1. Background
In recent years, antimicrobial resistance has emerged as a significant global health challenge, necessitating the search for novel and effective antibacterial agents. Pulp necrosis, the death of dental pulp tissue, can result from various causes, including bacterial infections, traumatic injuries, and exposure to chemical irritants (1). Notably, root canal infections and pulp pathologies often involve a polymicrobial consortium, consisting of multiple bacterial species (2). Facultative anaerobic bacteria are frequently present in pulp and root canal infections (3). Necrotic root canals exhibit a distinct microbial profile, with facultative anaerobes making up 57.14% and aerobes comprising 42.86% of the bacterial population (4).
Among oral pathogens, Streptococcus mutans, a gram-positive, facultatively anaerobic bacterium, is a primary and extensively studied etiological agent associated with dental caries and other oral infections (5). This species is a key member of the oral microbiome, particularly abundant in dental plaque biofilms, and is responsible for initiating the caries process through its acidogenic and aciduric properties. Streptococcus mutans accounts for a significant proportion of cariogenic bacteria in the oral cavity and has been implicated in various oral and systemic conditions, including dental caries, endocarditis, and bacteremia (6, 7). Its ability to produce extracellular polysaccharides, tolerate acidic environments, and form robust biofilms makes it a formidable contributor to oral disease, particularly in the presence of dietary sugars. Streptococcus mutans, as a key etiological agent in dental caries, poses a considerable public health concern due to its biofilm-forming capacity and resistance to conventional antimicrobial therapies. Consequently, there is a growing interest in exploring alternative treatment strategies that can combat bacterial infections while reducing the risk of resistance development (8).
Natural plant-derived compounds have garnered substantial attention for their potential antimicrobial properties and low likelihood of inducing resistance (9). Mentha (mint) plants are known to contain a diverse array of bioactive constituents, including polyphenols, flavonoids, and essential oils, which have demonstrated antibacterial activity against various pathogens. Additionally, alum (aluminum potassium sulfate) has been historically employed for its astringent and antimicrobial properties, though its potential in combination with botanical extracts remains largely unexplored (10).
The present study aims to investigate the synergistic antimicrobial effect of a hydroalcoholic extract of Mentha in conjunction with alum against Streptococcus mutans. The choice of Streptococcus mutans as the target pathogen is based on its clinical significance in oral health and the need for novel approaches to control its growth effectively. To comprehensively evaluate the antimicrobial properties of this combination, a variety of well-established antimicrobial susceptibility testing methods will be employed, including the well diffusion assay, minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and the checkerboard titration technique. These methods will allow for a thorough assessment of the antimicrobial efficacy of the individual components and their combined effects, potentially providing valuable insights into their synergistic interactions.
In this context, we present the results of the inhibition zone diameter of the hydroalcoholic Mentha extract and alum, as well as their respective MIC and MBC values against Streptococcus mutans. The combination of the hydroalcoholic Mentha extract and alum holds promise as an innovative and effective antibacterial approach, and the findings of this study may pave the way for the development of alternative, nature-based therapeutics to combat bacterial infections.
2. Objectives
Ultimately, this research seeks to expand the knowledge base on natural antimicrobial agents and explore the potential of this synergistic combination as an adjunctive therapy in dentistry or other fields. This effort aligns with the global fight against antimicrobial resistance, promoting public health by offering sustainable alternatives to conventional antibiotics.
3. Methods
3.1. Plant Material and Extract Preparation
Fresh Mentha plants were collected from a local herbal garden, and the leaves were thoroughly washed to remove any contaminants. The plant material was then dried in the shade and ground into a fine powder. A hydroalcoholic extract was prepared by macerating 50 grams of Mentha powder in 500 mL of 70% ethanol for 72 hours, with intermittent shaking. The extract was filtered, and the solvent was evaporated under reduced pressure at a controlled temperature of 40°C to obtain a dry residue. The extract was stored in an airtight container at 4°C, protected from light and air, until further analysis (11).
