1. Background
Functional dyspepsia (FD), a major category of dyspepsia, has no identifiable organic or metabolic causes (1). Functional dyspepsia is divided into two heterogeneous subgroups: (1) postprandial distress syndrome (PDS), characterized by meal-related symptoms such as postprandial fullness and early satiation, and (2) epigastric pain syndrome (EPS), characterized by epigastric pain or a burning sensation (2).
This highly prevalent disease impairs quality of life and requires substantial healthcare expenditure (3-5). Proton pump inhibitors (PPIs) are recommended as the first line of treatment for FD after the eradication of Helicobacter pylori (6). Proton pump inhibitors reduce inflammation and hypersensitivity, potentially making them more effective for treating EPS than PDS (2, 7). In PDS, although prokinetic agents may offer a pathophysiology-oriented treatment option, these medications are often associated with complications or adverse events (8). According to recent approaches to FD treatment, if patients do not respond to PPIs and prokinetics, psychotherapy and complementary and alternative medicine (CAM) therapies are recommended (6, 9, 10). Psychotherapy appears particularly beneficial for individuals with EPS or those experiencing pain (7). Although CAM therapies, including herbal medicines, have been integrated into new algorithms for dyspepsia management, their efficacy remains unclear due to the limited number of high-quality trials (6).
The World Health Organization (WHO) has repeatedly called for more rigorous scientific validation of traditional medicines (11). Numerous studies worldwide have introduced certain traditional herbal compounds that are effective in treating FD (12-14). Persian medicine, one of the oldest traditional medical systems, provides several recommendations for treating dyspepsia (15-17). Some herbs and compounds in Persian medicine have been researched and found to be effective and safe for treating FD (17-19). Persian medicine reference books highly recommend a well-known traditional herbal compound for the treatment of FD, particularly for PDS and bloating (20-22). This compound is also commonly used in Iranian folkloric medicine to treat dyspeptic symptoms; additionally, all four herbs in this compound are native to the region. We refer to this compound as Persian-FACT.
Persian-FACT contains the seeds of Pimpinella anisum L. (anise), Foeniculum vulgare Mill (fennel), Trachyspermum ammi L. Sprague (ajwain), and Cuminum cyminum L. (cumin). Anise has traditionally been used in both Persian and Chinese medicine, in simple or combined forms, to treat various conditions such as dyspepsia. Its effect on PDS has recently been documented (23). Fennel is a popular medicinal plant with various pharmacological activities, including antioxidant, anti-inflammatory, and gastroprotective effects (24, 25). Ajwain is traditionally used as a medicinal plant for the treatment of flatulence and atonic dyspepsia (26), and it has been proven effective in treating dyspeptic symptoms (27). Cumin seeds are traditionally used in treating FD, either alone or in combination, and the effect of cumin oil in combination with peppermint oil has been confirmed in FD treatment (28, 29).
Although the combination of these four herbal seeds is recommended for treating dyspepsia symptoms in Persian medicine textbooks (20-22) and its efficacy has been documented in reducing cardiometabolic risk factors in overweight and obese women (30), the efficacy of this compound in treating FD has not yet been investigated.
2. Objectives
This study was conducted to investigate the efficacy of this traditional herbal compound in the treatment of FD.
3. Methods
3.1. Ethical Statement
The study was approved by the Research Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.MED.REC.1400.570). The trial protocol was registered with the Iranian Registry of Clinical Trials on 13 April 2022 (IRCT20220304054179N1).
3.2. Trial Design
This study was an add-on, single-center, double-arm, parallel-group, double-blind, randomized controlled clinical trial with a 1:1 allocation ratio, conducted from May 2022 to December 2022. It took place at the Imam-Reza Clinic, affiliated with Shiraz University of Medical Sciences in Shiraz, Iran. No changes to the methods were made after the trial commenced.
