1. Background
The kidney plays a crucial role in maintaining the body's balance through various functions such as the removal of metabolic waste, regulation of fluid and electrolyte levels, maintaining acid-base equilibrium, and production of hormones. Many drugs, chemicals, and heavy metals can potentially interfere with these functions, causing disruptions to overall body metabolism. Typically, chemical substances can trigger immune responses and lead to cellular damage. Most cases of glomerular damage are attributed to circulating immune complexes, with most not originating in the glomerulus. Additionally, certain chemical substances can oxidize and damage the glomerulus. Aminoglycosides are among the most essential substances. Most chemical compounds that lead to kidney damage affect the proximal tubules. Proximal tubules are harmed by phospholipidosis, urine alpha-1-microglobulin (Uα1m), and low molecular weight proteins such as cadmium-metallothionein complexes (1-6).
Purslane, also known as Portulaca oleracea, is a leafy green vegetable popularly consumed in various parts of the world such as Europe, the Middle East, Asia, and Mexico. Its distinctive taste is characterized by its slightly tangy and salty flavor. The pharmacological effects of purslane, including diuretic (7), antioxidant (8), analgesic, anti-inflammatory (9), anti-bacterial (10), antifungal (11), antiplatelet (12), antifertility (13), wound healing (14), skeletal muscle-relaxant (15), and bronchodilator (16) effects have been reported. It has been used as a folk medicine for insomnia (17), hemorrhoids (18), appendicitis (19), dermatic diseases, urinary retention (20), gastritis (21), and diabetes (22).
Cadmium is connected to proteins in various tissues. Specific proteins that bind to heavy metals (metallothionein) have been found in organisms exposed to cadmium. Cadmium is carried in the bloodstream and distributed throughout the body but accumulates mainly in the liver and kidneys. Absorption of Cd is linked to metallothionein proteins (Cd-Mt) and then reabsorbed by the proximal tubules after passing through the glomeruli. Endocytosed Cd-Mt is released by proximal tubule cells through degrading vesicles, leading to a reduction in glutathione levels and the production of reactive oxygen species. This process also causes DNA damage, lipid peroxidation, protein cross-linking, and ultimately cell death induced by oxidants (23-25). Additionally, studies examining the tissue changes in the body have indicated that continuous exposure to cadmium can result in conditions such as anemia, osteoporosis, and bone fractures. Short-term exposure to cadmium can cause damage to the respiratory system, including pulmonary edema and irritations (26-28).
Recently, the kidney has been the focus of extensive research on the toxic effects of cadmium, an environmental pollutant. Many studies have investigated how cadmium enters renal epithelial cells and the mechanisms behind its toxicity in the kidney. Studies have shown that purslane can protect against nephrotoxicity, including a protective effect against cisplatin-induced nephrotoxicity in rats (29), and the nephroprotective effect of the aqueous extract of purslane and co-treatments of fish oil against gentamicin renal toxicity in rats (30). There is limited data available on the protective effects of purslane (P. oleracea) against toxicity induced by cadmium. We postulated that purslane would prevent Cd-induced nephrotoxicity based on the plant's constituents, its intrinsic and antioxidant components, possible therapeutic applications, and a number of recent similar publications.
2. Methods
2.1. Materials
The materials used in the study were cadmium sulfate from Merck in Germany, normal saline from Shahid Ghazi in Iran, alcohol from Asak in Iran, heparin from D. Braun Medical S.A. in Spain, and formalin 37% from Samad in Iran. In addition to the standard research equipment, a precise digital scale (model 2434, Sartorius, Germany) and a centrifuge (Beckman model, USA) were also used. Methanol solvent was obtained from Zagros Petrochemical Company, situated in the Special Economic Zone of South Pars Energy (Aslaviyeh).
2.2. Animals
The study involved male Wistar rats weighing between 200 and 250 grams that were bred and kept in the animal facility of the School of Pharmacy at Ahvaz Jundishapur University of Medical Sciences. The rats were kept in polycarbonate cages at a temperature of 25 ± 2ºC and a 12/12 hour light/dark cycle with access to food. Prior to the commencement of the study, the animals were adapted to the laboratory conditions for 10 days.