3.2. McFarland Standard Preparation
A 0.5 McFarland standard solution is prepared by adding 0.5 mL of 1.2% (w/v) barium chloride dihydrate solution to 99.5 mL of 1% sulfuric acid. This solution, stored in a dark environment for up to six months to minimize photodegradation, serves as a reference for determining bacterial suspension concentrations. The turbidity of the McFarland standard solution corresponds to an approximate bacterial concentration of 1.5 × 108 colony-forming units per milliliter (CFU/mL). To prepare a bacterial suspension with an equivalent concentration, fresh and pure bacterial colonies are inoculated into a tube containing sterile phosphate-buffered saline (PBS).
By visually comparing the turbidity of the resulting suspension to that of the 0.5 McFarland standard solution, a suspension containing approximately 1.5 × 108 CFU/mL of bacteria can be achieved (12).
3.3. Bacterial Strain and Culture Conditions
Streptococcus mutans (PTCC 16836) was obtained from the Persian Type Culture Collection (PTCC) and maintained on LB (Luria-Bertani) agar slants at 4°C. Prior to each experiment, a fresh subculture of the bacterium was prepared by inoculating a loopful of the strain into LB broth and incubating it overnight at 37°C (13). The standard Streptococcus mutans (PTCC 16836), obtained in lyophilized form from the Iran Scientific and Industrial Research Organization, was revived by culturing it on LB agar medium for 24 hours at 37°C under microaerophilic conditions. Following incubation, the bacterial suspension was adjusted to a 0.5 McFarland standard and diluted 1:100 in LB broth to achieve a final concentration of approximately 1.5 × 106 CFU/mL. One hundred microliters of this diluted bacterial suspension were added to each well containing the serially diluted test compounds, including positive and negative controls.
3.4. Determination of Inhibition Zone Diameter
The agar well diffusion method was employed to evaluate the in vitro antimicrobial activity of the hydroalcoholic Mentha extract and alum (potassium aluminum sulfate) against Streptococcus mutans. This technique assessed the ability of these agents to inhibit bacterial growth. LB agar plates were inoculated with an overnight culture of Streptococcus mutans using a sterile cotton swab to achieve a uniform lawn of growth. Wells were then created in the agar using a sterile cork borer (6 mm in diameter).
Next, 100 μL of various concentrations of the Mentha extract and alum solutions were added to separate wells. The plates were incubated at 37°C for 24 hours, after which the inhibition zone diameters were measured in millimeters (mm) (14). All experiments were performed in triplicate to ensure reproducibility, and the results were expressed as mean ± standard deviation. Statistical analysis was conducted using one-way ANOVA, followed by Tukey's post-hoc test, to determine significant differences between treatments (P < 0.05).
3.5. Determination of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
The MIC and MBC values of the hydroalcoholic Mentha extract and alum were determined using the microdilution method in 96-well microtiter plates. Two-fold serial dilutions of each substance were prepared in LB broth to achieve a concentration range covering a suitable spectrum.
The bacterial inoculum was standardized to a 0.5 McFarland standard and subsequently diluted in LB broth to achieve a final concentration of approximately 1 × 106 CFU/mL. Aliquots of the bacterial suspension were added to each well containing the test substances, along with positive and negative controls. After incubation at 37°C for 24 hours, the MIC was determined as the lowest concentration of the test substance that completely suppressed visible bacterial growth, assessed by either optical density (OD) measurements or direct visual observation. For the MBC determination, aliquots from the wells showing no visible growth were subcultured onto LB agar plates and incubated at 37°C for an additional 24 hours. The minimum bactericidal concentration was identified as the lowest concentration of the test substance that resulted in no visible bacterial growth on the agar plates after subculturing (15).
3.6. Checkerboard Titration
The Checkerboard Titration method was employed to evaluate the combined antimicrobial effect of the hydroalcoholic Mentha extract and alum against Streptococcus mutans. The same microdilution technique used for MIC determination was applied, but this time, the two substances were combined in various ratios and concentrations. The fractional inhibitory concentration index (FICI) was calculated to determine whether the interactions between the two agents were synergistic, additive, or antagonistic (16).
