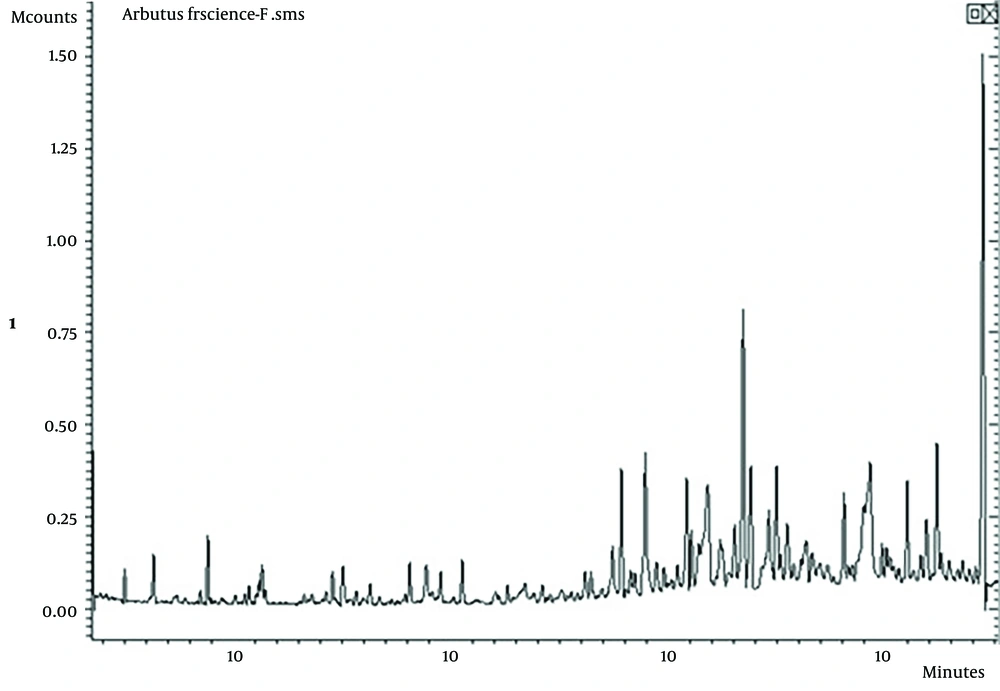
The hydrodistillation process was performed on the air-dried fruits of A. andrachne, yielding 0.11% (w/w) essential oil. GC/MS, a widely accepted method for identifying and quantifying volatile compounds, was employed to determine the chemical composition of the essential oils from A. andrachne. The examination revealed that thirty-six compounds accounted for 97.53% of the total oil extracted from the fruits. The extracts' GC-MS spectra are presented in Figure 1.
The study revealed that the main components of the essential oil from A. andrachne fruits are pentadecanoic acid, 14-methyl-, and methyl ester, making up a substantial 19.87% of the oil. The second most abundant component, khusimone, with a percentage of 11.29%, holds promising potential for various applications. This oil's oxygenated sesquiterpenes, comprising a significant 41.98% of its volatile parts, are particularly noteworthy. They are made up of khusimone (11.29%), 10-epi-g-Eudesmol (5.00%), and 10-epi-cis-Dracunculifoliol (3.71%). A considerable amount of non-aromatic compounds was found (31.93%), such as pentadecanoic acid, 14-methyl ester (19.87%), and hillyl acetate (4.52%) (Table 1).