2.3. Plants
Purslane is a succulent plant that grows annually and belongs to the Portulacaceae family. Fresh purslane was collected from the campus of Jundishapur University of Ahvaz. It was identified by the Department of Pharmacognosy and was impounded for reference in the herbarium of this department, School of Pharmacy, Ahvaz, Iran, in summer (voucher No. 21156).
2.4. Preparation of Extract
After being cleaned and dried in a shaded area at room temperature, the stems and leaves of the plants were ground into powder. A 200-gram powder was soaked in a 70% v/v methanol solution for 48 hours at room temperature, followed by shaking it using a shaker for two hours. The mixture was then left to condense in a bath for three hours, filtered through Whatman No.1 filter paper, and concentrated in a rotary evaporator at 40°C. The concentrated extract was kept in the refrigerator and diluted with physiological saline when using (29, 30).
2.5. Experimental Procedure
At first, the maximum tolerable dose (MTD) of the purslane extract was determined in the rats, and the modified method was applied for running (29, 30). Animals were randomly divided into 8 groups (n = 6). In this research, the MTD was defined as the highest amount of purslane extract administered over a two-week period that was anticipated not to impact the longevity of the animals besides renal effects. The dosages were chosen to result in less than a 10% decrease in weight compared to the control group, without leading to fatalities, signs of harm, or pathological consequences aside from those potentially associated with renal responses.
Group 1 (negative group) received 1 mL physiological saline for 4 weeks. Group 2 (positive group) received 2 mg/kg cadmium sulfate intraperitoneally (IP) in 1 mL physiological saline solvent for 2 weeks. Groups 3, 4, and 5 were treated simultaneously daily for two weeks. Groups 6, 7, and 8 were given pretreatment for two weeks. Groups 3, 4, and 5 were administered purslane extract IP at 400, 600, and 800 mg/kg/day respectively, three hours before the daily cadmium (2 mg/kg/day) IP injection for two weeks. The pretreatment groups (6, 7, and 8) received the extract at doses of 400, 600, and 800 mg/kg IP daily for two weeks, followed by daily administration of cadmium sulfate (2 mg/kg, IP) after 24 hours of the last extract prescription for 2 weeks. Animals were stupefied by chloroform one day after the last injection, and blood samples (2 mL) were collected from their hearts; then, they were centrifuged at 3000 rpm for 15 minutes, and blood urea nitrogen (BUN) and serum creatinine (Scr) were analyzed for evaluating renal function (31, 32).
2.6. Histology Evaluation
After removing the tissues and draining the kidneys, they were immersed in a buffered formalin solution (10%) to be fixed and ready for additional examination for at least 24 hours. The tissues were then embedded, dehydrated in paraffin, and stained with hematoxylin and eosin for examination under a microscope (29, 33). This protocol on rats was approved by the ethics committee at Ahvaz Jundishapur University of Medical Sciences. In histological evaluations, the rate of desquamation and necrotic changes in cortical tubules have been shown by grades of mild (< 50% damage), moderate (50% - 75% damage), and severe (> 75% damage) (29).
2.7. Statistical Analysis
The statistical analysis was conducted using the SPSS software package for Windows (SPSS Inc., Chicago, IL, USA, 2001). The findings were expressed as mean ± standard deviation. In order to compare the means of the experimental groups within each group of animals, the one-way analysis of variance (ANOVA) was used. Additionally, the Tukey test (Tukey’s Post-hoc) was utilized to assess and identify variations between means. The statistical significance threshold was specified as P < 0.05.