3.3. Persian-FACT
3.3.1. Persian-FACT Ingredients
Persian-FACT contains the seeds of four plants, which are listed in Table 1. A comparison of these seed sizes is illustrated in Figure 1.
| Scientific Name | Persian Name/ Common Name | Voucher Number | Ratio to 7 |
|---|---|---|---|
| Foeniculum vulgare Mill. | Rāziāneh/ fennel | PM 1424 | 2 |
| Pimpinella anisum L. | Anisun/ Anise | PM 1425 | 2 |
| Cuminum cyminum L. | Zireh /Cumin | PM 1423 | 1 |
| Trachyspermum ammi (L.) Sprague | Zeniān /Ajwain | PM 1426 | 1 |
| Additive | Nabāt/Rock candy | - | 1 |
We adjusted the daily dose of the compound to 14 g, consisting of 4 g of anise, 4 g of fennel, 2 g of ajwain, and 2 g of cumin, which is equivalent to half of the amount recommended in Persian medicine books. Therefore, 7 ± 0.1 g of Persian-FACT was measured into each sachet to be consumed twice daily.
3.3.2. Preparation of Persian-FACT
To prepare the herbal compound, we purchased fresh plant seeds from the medicinal plant market in Shiraz, Southern Iran, in the spring of 2022. The seed samples were authenticated by the herbarium botanist at the Shiraz Faculty of Pharmacy, with the specific voucher sample numbers provided in Table 1.
The seeds were quickly washed with cold distilled water and then air-dried. To reduce contamination, the contents were exposed to UV light. The seeds and rock candy were crushed separately using a mechanical grinder, sieved with a 40-mesh screen, and mixed with a cubic mixer. The pharmaceutical form of the compound aligns with the guidelines in Persian traditional medicine books, which aim to maximize its effect on the stomach while minimizing systemic side effects by reducing absorption. This form, known as "safoof" in Persian medicine, indicates that the substance is crushed and coarse, rather than ground into a fine powder.
The placebo was prepared using rolled oats that were heated until they turned light brown. The oats were then crushed, sieved with a 40-mesh screen, and mixed with rock candy in a ratio of 1 to 6 rolled oats. To match the texture and properties of the Persian-FACT, we added it to the placebo in an amount equal to ten percent of the placebo's weight. Both the Persian-FACT and placebo were filled into sachets using an herbal medicine packaging machine. Each box for the patients contained 28 sachets.
3.3.3. Assessment of Microbial Contamination of Persian-FACT
To assess the microbial content, we suspended 10 g of the prepared Persian-FACT in 90 mL of buffered sodium chloride peptone broth. Then, 1 cc of the prepared suspension was mixed with 15 cc of liquefied casein-soybean digest agar at a temperature not exceeding 45°C in a petri dish and incubated at 30 - 35°C for 48 hours to count the probable aerobic bacteria. Additionally, Sabouraud dextrose medium was incubated at 20 - 25°C to count yeast and mold. According to WHO standards for internal use formulations, the acceptable limit is less than 103 CFU/g for total aerobic microorganisms, yeast, and mold. No aerobic microbes or fungal growth were observed in the prepared Persian-FACT, and the sample met WHO standards for being uncontaminated.
3.4. Participants
Adults aged 18 to 65 years with a confirmed diagnosis of FD, as referred by a gastroenterologist, were included in the study. We excluded patients with organic causes of dyspepsia, pregnant women, those planning to become pregnant during the study period, lactating mothers, patients with a history of chronic liver disease, renal disease, diabetes, or malignancies, those taking antithrombotic agents such as warfarin, Plavix, aspirin, and other anticoagulants, and individuals with a history of allergic reactions to anise, fennel, ajwain, cumin, or oat.
3.5. Intervention
The selected patients were invited to the clinic, where a physician explained the study method to them before they entered the study. After signing the informed consent form, all participants completed a questionnaire regarding their demographic characteristics and symptom scores.
Each patient received one package containing sachets of either Persian-FACT or a placebo for each treatment cycle. Patients were instructed to take the contents of their sachets orally twice a day, once while fasting in the morning and again at night before bedtime, for two weeks. They were allowed to drink water with the contents of the sachet to aid swallowing, with a maximum of one glass per dose.