3.7. Statistical Analysis
All experiments were performed in triplicate, and the data are presented as mean ± standard deviation (SD). Statistical analysis was conducted using appropriate parametric tests, such as one-way analysis of variance (ANOVA) for normally distributed data involving multiple groups, or Student's t-test for normally distributed data with two groups, to assess significant differences between the groups. A P-value threshold of 0.05 was used to determine statistical significance.
4. Results
4.1. Inhibition Zone Assay
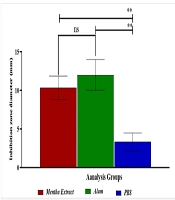
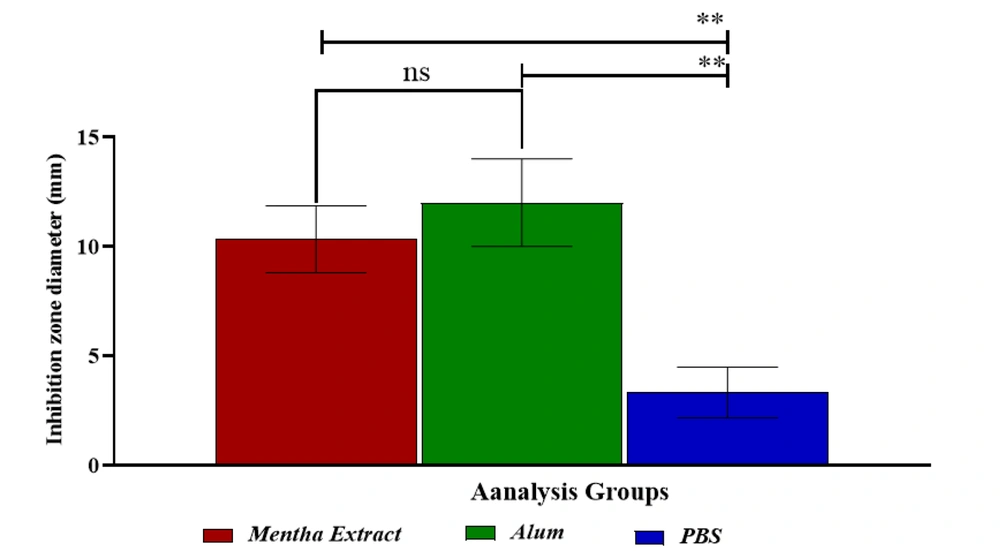
The aim of the present study was to assess the antimicrobial effects of hydroalcoholic extracts of Mentha and alum against standard strains of Streptococcus mutans (PTCC 16836) under laboratory conditions. The well diffusion method was used to measure the diameter of the growth inhibition zone for Mentha extract at a concentration of 80 mg/mL and alum at a concentration of 10 mg/mL against Streptococcus mutans. The results, presented in Figure 1 and Table 1, show the values of the growth inhibition zone in millimeters.
| Substance | Inhibition Zone Diameter (mm) | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|---|
| Mentha extract | 10.33 ± 1.53 | 8.20 ± 5.57 | 10.000 ± 0.000 |
| Alum | 12.04 ± 2.02 | 0.35 ± 0.20 | 0.625 ± 0.006 |
| PBS | 3.33 ± 1.15 | - | - |
Inhibition Zone Diameter, MIC and MBC Values the Mentha extract and Alum Against Streptococcusmutans a
The hydroalcoholic extract of Mentha exhibited the greatest in vitro inhibitory effect against Streptococcus mutans at a concentration of 50 mg/mL, with a zone of growth inhibition measuring 10.33 ± 1.53 mm. Notably, alum exhibited a larger zone of inhibition (12.04 ± 2.02 mm). These findings indicate that both the hydroalcoholic Mentha extract and alum demonstrate antimicrobial activity against Streptococcus mutans. The diameter of the inhibition zone represents the area of bacterial growth inhibition around the test substance, with a larger zone indicating stronger antimicrobial activity.
4.2. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration Assay
To further assess the antimicrobial activity of the hydroalcoholic Mentha extract and alum against Streptococcus mutans, the MIC and MBC were determined. As shown in Table 1, these values were obtained through serial dilutions of the extract and alum, starting at concentrations of 80 mg/mL and 10 mg/mL, respectively
The results revealed that the MIC and MBC values of the extract against Streptococcus mutans were approximately 8.21 ± 5.57 mg/mL and 10.05 ± 0.06 mg/mL, respectively. In comparison, alum exhibited lower MIC and MBC values of 0.35 ± 0.20 mg/mL and 0.63 ± 0.02 mg/mL.