3. Results
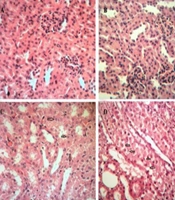
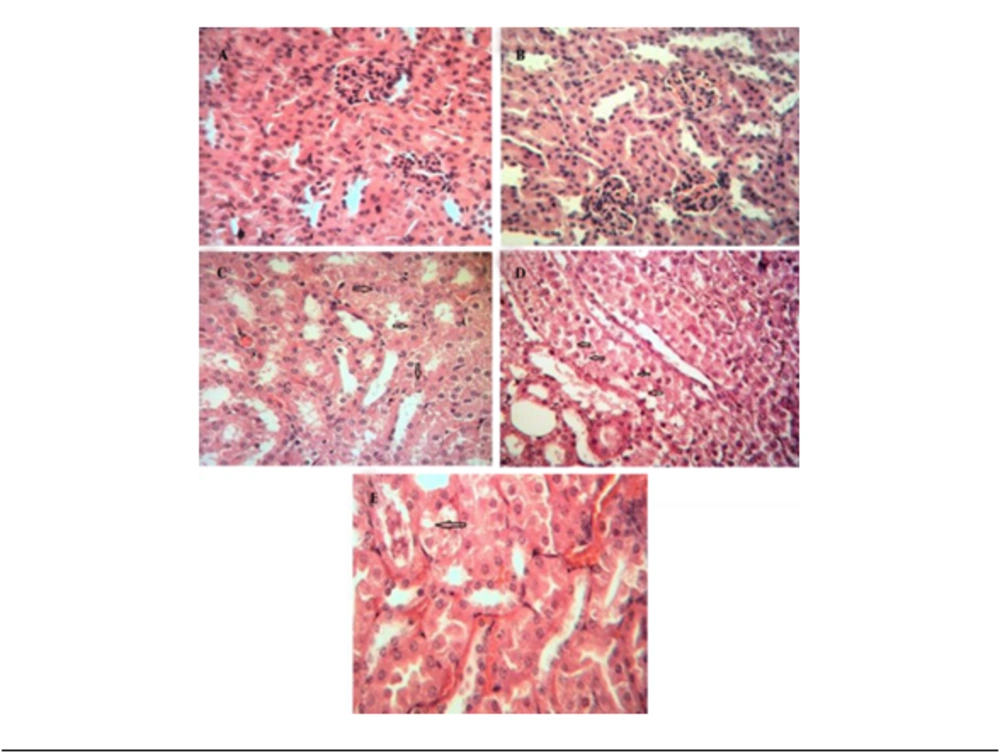
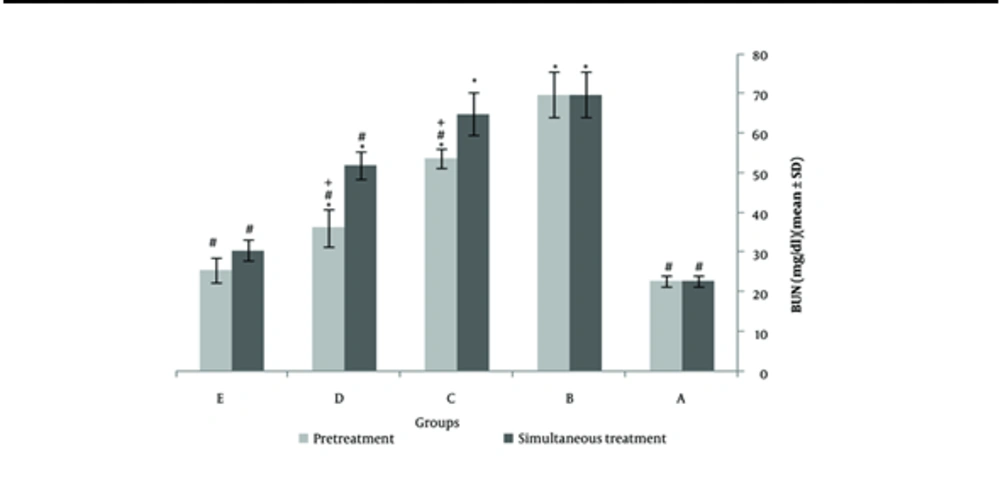
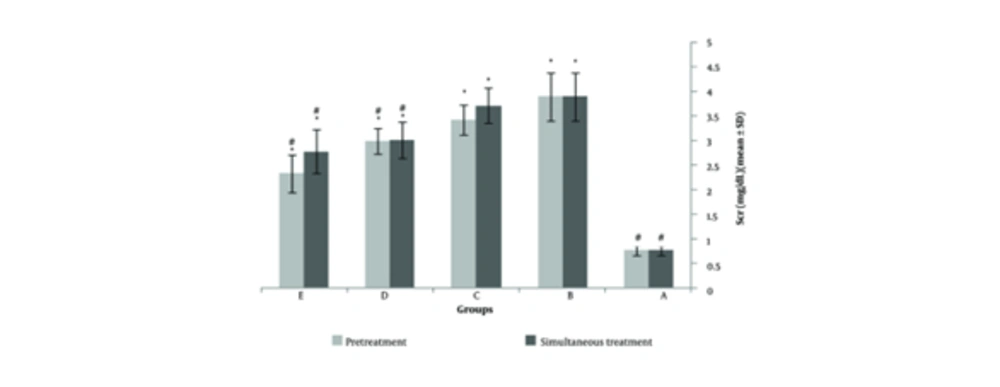
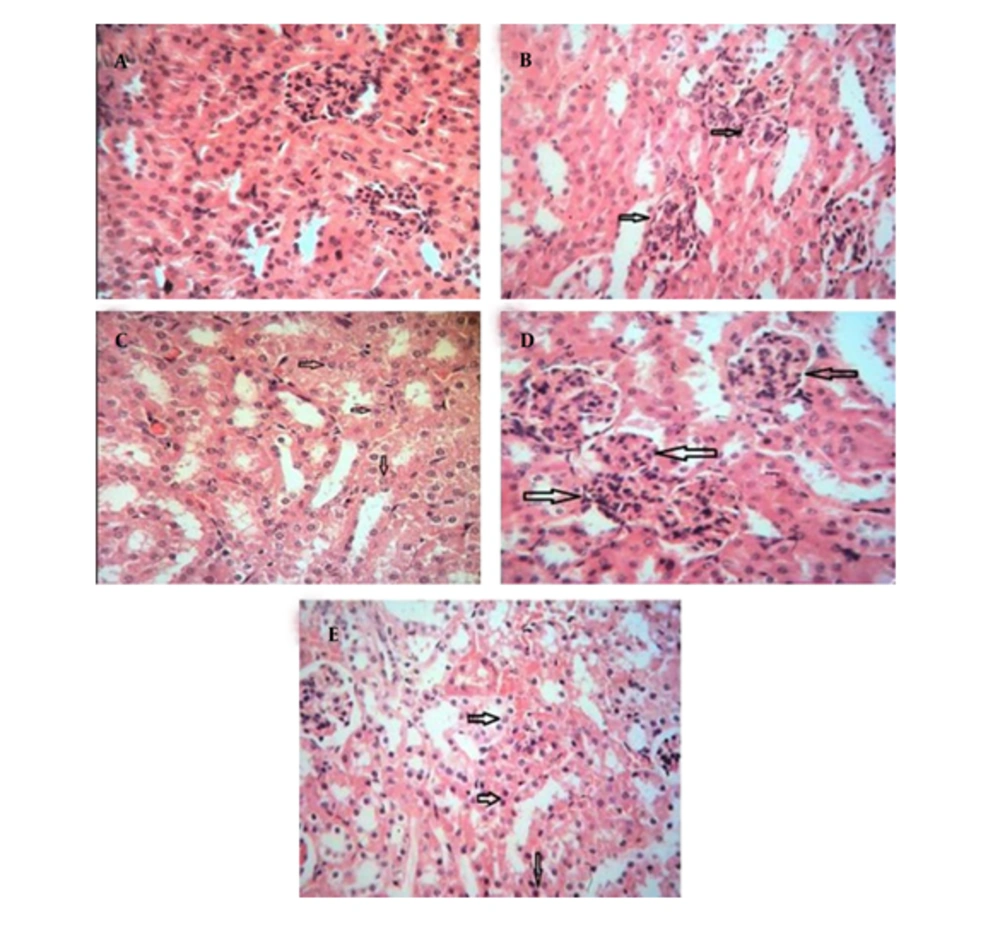
Two weeks of cadmium sulfate treatment caused damage to the proximal tubules in rats by increasing and subsequently depleting reactive oxygen species (ROS) and antioxidant agents. This led to a significant increase in BUN and Scr levels (P < 0.05) in these rats compared to the control group (Tables 1 and 2). Severe necrosis and swelling of epithelial cells in the proximal tubular area were observed after the administration of 25 mg/kg/ day of cadmium sulfate for14 days (Figure 1B). Following the administration of a mixture containing 400 mg/kg of pursulan extract, tubular damage was noted (Figure 1C). Minimal changes were noted in the structure of tubular cells in the simultaneous condition, as shown in Figure 1A, D, and E. Groups in Figure 1A, D, and E were given different treatments: Normal saline, 600 