A follow-up phone call was made one week after the first visit to assess symptom severity using the questionnaire. Patients were reminded to take the compound regularly and report any potential adverse events. Two weeks after the first visit, the patients returned for a follow-up visit, and their outcomes were evaluated using the questionnaire. All patients were advised to maintain their previous diet and physical activity levels throughout the study.
3.6. Outcomes
3.6.1. Questionnaire
Symptoms were assessed using the Gastrointestinal Symptom Rating Scale (GSRS) and the Rome IV questionnaire. The reliability and validity of the GSRS are well-documented, and in this study, we used the Persian versions of both the GSRS and the Rome IV questionnaire (31, 32). These questionnaires evaluate the severity of dyspepsia symptoms, including PDS and EPS, as well as bloating, gastroesophageal reflux disease (GERD), and constipation. All questions are scored on a seven-point Likert scale.
3.6.2. Primary Outcome Measure
The primary outcome measure was the mean severity score of dyspepsia, PDS, and EPS at baseline, and at the first and second weeks after the intervention. We evaluated the difference in mean severity scores between the two groups, Persian-FACT and placebo, across the follow-up times.
3.6.3. Secondary Outcome Measure
The secondary outcome measure was the mean severity score of bloating, GERD, and constipation at baseline, and at the first and second weeks after the intervention. Any unexpected symptoms and complaints from the participants were recorded to investigate potential adverse events. Systolic and diastolic blood pressure were measured and recorded by the physician at the beginning of the trial and two weeks after the intervention to assess possible hypertension. Blood parameters, including the levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, blood urea nitrogen (BUN), and creatinine (Cr), were measured using the photometric method with standard kits at the beginning of the trial and four weeks after the intervention to assess possible renal or hepatic complications.
3.7. Sample Size
We initially planned to collect data from 150 dyspeptic patients. However, for ethical reasons, we decided to conduct an interim analysis. Upon analysis, we observed a significance level of 0.05 with a power of 0.80 and a large effect size (effect size = 0.68) in the dyspepsia symptom score when 55 patients completed two weeks of observation. Since this met our desired confidence level, we decided to stop data collection.
3.8. Randomization
Block randomization was performed, taking into account variables such as age, sex, and dyspepsia syndrome (PDS vs. EPS). The random sequence was generated using Random Allocation Software. The statistician provided the block randomization table to the pharmacist at the drug packaging center, who then labeled the packages with a code. The pharmacist had no role in the study. To enhance blinding, a three-digit code was used to conceal patient allocation. After visiting the physician and completing the questionnaire, patients received a package with a code determined by the block randomization table, based on their age, gender, and dyspepsia syndrome.
3.9. Blinding
The participants and the questionnaire were blinded to the allocation. Only the study statistician and the pharmacist had access to the preset randomization assignments. Decoding was carried out during the data analysis phase.
3.10. Statistical Analysis
The Kolmogorov-Smirnov test was used to assess the normality assumption of the data. Demographic information and baseline values were compared between the study groups using chi-Square, Fisher’s Exact, and Mann-Whitney U tests. The distribution showed abnormalities according to the Kolmogorov-Smirnov test (P-value < 0.001). Therefore, a non-parametric analysis using the Generalized Estimating Equations (GEE) model for repeated measures was conducted. This analysis included the interaction of group and time to examine the effect of treatments on the outcome measures throughout the study follow-up. The models were adjusted for potential confounding variables such as age, gender, marital status, job, and education. All analyses were performed using IBM SPSS v.24, and a P-value < 0.05 was considered statistically significant.