| KI Exp a | KI Litb | Compound | % Content c | Identified by |
|---|---|---|---|---|
| 1027 | 1029 | Limonene | 1.84 | MS-KI d |
| 1093 | 1100 | 1-Undecyne | 0.92 | MS-KI |
| 1181 | 1177 | E-Isociral | 1.24 | MS-KI |
| 1208 | 1205 | Verbenone | 0.66 | MS-KI |
| 1268 | 1268 | Vitispirane | 1.51 | MS-KI |
| 1281 | 1279 | Trans-Ethyl chrysanthemumate | 0.87 | MS-KI |
| 1351 | 1342 | Trans-Carvylacetate | 0.59 | MS-KI |
| 1431 | 1433 | Naphthalene, 2-methoxy- | 1.02 | MS-KI |
| 1438 | 1436 | Dihydro-β-Ionone | 1.09 | MS-KI |
| 1461 | 1455 | Khusimene | 1.34 | MS-KI |
| 1471 | 1471 | Dehydro-Sesquicineole | 4.05 | MS-KI |
| 1480 | 1477 | β-Chamigrene | 0.67 | MS-KI |
| 1495 | 1498 | α-Selinene | 6.55 | MS-KI |
| 1507 | 1500 | α-Muurolene | 1.36 | MS-KI |
| 1516 | 1512 | α-Alaskene | 0.80 | MS-KI |
| 1543 | 1541 | 10-epi-cis-Dracunculifoliol | 3.71 | MS-KI |
| 1548 | 1542 | Cis-Dracunculifoliol | 1.87 | MS-KI |
| 1565 | 1568 | Lauric acid n-amyl ester | 3.11 | MS-KI |
| 1580 | 1572 | Caryophyllenyl alcohol | 1.73 | MS-KI |
| 1583 | 1578 | Spanthuelenol | 1.14 | MS-KI |
| 1595 | 1592 | Syn-anti-anti-Helifolen-12-al A | 2.15 | MS-KI |
| 1605 | 1604 | Khusimone | 11.29 | MS-KI |
| 1610 | 1608 | β-Atlantol | 0.02 | MS-KI |
| 1614 | 1622 | 10-epi-γ-Eudesmol | 5.00 | MS-KI |
| 1636 | 1638 | Epi-α-Cadinol | 1.80 | Ms only |
| 1645 | 1649 | β-Eudesmol | 3.73 | MS-KI |
| 1651 | 1656 | Exalatacin | 1.03 | MS-KI |
| 1658 | - e | Unkown | 2.51 | - |
| 1681 | 1672 | Tetradecanol<n-> | 1.96 | MS-KI |
| 1726 | 1722 | Celestolide | 2.88 | MS-KI |
| 1751 | 1809 | Isopropyl myristate | 0.61 | MS only |
| 1804 | 1802 | 2,2-dimethyl-7-sec-butyl-2H,5H, pyrano [4,3-b]-Pyran-5-one | 3.27 | MS-KI |
| 1829 | 1837 | Galaxolide 1 and 2 | 2.38 | MS-KI |
| 1844 | 1838 | Hillyl acetate | 4.52 | MS-KI |
| 1850 | 1869 | Pentadecanoic acid | 0.94 | MS-KI |
| 1890 | 1877 | Pentadecanoic acid, 14-methyl-, methyl ester | 19.87 | Ms only |
| Variables | Values (%) | |||
| Total identified | 97.53 | |||
| MT | ||||
| Hydrocarbons MT | 1.84 | |||
| Oxygenated MT | 4.39 | |||
| ST | ||||
| Hydrocarbons ST | 10.72 | |||
| Oxygenated ST | 41.98 | |||
| DT | ||||
| Hydrocarbons ST | 0.00 | |||
| Oxygenated ST | 2.38 | |||
| Non-terpenoid non-aromatic compounds | 31.93 | |||
| Non-terpenoid aromatic compounds | 4.29 | |||
Abbreviation: KI, kovats indices; MT, monoterpenes; ST, sesquiterpenes; DT, diterpenes.
a Experimental KI relative to (C8-C20) n-alkanes.
b Literature KI (Adams, 2007).
c The percentage composition was ascertained through peak area analysis.
d Mass spectrum-arithmetic retention index matching.
e KI value not available in literature.