mg/kg purslane extract, and 800 mg/kg purslane extract simultaneously. The group given the highest dosage of purslane extract (800 mg/kg, group E) had the lowest BUN and Scr levels compared to the other groups, as indicated in the pretreatment and simultaneous administration of purslane extract (Figures 2 and 3; Tables 1 and 2).
| Groups | BUN (mg/dL) | Cr (mg/dL) | BUN/ Cr (mg/dL) |
|---|---|---|---|
| G1: Normal saline | 22.55 ± 1.30 a | 0.76 ± 0.10 a | 29.68 |
| G2: Cadmium (2 mg/kg) | 69.70 ± 5.80 | 3.90 ± 0.49 | 17.87 |
| G3: Extract 400 mg/kg + cadmium (2 mg/kg) | 64.87 ± 5.51 | 3.71 ± 0.36 | 17.49 |
| G4: Extract 600 mg/kg + cadmium (2 mg/kg) | 51.89 ± 3.62 a | 3.01 ± 0.36 a | 17.24 |
| G5: Extract 800 mg/kg + cadmium (2 mg/kg) | 30.44 ± 2.56 a | 2.78 ± 0.45 a | 10.97 |
Abbreviation: BUN, blood urea nitrogen.
a P < 0.05 significantly different compared with normal cadmium-treated group.
| Groups | BUN (mg/dL) | Cr (mg/dL) | BUN/ Cr(mg/dL) |
|---|---|---|---|
| G1: Normal saline | 22.55 ± 1.30 a | 0.76 ± 0.10 a | 29.68 |
| G2: Cadmium (2 mg/kg) | 69.70 ± 5.80 | 3.90 ± 0.49 | 17.87 |
| G6: Extract 400 mg/kg + cadmium (2 mg/kg) | 53.54 ± 2.51 a | 3.42 ± 0.31 | 15.65 |
| G7: Extract 600 mg/kg + cadmium (2 mg/kg) | 36.06 ± 4.60 a | 2.99 ± 0.26 | 12.06 |
| G8: Extract 800 mg/kg + cadmium (2 mg/kg) | 25.27 ± 3.12 a | 2.33 ± 0.38 a | 10.85 |
Abbreviation: BUN, blood urea nitrogen.
a P < 0.05 significantly different compared with normal cadmium-treated group.
Histological analysis under simultaneous conditions. A, healthy tubules; B, proximal tubules area showing severe necrosis and swelling of epithelial cells following administration of 2 mg/kg/day cadmium sulfate for 14 days; C, some degree of tubular damage noted after combined treatment with 400 mg/kg of purslane extract; D, mild to moderate tubular damage observed after simultaneous treatment with 600 mg/kg of purslane extract; E, minimal tubular damage seen after pre-treatment with 800 mg/kg of purslane extract. The arrows indicate the areas of damage and alterations.