4. Results
4.1. Participants’ Flow
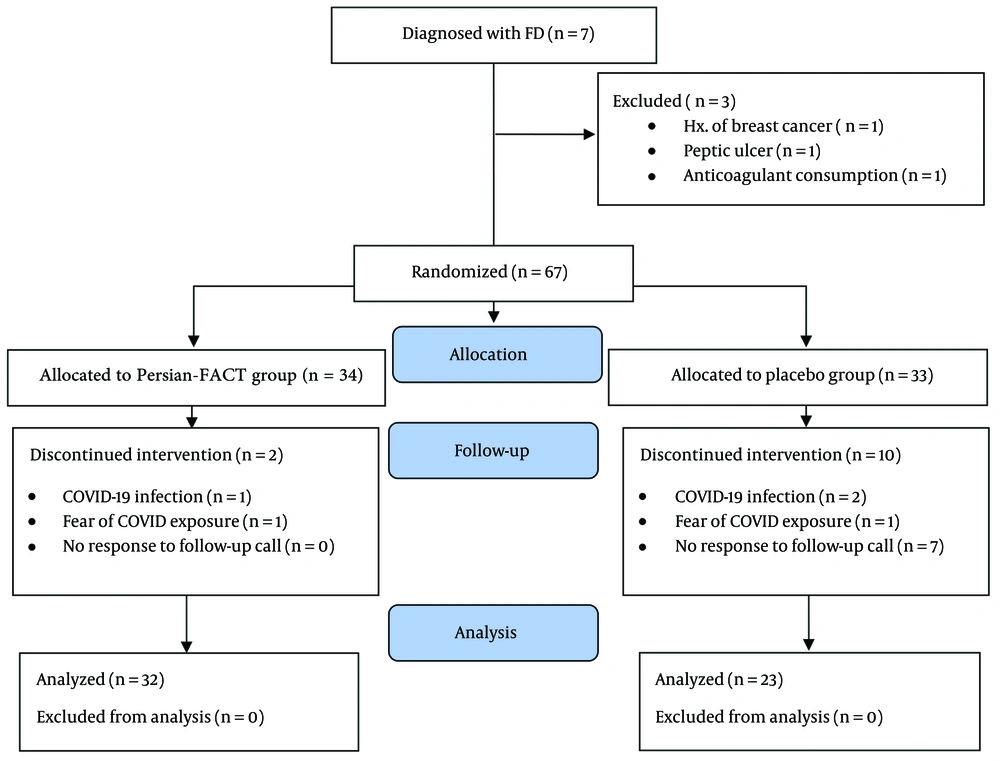
Of the 70 FD patients assessed for eligibility, 67 were randomly assigned to receive either Persian-FACT (n = 34) or a placebo (n = 33). Among these, 32 patients in the Persian-FACT group and 23 in the placebo group completed the baseline, 1-week, and 2-week visits. The CONSORT flow diagram depicting the assessment, enrollment, and dropout of the patients is shown in Figure 2.
4.2. Baseline Characteristics
No significant difference was observed between the Persian-FACT and placebo groups in baseline symptom severity scores, as shown in Table 2. This was an add-on study. In the Persian-FACT group, 4 patients (17.4%) and in the placebo group, 4 patients (22.2%) used pantoprazole alongside Persian-FACT or placebo during the intervention, and this difference was not significant (P = 0.713).