4.2. In vitro Antioxidant Potential of Arbutus andrachne Fruits Crude Extracts
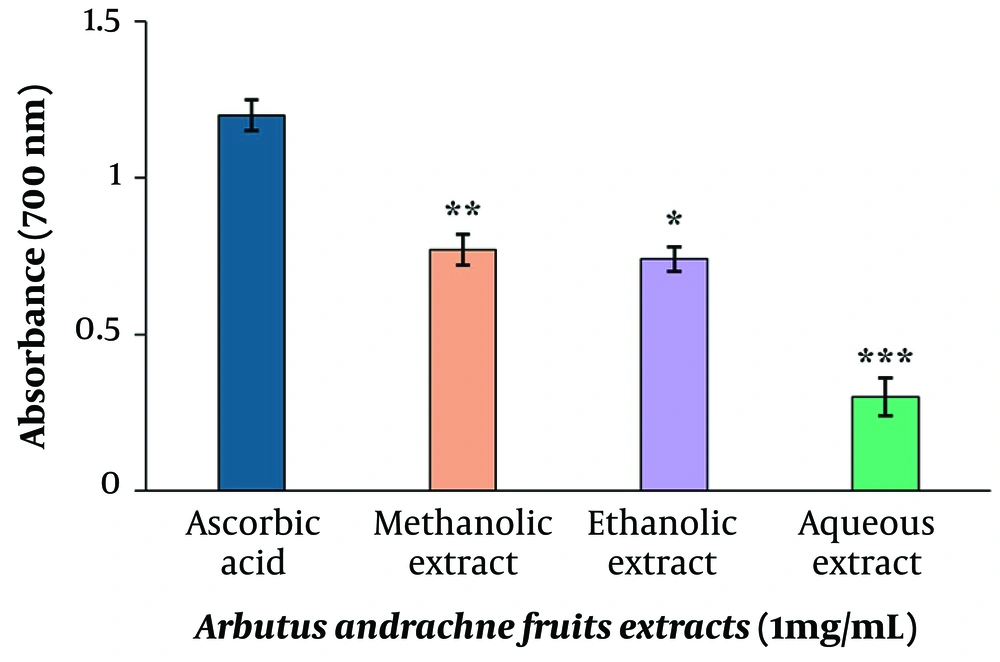
The study's findings indicated a distinct order of antioxidant activity in the methanolic, ethanolic, and aqueous extracts of A. andrachne fruits compared to ascorbic acid (positive control). This order, descending from methanolic to ethanolic to aqueous, underscores the higher antioxidant potential of the first two extracts over the latter (Figure 2). The results, expressed in terms of Fe2+ equivalents, were obtained using a standard curve equation derived from known concentrations of Fe2+ ions. The absorbance values at 700 nm from the FRAP assay were then converted to Fe2+ equivalents (y = 0.0293x + 0.2340, R2 = 0.9866). The methanolic extract showed 18.27 µmol/L Fe2+ equivalents, the ethanolic extract 17.26 µmol/L Fe2+ equivalents, and the aqueous extract 2.25 µmol/L Fe2+ equivalents.
The antioxidant potential of Arbutus andrachne fruit extracts using the Ferric educing antioxidant power assay (FRAP) at n absorbance of 700 nm. Methanolic, ethanolic, and aqueous extracts (1 mg/mL) were prepared, and ascorbic acid (1 mg/mL) was used as a positive control. The mean ± SEM of three replicates was reported, and the results were analyzed statistically. The obtained results indicated that the antioxidant activity of the extracts was concentration-dependent and significantly different from the positive control group (*P < 0.05, **P < 0.01, ***P < 0.001).