The impact of purslane extract on blood urea nitrogen (BUN) levels in response to Cd-induced nephrotoxicity under both simultaneous and protective conditions. The best effect in BUN reduction was observed in the 800 mg/kg of extract injection duration pretreatment. A, normal saline receiver groups 1 mL for 4 weeks; B, cadmium sulfate receiver groups 2 mg/kg/day for 2 weeks; C, 400 mg/kg of purslane extract receiver groups; D, 600 mg/kg of purslane extract receiver groups; E, 800 mg/kg of purslane extract receiver groups (*Significantly different from the normal saline receiver group; #Significantly different from the cadmium receiver group; + significantly different from the simultaneous treatment; P < 0.05; N = 8; ANOVA, Tukey’s Post-hoc).
Illustrates the impact of purslane extract on serum creatinine (Scr) levels after nephrotoxicity induced by cadmium in both simultaneous and protective conditions. The best effect in Scr reduction was observed in 800 mg/kg of extract injection duration pretreatment. A, normal saline receiver groups 1 ml for 4 weeks; B, cadmium sulfate receiver groups 2 mg/kg/day for 2 weeks; C, 400 mg/kg of purslane extract receiver groups; D, 600 mg/kg of purslane extract receiver groups; E, 800 mg/kg of purslane extract receiver groups (* significantly different from the normal saline receiver group; # significantly different from the cadmium receiver group; P < 0.05; N = 8; ANOVA, Tukey’s Post-hoc).
According to Table 1, when administered simultaneously, the levels of BUN and Scr concentrations in the group receiving 400 mg/kg (G3) did not exhibit significant differences (P < 0.05) compared to the cadmium group (G2). In Table 2, it is demonstrated that there is a noteworthy difference in BUN concentrations among all groups treated with purslane extracts compared to the cadmium group during the pretreatment phase. Figure 4A shows healthy tubules. Necrosis and swelling of epithelial cells in the proximal area were observed after cadmium exposure (Figure 4B). The extent of damage to the tubules decreased after the administration of 400 mg/kg of the extract (Figure 4C).Only the group receiving 800 mg/kg purslane extract showed significant changes in Scr values. In instances of tubular necrosis and swelling of epithelial cells, histopathological examination revealed that the healing process was influenced by the dosage administered (Figure 4D, E). The levels of BUN and Scr decreased in animals given 400 and 600 mg/kg of the extract. This suggests that using purslane extract led to lower levels of BUN, Scr, and tubular necrosis in a dose-dependent manner. Additionally, rats pre-treated with the extract showed a more significant reduction in BUN and Scr compared to rats given the extract simultaneously (Figures 2 and 3; Tables 1 and 2).
Histological examination before treatment. A, healthy tubules; B, severe necrosis and swelling of epithelial cells in the proximal tubules after exposure to 2 mg/kg/day cadmium sulfate for 14 days; C, after exposure to a 400 mg/kg extract of purslane, some damage to the tubules was observed; D, some damage to tubules observed after treatment with 600 mg/kg extract of purslane; E, no damage to tubules noted after pretreatment with 800 mg/kg extract of purslane (notable healing). The arrows point to the areas of damage and changes. The healing observed is more significant when compared to simultaneous treatment conditions.
Table 1 shows the average BUN and creatinine levels in the groups that received cadmium and purslane extract simultaneously (mean ± SD) (n = 3). The diagnosis of acute kidney injury (AKI) was typically based on Scr and BUN levels. An increase in Scr of at least 0.3 mg/dL or 1.5 times the baseline value is considered indicative of AKI. A significant decrease was observed with the simultaneous administration of 800 mg/kg. The reduction with 400 and 600 mg/kg was only partial and minor. At 800 mg/kg, the levels were similar to those in the group that received normal saline.
Table 1 presents the BUN and Scr levels for the groups who were given cadmium and purslane extract at the same time. The findings indicate that group 1 had the lowest BUN and Scr, whereas group 2 had the highest. The levels of BUN and Scr were significantly different in these two groups. A significant difference in BUN concentration was noted when groups 4 and 5 were compared to group 2 (P < 0.05). The group administered 800 mg/kg of the extract did not show a statistically significant variance in BUN levels compared to the group that received normal saline, with a significance level of P > 0.05. Furthermore, there was a significant variance (P < 0.05) in the Scr levels between groups 4 and 5 when compared to group 2, the cadmium-treated group. The effects of purslane are influenced by the dosage, as indicated in the table, showing the strongest impact at 800 mg/kg.