| Characteristics | Persian-FACT | Placebo | P-Value |
|---|---|---|---|
| Age (y) | 49.59 ± 11.99 | 43.13 ± 12.38 | 0.064 b |
| Duration of dyspepsia (y) | 6.77 ± 3.62 | 6.43 ± 3.69 | 0.781 b |
| Education level | 0.660 c | ||
| Academic | 13 (40.6) | 8 (34.8) | |
| Non academic | 19 (59.4) | 15 (65.2) | |
| Gender | 0.073 c | ||
| Woman | 21 (65.6) | 20 (87.0) | |
| Man | 11 (34.4) | 3 (13.0) | |
| Marital status | > 0.999 d | ||
| Single | 6 (20.0) | 5 (21.7) | |
| Married | 24 (80.0) | 18 (78.3) | |
| Living area | 0.697 c | ||
| Large city | 20 (64.5) | 16 (69.6) | |
| Small city | 11 (35.5) | 7 (30.4) | |
| Job | 0.537 c | ||
| No | 14 (43.8) | 12 (52.2) | |
| Yes | 18 (56.3) | 11 (47.8) | |
| Consumption of dyspepsia drugs through intervention | 0.713 c | ||
| No | 19 (82.6) | 14 (77.8) | |
| Yes | 4 (17.4) | 4 (22.2) | |
| Familial history | 0.879 c | ||
| No | 12 (41.4) | 10 (43.5) | |
| Yes | 17 (58.6) | 13 (56.5) | |
| Baseline dyspepsia symptom rating scale | 13.84 ± 6.89 | 13.91 ± 5.83 | 0.857 b |
| Baseline PDS symptom rating scale | 7.47 ± 4.30 | 5.57 ± 4.28 | 0.104 b |
| Baseline EPS symptom rating scale | 6.38 ± 4.45 | 8.35 ± 3.16 | 0.082 b |
| Baseline postprandial fullness rating scale | 4.56 ± 2.24 | 3.78 ± 2.58 | 0.302 b |
| Baseline bloating symptom rating scale | 19.63 ± 9.04 | 15.96 ± 7.84 | 0.092 b |
| Baseline GERD symptom rating scale | 3.56 ± 2.77 | 4.48 ± 2.31 | 0.241 b |
| Baseline constipation symptom rating scale | 13.22 ± 7.01 | 10.83 ± 7.30 | 0.221 b |
Abbreviations: PDS, postprandial distress syndrome; EPS, epigastric pain syndrome; GERD, gastroesophageal reflux disease.
a Values are expressed as No (%) or mean ± SD.
b Mann-Whitney.
c Chi-Square.
d Fisher’s exact.
4.3. Outcomes
The difference in the mean severity scores between the Persian-FACT and placebo groups is presented in Table 3.
| Sub (Scale); Group | 1 Week (95% CI) | P-Value | 2 Weeks (95% CI) | P-Value |
|---|---|---|---|---|
| Postprandial fullness (1Q) | 0.097 | 0.031 | ||
| Persian-FACT | 1.69 (0.94 - 2.45) | 1.60 (0.87 - 2.31) | ||
| Placebo | 2.69 (1.55 - 3.81) | 2.82 (1.76 - 3.89) | ||
| PDS(2Q) | 0.172 | 0.028 | ||
| Persian-FACT | 3.03 (1.62 - 4.44) | 2.76 (1.54 - 3.98) | ||
| Placebo | 4.31 (2.31 - 6.31) | 4.63 (2.68 - 6.58) | ||
| EPS(2Q) | 0.001 | 0.001 | ||
| Persian-FACT | 2.92 (1.34 - 4.51) | 2.71 (1.14 - 4.27) | ||
| Placebo | 5.67 (3.83 - 7.50) | 5.45 (3.65 - 7.20) | ||
| Dyspepsia(4Q) | 0.019 | 0.019 | ||
| Persian-FACT | 6.25 (3.74 - 8.75) | 6.01 (3.64 - 8.38) | ||
| Placebo | 9.61 (6.53 - 12.69) | 9.37 (6.2 - 12.45) | ||
| Bloating(4Q) | 0.031 | 0.072 | ||
| Persian-FACT | 5.31 (3.06 - 7.57) | 5.48 (3.42 - 7.55) | ||
| Placebo | 8.68 (5.70 - 11.65) | 8.18 (5.18 - 11.18) | ||
| GERD(1Q) | 0.036 | 0.036 | ||
| Persian-FACT | 1.20 (0.47 - 1.93) | 1.18 (0.41 - 1.94) | ||
| Placebo | 2.43 (1.29 - 3.58) | 2.41 (1.27 - 3.56) | ||
| Constipation(3Q) | 0.049 | 0.152 | ||
| Persian-FACT | 4.74 (2.88 - 6.60) | 4.77 (2.75 - 6.80) | ||
| Placebo | 7.98 (4.95 - 11.01) | 7.43 (4.24 - 10.63) |
Abbreviations: Q, question; EPS, epigastric pain syndrome; GERD, gastroesophageal reflux disease; CI, confidence intervals.