4.3. In vitro Antiproliferative Activity of Arbutus andrachne Fruits Crude Extracts
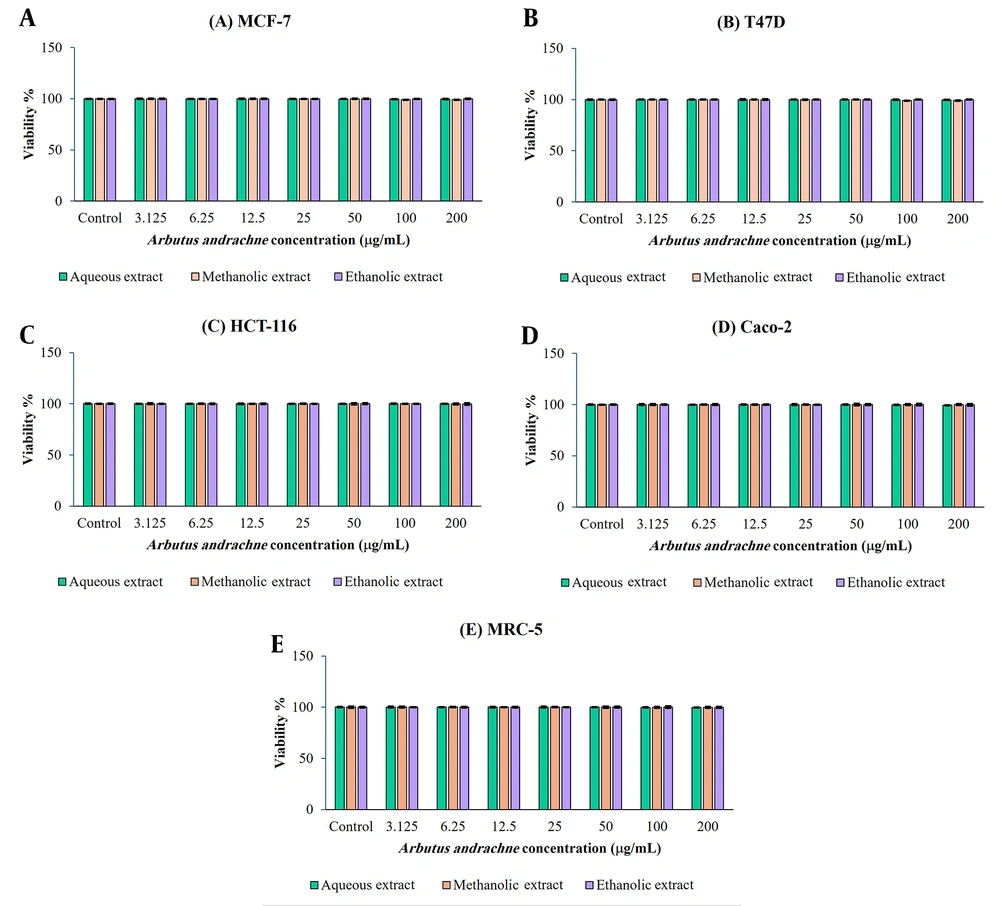
The antiproliferative activity of crude extracts obtained from the fruits of A. andrachne was assessed against different human cancer cell lines, including MCF-7, T47D, HCT-116, and Caco-2, as well as a normal fibroblast cell line, MRC-5.
The findings revealed that all tested extracts from the fruits of A. andrachne exhibited only weak antiproliferative activity against the MCF-7, T47D, HCT-116, and Caco-2 cancer cell lines, indicating a potential selectivity towards cancer cells. Importantly, A. andrachne extracts had no significant antiproliferative activity against the MRC-5 human normal skin fibroblast cell line, suggesting a potential safety profile (Figure 3).
The antiproliferative potential of aqueous, methanolic, and ethanolic extracts obtained from the fruits of Arbutus andrachne. The extracts were tested against various cancerous cell lines, including MCF-7, T47D, HCT-116, and Caco-2, as well as noncancerous cell lines such as MRC-5. The results were presented as the mean of three replicates, with a standard error of the mean (SEM). (P-value < 0.05) when compared to the untreated control group.
Furthermore, the standard cytotoxic drug doxorubicin demonstrated remarkably high cytotoxic activity against both cancerous and non-cancerous cell lines, underscoring its effectiveness. Table 2 shows the IC50 (µg/mL) values for the crude extracts derived from the fruits of A. andrachne and doxorubicin against the tested cell lines.
| Treatment | MCF7 | T47D | HCT-116 | Caco-2 | MRC-5 |
|---|---|---|---|---|---|
| Aqueous extracts | NI | 242.8 ± 1.54 | NI | 584.6 ± 1.92 | NI |
| Methanolic extracts | 205.8 ± 4.02 | 420.8 ± 1.60 | NI | 825.1 ± 3.15 | NI |
| Methanolic extracts | 205.8 ± 4.02 | 420.8 ± 1.60 | NI | 825.1 ± 3.15 | NI |
| Doxorubicin b | 1.15 ± 0.20 | 2.1 ± 1.30 | 1.3 ± 0.54 | 5.57 ± 0.35 | 28.82 ± 3.23 |
Abbreviation: NI denotes non-inhibitory.
a The data were independently measured three times, and the mean ± SEM was presented.
b Doxorubicin was used as a standard cytotoxic drug.