Table 2 shows the average BUN and creatinine levels in the groups administered cadmium and purslane extract before treatment. A noticeable decrease was observed when the extract was administered at 800 mg/kg. The reduction in levels at 400 and 600 mg/kg concentrations was only partial and minimal. Comparing the data from both tables indicates that the reduction in BUN and Scr levels was significantly more efficient during pretreatment. The levels were closer to the group receiving normal saline when the extract was given 800 mg/kg before treatment.
The results of measuring BUN and Scr in the cadmium and purslane extract groups (pretreatment) are presented in Table 2. Group 1 showed the lowest levels of Scr and BUN, while group 2 had the highest levels. There was a noticeable difference in Scr and BUN levels between these two groups. A significant variation was seen when comparing the BUN levels of groups 6, 7, and 8 with group 2, which was treated with cadmium (P < 0.05). It was observed that the level of BUN in the group that received 800 mg/kg of the extract was not significantly different from the group that received normal saline (P > 0.05). The effects of the purslane extract depend on the dosage, as evidenced by the table, with the highest impact seen at 800 mg/kg of the extract. Analysis of creatinine levels in groups 8 and 2, the cadmium-treated group, showed a notable difference (P < 0.05).
Upon comparing Tables 1 and 2, it is clear that the extract has a more significant effect when given before treatment. A distinct variation in pre-treatment time (G6) is observed when using 400 mg/kg extract. This decrease was not observed with simultaneous administration. The impact values of the three extract concentrations indicate a more pronounced reduction when administered before treatment.
5. Discussion
Purslane is a yearling plant of the Portulaca genus from the Portulace family that has several names in different languages. This grassy plant has small and yellow flowers with black seeds that grow in mild weather. Purslane contains water (90% w/w), alkaloids (34), flavonoids, coumarins (35), α-linolenic acid, β-carotene (36), α-tocopherols, ascorbic acid, glutathione (37), and omega-3 fatty acids (38).
Researchers and regulatory agencies have been advocating for more accurate and specific early indicators of drug-induced toxicity to serve as biomarkers that are directly released into the blood or urine by the kidney, as creatinine and BUN are not sufficient in this aspect. The development of a kidney-specific biomarker could facilitate the early detection of drug-induced kidney toxicity, leading to the avoidance of nephrotoxic drugs and the prompt initiation of targeted treatments to repair the injured kidney. An ideal biomarker for AKI would be able to differentiate drug-induced kidney toxicity from other causes of AKI, allow for the early detection of drug-induced kidney toxicity before an increase in Scr and/or BUN occurs, and predict the long-term outcome of the kidney and mortality (29, 30). This article shows that plant extracts and herbal remedies could potentially reduce kidney damage from cadmium due to their antioxidant properties. The theory that oxidative damage plays a role in cadmium-induced kidney toxicity serves as the basis for explaining how cadmium harms the kidneys.
In the present study, cadmium was selected as a nephrotoxic agent to induce kidney damage, assessed by BUN, Scr, and histopathological examinations. Cd damages the proximal tubule (S1 and S2 segments) due to increased oxidative stress and the exhaustion of GSH stores; thus, antioxidant systems and rich sources of GSH can protect the kidney against these acute actions of Cd (39, 40). Oxidative stress and depletion of glutathione and other antioxidants are the mainsprings of Cd toxicity because they have a high affinity for sulfhydryl groups, leading to inactivation of antioxidant agents and infirmity in defense systems (41, 42).
The proximal tubule is identified by a tall brush border and a well-developed vacuolar lysosomal system. The morphological changes indicate cell damage, such as necrosis, desquamation, and swelling of tubular epithelial cells, in this specific part of the kidney (43).