4.3.1. Primary Outcome
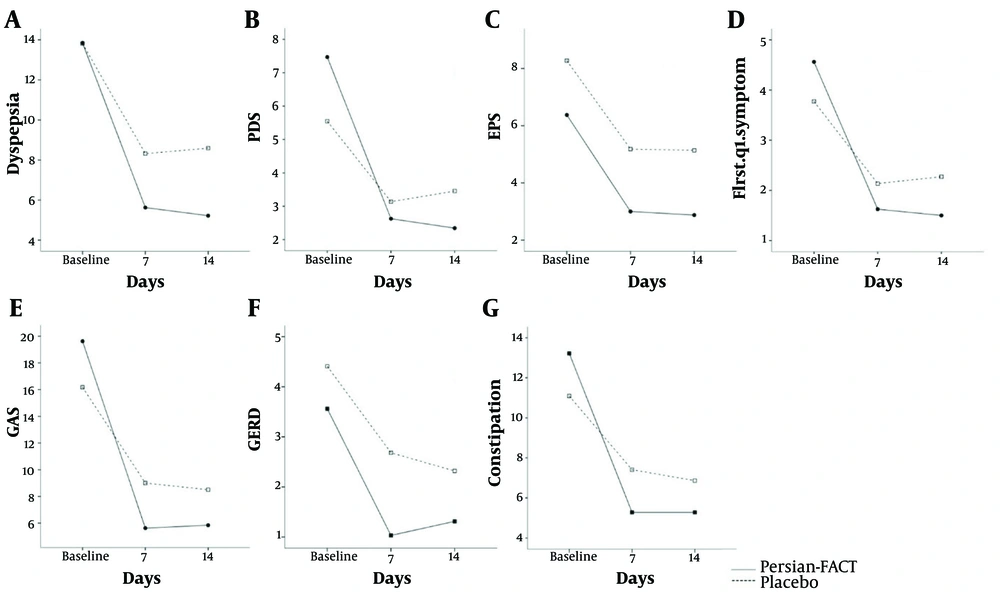
According to the GEE analysis results, the severity of dyspepsia symptoms decreased in both the Persian-FACT and placebo groups. However, this reduction was significantly greater in the Persian-FACT group during the first and second weeks of the intervention (mean difference = 3.36, 95% CI = 0.55 to 6.17, P-value = 0.019, Figure 3A).
In the dyspepsia subgroups, the Persian-FACT group had significantly lower PDS symptom severity in the second week of intervention compared to the placebo group (mean difference = 1.87, 95% CI = 0.20 to 3.55, P-value = 0.028, Figure 3B). However, this reduction was not significant during the first week of intervention (P-value = 0.172). The severity of EPS symptoms was significantly reduced in the Persian-FACT group compared to the placebo group from the first week (mean difference = 2.74, 95% CI = 1.09 to 4.40, P-value = 0.001, Figure 3C).
The Persian-FACT group also had significantly lower postprandial fullness two weeks after the intervention compared to the placebo group (mean difference = 1.23, 95% CI = 0.11 to 2.35, P-value = 0.031, Figure 3D), but this reduction was not significant during the first week of the intervention (P-value = 0.097).
4.3.2. Secondary Outcome
One week after the intervention, the Persian-FACT group showed a significant reduction in bloating (mean difference = 3.367, 95% CI = 0.313 to 6.421, P-value = 0.031, Figure 3E) and constipation symptoms (mean difference = 3.238, 95% CI = 0.030 to 6.507, P-value = 0.049, Figure 3G) compared to the placebo group. However, this difference was not significant in the second follow-up for bloating (P-value = 0.072) and constipation (P-value = 0.152). Gastroesophageal reflux disease symptom severity in the Persian-FACT group was significantly lower than in the placebo group (mean difference = 1.238, 95% CI = 0.084 to 2.392, P-value = 0.036, Figure 3F) in both the first and second weeks of the intervention.