5. Discussion
The essential oil constituents of A. andrachne fruits were identified using gas chromatography-mass spectroscopy (GC-MS). The provided GC chromatogram (Figure 1) illustrates the separation of compounds in a sample over time, with retention times on the x-axis and detector response intensity on the y-axis. Early eluting peaks (0 - 10 minutes) suggest the presence of more volatile and smaller molecular weight compounds, while mid-eluting peaks (10 - 30 minutes) indicate compounds of intermediate volatility. Notable peaks around 30 minutes, which are key to your findings, point to significant components in the sample, and later peaks (30 - 40+ minutes) correspond to less volatile, higher molecular weight compounds. The height of each peak reflects the concentration of the respective compound, with a significant peak around 30 minutes indicating a predominant component. Identifying specific compounds requires comparing retention times and mass spectra with known standards from libraries such as NIST, Wiley, or Adam-2007 (19).
It is essential to analyze and compare our findings with previous reports on the isolation of pentadecanoic acid, 14-methyl-, methyl ester, and khusimone from Arbutus spp., as well as other studies related to A. andrachne. A comprehensive literature review revealed no reports on the isolation of pentadecanoic acid, 14-methyl-, methyl ester from other Arbutus spp. However, its presence in the essential oils of A. andrachne fruits as a primary component (19.87%) is noteworthy and suggests a unique chemical profile for this species. Additionally, khusimone, accounting for 11.29% of the essential oils, further highlights the distinct composition of A. andrachne. Our findings of significant oxygenated sesquiterpenes (41.98%) and non-aromatic compounds (31.93%) add valuable insights into the comprehensive chemical makeup of A. andrachne essential oils.
The comparison between the essential oils derived from the fruits of A. andrachne in our study and those obtained from the wood as reported in Turkey provides significant insights into the plant's chemical diversity. The study on A. andrachne wood identified 25 compounds making up 80.5% of the oil, with cinnamyl alcohol (21.97%), 4-tert-butylcyclohexyl acetate (16.59%), and isobornyl acetate (15.37%) as the primary constituents (22). In contrast, our analysis of the fruit essential oil revealed pentadecanoic acid, 14-methyl-, methyl ester (19.87%) and khusimone (11.29%) as major components, highlighting a different chemical profile.
In Jordan, the liquid chromatography-mass spectrometry (LC-MS) analysis of the methanolic leaf extract of A. andrachne displayed the presence of various constituents such as arbutin, rutin, linalool, linoleic acid, gallic acid, lauric acid, myristic acid, hydroquinone, β-sitosterol, ursolic acid, isoquercetin, (+)-gallocatechin, kaempferol, α-tocopherol, quercetin, myricetin, and catechin gallate, according to Jaffal et al (23).
The variability in essential oil composition among different species offers intriguing insights into each plant's unique biological and ecological traits. For instance, in a study conducted in Morocco, the essential oils of A. unedo were found to possess decenal (13.47%), α-terpineol (7.8%), and palmitic acid (6.00%) as the primary volatile compounds (24). This distinct chemical profile contrasts with our findings, highlighting the diversity within the Arbutus spp. and prompting further exploration into the factors shaping such differences.
The current investigation evaluates the antioxidant and antiproliferative potential of crude extracts derived from the fruits of A. andrachne. The degree of antioxidant capacity of extracts was assessed using the ferric-reducing antioxidant power assay (FRAP), one of the most prevalent techniques for assessing the antioxidant power of plant extracts. FRAP is a method based on converting a ferric-tripyridyl triazine complex (colorless) to a ferrous-tripyridyl triazine complex (blue colored) (25). The findings of this study imply that the methanolic fruit extract of A. andrachne exhibited the highest antioxidant potential, with the ethanolic extract showing moderate antioxidant activity. In contrast, the aqueous extract demonstrated the lowest antioxidant activity.
Research conducted in Syria aimed to determine the amount of phenolic content and antioxidant properties in different parts of A. andrachne, including flowers, leaves, bark, and fruits. The results revealed that the flowers contain the highest phenolic compounds (38.32 mg/g) and exhibit the most significant antioxidant activity (19.35 µM Fe²⁺/g) compared to the other parts analyzed. This indicates a clear correlation between phenolic content and antioxidant activity, with flowers showing the highest values in both measures (26).