Purslane is a good source of β-carotene, omega-3 fatty acids, α-tocopherols, essential amino acids, vitamins, ascorbic acid, and glutathione, as well as phenolics, coumarins, and alkaloids that are important chemicals. The antioxidant and/or anti-inflammatory effects of omega-3 fatty acids through scavenging of free radicals and inhibiting lipid peroxidation have been shown. These constituents of purslane have antioxidant activity (35, 44). Thus, as a study demonstrated this effect on rats with vitamin A deficiency (45), purslane extract can reduce oxidative stress. Since purslane increases the activity of enzymes like paraoxonase-1 and superoxide dismutase and also decreases the LDL value, it can inhibit lipid peroxidation and decrease oxidative damage (46). In addition to oxidative stress, other mechanisms related to energy, amino acid, choline, and nucleotide metabolism pathways may be involved, which should be considered. The primary biological damage caused by active oxygen metabolites is their interaction with unsaturated lipids, leading to peroxidation. This process alters membrane fluidity, making the membrane permeable to molecules, including enzymes.
Several studies in recent years have shown that various natural substances, including phytochemicals, plant extracts, herbal mixtures, and animal-derived substances, can offer protection against cadmium-induced kidney damage. The protective effects are carried out through different therapeutic pathways such as inhibiting oxidative stress, inflammation, apoptosis, fibrosis, and necroptosis. Furthermore, these natural products can also control autophagy, preserve cell polarity, and influence other cellular functions by targeting multiple signaling pathways and molecular receptors. The leading cause of kidney damage is inflammation and fibrosis. Therefore, the bioactive compounds, such as vegetables, containing omega-3 polyunsaturated fatty acids, phytosterols, and phenolic compounds, are effective against kidney failure. In addition, another mechanism of cadmium-induced nephrotoxicity is activating pro-inflammation factors, and purslane showed that it has anti-inflammatory effects and may reduce renal damage (3, 47, 48). Eleven compounds, including two new ketone alkaloids, were isolated from P. oleracea recently. When their anti-inflammatory properties were examined, it was discovered that they could considerably reduce the release of TNF-α and IL-1β (49). The combined use of P. oleracea and lifestyle modification (LM) for the treatment of non-alcoholic fatty liver disease (NAFLD) was partially supported by clinical evidence found in a recent meta-analysis (50).
Serum markers like Scr and BUN may not be adequate for accurately detecting kidney injury. These markers are not optimal because they mainly reflect changes in the glomerular filtration rate, which is a general measure of proximal tubular injury and only becomes apparent after significant kidney damage. They lack sensitivity and may lead to drugs passing preclinical safety checks only to be later found nephrotoxic in patients during clinical trials or post-market use. Additional non-invasive urinary biomarkers, such as kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL), could be essential in identifying kidney injury. Our research discovered a significant reduction in renal damage indicators like BUN and Scr when using ethanolic extract of purslane before or at the same time as treatment. The most impact was observed with a dose of 800 mg/kg of the extract. The study indicates that the protective benefits of the extract vary based on the dosage used in both treatment approaches. Additionally, our findings suggest that pretreatment proves more effective than simultaneous treatment, aligning with prior research on the effects of purslane extract on cisplatin-induced kidney damage. Data reveals that pretreatment yields superior results compared to simultaneous treatment, possibly due to the combined effect of the extract and its water solubility (29, 51).
Our findings validate the traditional Chinese medicine's theory that P. oleracea has a variable chemical composition that includes organic acids, terpenoids, alkaloids, flavonoids, and other classes of natural compounds. Numerous substances demonstrate potent anti-inflammatory and anti-cancer transforming properties within the gastrointestinal tract (52).
5.1. Conclusions
This study suggests that vegetable antioxidants such as vitamins A, C, and E, β-carotene, and glutathione, along with omega-3 fatty acids, may reduce cadmium-induced nephrotoxicity and could be a new product to safeguard individuals exposed to cadmium in industrial settings. The preventive properties of purslane are more potent when used before exposure, rather than simultaneously, and the effectiveness is based on the dosage. Due to the inevitable exposure to cadmium, further research using animal models is needed to fully understand how cadmium induces the production of ROS and to discover potential therapeutic targets.