4.4. Adverse Event Report
Three adverse events occurred in both the Persian-FACT and placebo groups, all of which resolved spontaneously within a few hours. The percentage of these events in the Persian-FACT and placebo groups, respectively, were as follows: Dry mouth (9% vs. 4%), headache (3% vs. 4%), and transient abdominal pain (6% vs. 4%). There were no significant changes in systolic blood pressure (P-value = 0.383) or diastolic blood pressure (P-value = 0.266). Similarly, serum levels of liver enzymes, including AST (P-value = 0.862), ALT (P-value = 0.505), and alkaline phosphatase (P-value = 0.926), as well as renal function tests, including BUN (P-value = 0.685) and Cr (P-value = 0.383), did not show significant changes in the participants.
5. Discussion
To the best of our knowledge, this is the first attempt to generate RCT data for Persian-FACT in the treatment of FD. The most significant finding of the present study was the improvement in symptoms of epigastric pain and burning sensation, postprandial fullness, and early satiation after consuming Persian-FACT (7 g twice daily). Other common gastrointestinal symptoms, including constipation, GERD, and bloating, were also significantly reduced with Persian-FACT (7 g twice daily). This study paid special attention to the subgroups of dyspepsia and coexisting conditions, as well as their recovery times.
Although the ratio of the seeds in the compound follows the recommendations of Persian medicine reference books (20-22), the daily dosage was adjusted based on the permissible doses of the seeds in recent herbal pharmacological references. According to the PDR for Herbal Medicines, the recommended dose for anise is 3 g daily, with a dessertspoon (10 g) for gastrointestinal complaints (33). Another herbal medicines reference allows for 1 to 5 g of anise seeds daily (34). The recommended dose for fennel by the PDR is 5 to 7 g daily (33), while the permissible amount of ajwain seeds is 3 - 6 g daily (35). The PDR recommends a daily dose of 1 to 5 g of cumin (33). Therefore, the daily dose of the compound was calculated to be 14 g (4 g anise, 4 g fennel, 2 g ajwain, and 2 g cumin), which is equivalent to half the amount recommended in Persian medicine books.
The duration of treatment is not precisely mentioned in traditional medicine books, so we conducted weekly follow-ups to assess the improvement process. These follow-ups indicated that the treatment duration should be tailored according to the subtype of dyspepsia. Symptoms of EPS, GERD, constipation, and bloating decreased earlier (within one week), while more time was needed to treat PDS (at least two weeks). This study demonstrated that taking Persian-FACT for less than two weeks was sufficient for treating constipation and flatulence, but for dyspepsia with postprandial distress and early satiety, at least two weeks of consumption was necessary.
The therapeutic effects of all four herbs in Persian-FACT have been well documented for FD. Ghoshegir et al. studied anise and confirmed its efficacy in FD. In their clinical trial, anise was compared with a placebo, with the anise group consuming 3 g of powder after each meal (9 g/day) for 4 weeks, which was more than the dosage used in our study (4 g/day) and for a longer duration (23). Ethnobotanical data on fennel collected by Jadid et al. showed the efficacy of this plant in treating dyspepsia in India, China, Turkey, and Northern Morocco (36). Recent review articles, including studies by Mehra et al., have emphasized the protective effects of fennel on gastrointestinal function (37). Birdane et al. demonstrated the gastroprotective effect of fennel in a clinical trial involving rats (25). A review article by Bandi et al. mentioned that fennel prevents spasms and flatulence and that chewing a few fennel seeds reduces symptoms of acidity. Fennel also has anti-ulcer properties and can relieve constipation (38). Valussi described fennel as a functional food effective in treating dyspepsia, bloating, flatulence, and poor appetite (39). In a clinical trial conducted by Shafiezadeh et al., ajwain significantly decreased the severity of FD (27). The therapeutic effects of ajwain on reflux, abdominal cramps, and pain have been demonstrated in a review article by Ullah et al. in 2024 (40). Ahmadi-Jouybari et al. showed that a 100 cc decoction of cumin alongside conventional therapy increased H-pylori eradication (41). Mahboubi reported the actions of cumin in improving digestive system function and eliminating accumulated gases (28). May et al. confirmed the efficacy of caraway combined with peppermint in treating dyspepsia (29). Krupavaram et al. explained that in Ayurvedic medicine, cumin seeds are widely used to treat various gastrointestinal illnesses, including indigestion, chronic diarrhea, dyspepsia, and acute gastritis (42).