Another study was conducted to determine the radical scavenging activity (RSA) and total phenolic content (TPC) of A. unedo fruits and leaves collected in Croatia. The total phenolic contents of leaves ranged from 67.07 to 104.74 mg gallic acid equivalents (GAE)/g dry weight (DW), while the TPC of fruits ranged from 16.78 to 25.86 mg GAE/g DW. The RSA of fruits and leaves ranged between 74.30 to 104.04 μmol Trolox equivalents (TE)/g DW and 408.92 to 430.98 μmol TE/g DW, respectively (27). These findings highlight the significant antioxidant potential and phenolic content in different parts of A. unedo. This trend is similar to that observed in A. andrachne in a previous study (26), where flowers demonstrated the highest phenolic content and antioxidant activity values.
Furthermore, the antioxidant activity of ethanol, dichloromethane, and n-hexane extracts from A. andrachne L. was investigated. The findings demonstrated that the ethanolic extract showed the highest level of DPPH activity (IC50 146.60 μg/mL), while the n-hexane extract exhibited the lowest activity (IC50 333.30 μg/mL) (28). The relevance of this study lies in its indication of the antioxidant activity of A. andrachne extracts, providing a basis for comparison with the present study's findings. It suggests that A. andrachne possesses antioxidant properties across various plant parts, which may vary in potency depending on the extraction method and solvent used. Consequently, while the specific plant parts investigated differ, the collective findings contribute to understanding the overall antioxidant potential of A. andrachne as a botanical resource.
The present study evaluated the antiproliferative activity of extracts from A. andrachne fruits using an MTT assay. However, the results showed that none of the tested extracts significantly affected the tested cancerous and non-cancerous cell lines.
Compared to the current investigation, the methanol extract of A. andrachne exhibited no significant cytotoxic effects on either Hep3B or HepG2 cells (human hepatocellular carcinoma) at any concentration or treatment time. Conversely, it significantly enhanced proliferation in Hep3B cells at a concentration of 100 µg/mL after 48 hours (14).
Another study investigated the interaction between the ethanol, dichloromethane, and n-hexane extracts from A. andrachne L. and DNA molecules. It found that increasing doses of A. andrachne L. extracts had a protective effect against hydroxyl radical-mediated plasmid DNA damage. The ethanol, dichloromethane, and n-hexane extracts exhibited similar protective effects on plasmid DNA (28).
Several studies have been conducted to evaluate the cytotoxic activity of A. unedo L., a plant species found in Morocco. One such study investigated the n-hexane extract obtained from the leaves of A. unedo and found that it exhibited a significant cytotoxic effect against L20B, RD, and Vero (poliovirus) cell lines (29). In contrast, another study analyzed the ethanolic extract from A. unedo leaves and found that at a concentration of 6.02 ± 0.76 mg/mL, there were no toxic effects on peritoneal macrophages (30).
Finally, given the potential for side effects associated with herbal treatments, it is crucial to provide thorough explanations and remind therapists to exercise caution. While herbal remedies can offer therapeutic benefits, they also carry risks of adverse reactions, medication interactions, and variability in potency and purity. Therefore, therapists should be aware of potential side effects, prioritize patient safety, provide clear guidance on identifying and managing adverse reactions, and stay informed about the latest research and evidence-based guidelines related to herbal medicine to ensure the highest standard of care for their patients.
5.1. Conclusions
The study explored the antioxidant and antiproliferative properties of A. andrachne extracts. The methanolic extract demonstrated the highest antioxidant activity, indicating its potential as a source of natural antioxidants for various industries. The extracts showed weak antiproliferative activity against cancerous cell lines and no significant effect on normal fibroblast cell lines. These findings suggest the potential of A. andrachne extracts as natural sources of antioxidants for the food and pharmaceutical industries.
Future research could investigate the mechanisms underlying the antioxidant properties of the extracts, identify and isolate specific bioactive compounds, explore formulations incorporating the extracts into various products, conduct in vivo studies, translate preclinical findings into clinical trials, and explore other applications such as cosmetics and nutraceuticals.