Medicinal plants in combination can act synergistically, affecting multiple targets simultaneously, which is crucial in treating multifactorial diseases such as functional gastrointestinal disorders (12). The combination of anise, fennel, cumin, and ajwain has been introduced in some studies for treating dyspepsia. Larijani et al. reviewed treatments for flatulence from the perspective of traditional Persian medicine and introduced herbs and herbal compounds effective in relieving gastrointestinal discomfort. They highlighted compounds abundantly mentioned in traditional Persian medicine reference books for treating dyspepsia and suggested that clinical studies be conducted to confirm their efficacy (43). Aghabeiglooei et al. used this combination, with slightly different dosages, under the name "Komouni Formulation" to reduce cardiometabolic risk factors in overweight and obese women. They emphasized the possible effects of this compound in reinforcing digestion and the gastrointestinal system and improving poor digestion (30).
The Asian Pacific Association of Gastroenterology working group recommends herbal treatment, especially for PDS and dyspepsia with bloating (44), due to the potential complications or adverse events (such as QT prolongation and cardiac arrhythmia) associated with prokinetics used in PDS. Therefore, Persian-FACT, which is prescribed in Persian medicine particularly for PDS symptoms and dyspepsia with bloating, is a good choice (20-22, 43).
In the treatment of FD patients, the integration of traditional and modern medicine allows for leveraging the strengths of both, leading to more scientific and accurate treatments (45). Consequently, it may be more logical to use traditional medicine in cases where standard treatment fails. Considering that PPIs are somewhat effective in treating hypersensitivity and pain in EPS, and according to recommendations from Persian medicine books, it would be better to prescribe this compound for cases of PDS, particularly for patients with postprandial fullness and bloating.
5.1. Limitations
The present study has certain limitations that warrant discussion. One potential limitation is that the study was conducted at a single center. Additionally, the study was limited by the COVID-19 pandemic, which resulted in the loss of follow-up for 5 participants. In contrast, a review of 49 research studies by Heiran et al. found an average sample size of 142 (18). Therefore, despite achieving significant results with our current sample size, we propose conducting further studies with a larger sample size under stable conditions.
Diet directly affects gastric function and dyspepsia outcomes, so lifestyle differences among participants are an important limitation of our study. All patients were instructed not to modify their diet and physical activity during the study period. However, it would be beneficial to monitor the recurrence of symptoms after discontinuing the compound with more weekly follow-ups. This could help us assess the exact duration of the treatment. Therefore, studies with longer follow-up periods are suggested for future research.
Notably, a slight reduction in symptoms was observed in the placebo group. This could be attributed to the significant presence of volatile constituents in the components of Persian-FACT, which were added to the placebo at a rate of ten percent. This finding underscores the potential influence of such constituents on the study's outcome, in addition to the placebo effect (46). Therefore, it is suggested that future research compare this compound with standard medications such as prokinetics in PDS patients.
Persian-FACT likely contains phenolic and flavonoid components, based on the seeds used in the compound (40, 47-49). The possible presence of these components could help explain the mechanism of action of Persian-FACT in treating FD. A phytochemical assessment of this compound is recommended in future studies.
It is important to note that these seeds may contain substances that mimic estrogenic actions. Therefore, this compound is not recommended during pregnancy or for individuals with estrogen-related cancers.
5.2. Conclusions
Persian-FACT is effective in improving dyspepsia symptoms. For the management of dyspeptic patients suffering from PDS, this herbal compound should be used for at least two weeks.