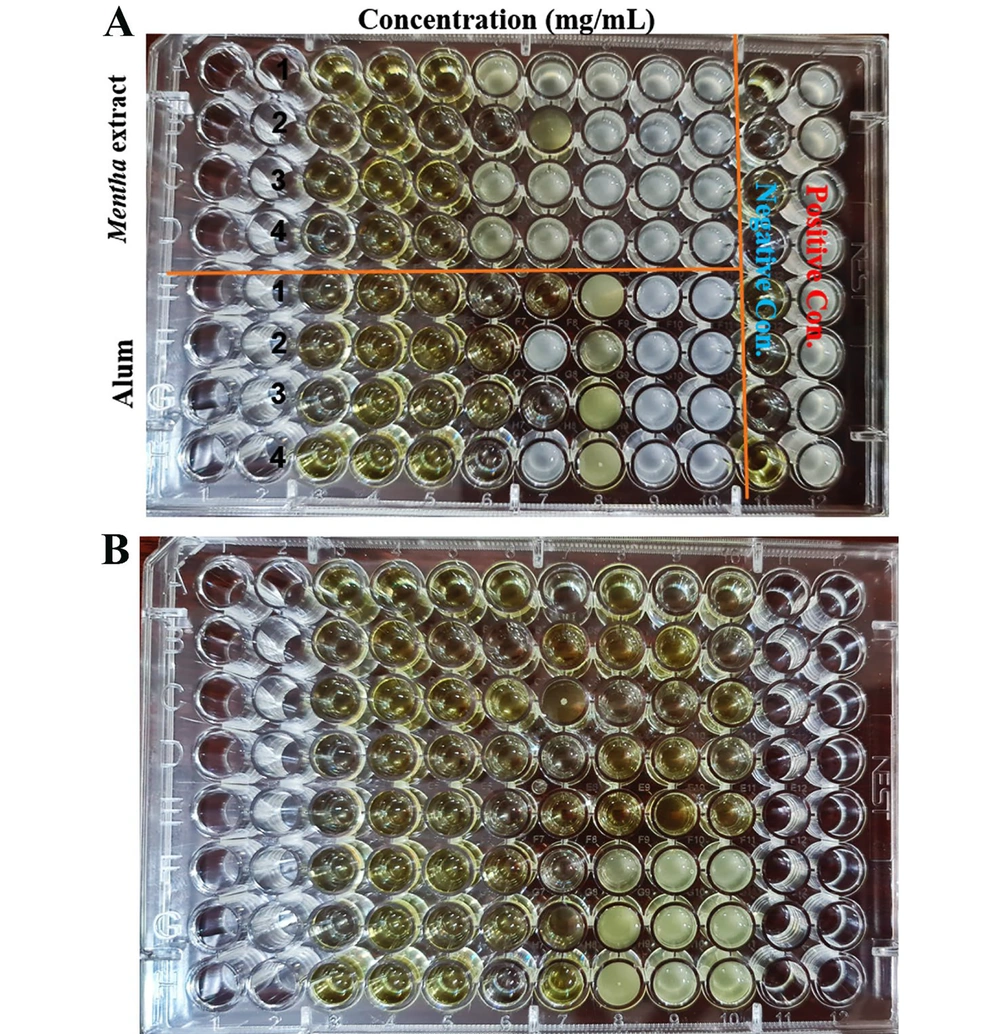
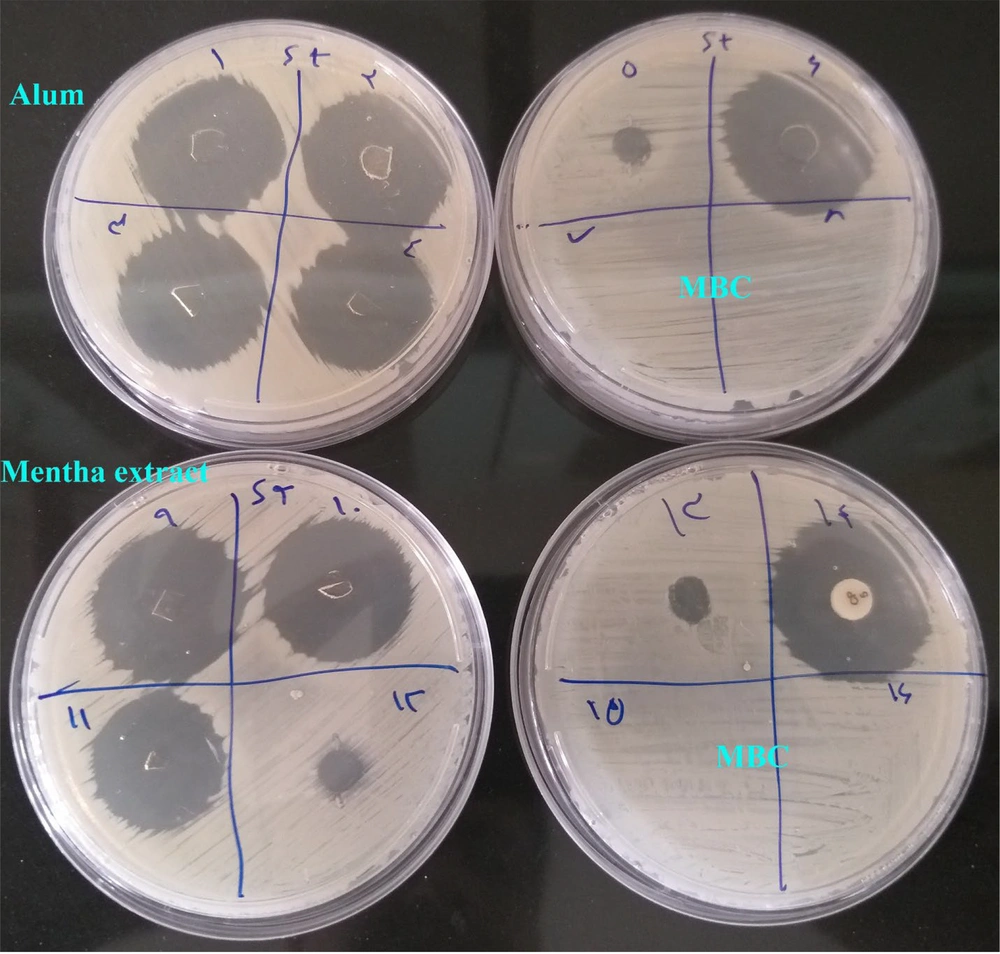
A 96-well microplate was used for the MIC assay (Figure 2A), where the absence of visible bacterial growth in specific wells indicated the inhibitory effect of the extract and alum at those corresponding concentrations. For MBC determination, LB agar plates were utilized (Figure 3).
The absence of bacterial growth on these plates following subculturing confirmed the bactericidal effect of the test substances at specific concentrations. The specific MIC and MBC values for the Mentha extract and alum are presented in Table 1.
4.3. Synergistic Assay via Checkerboard Titration Test
To investigate the synergistic effects of Mentha extract and alum, the Checkerboard Titration method was utilized. First, concentrations of 10, 5, 2.500, 1.250, 0.625, 0.312, 0.156, and 0.087 mg/mL were prepared from a stock solution of 20 mg/mL alum. To assess the synergism between the two compounds, 100 µL of LB Broth was added to all wells in a 96-well microplate with 64 wells (8 × 8). Then, 100 µL of a 160 mg/mL stock of peppermint extract was added to the first vertical column, resulting in a concentration of 80 mg/mL Mentha extract in the first vertical row. Serial dilution was performed by transferring 100 µL from each well to the next until 100 µL was transferred from the last well outside the plate.
Next, 100 µL of a 10 mg/mL alum stock was added to the first horizontal row (A). A 5 mg/mL concentration was added to the second horizontal row (B). For rows C through H, concentrations of alum were added as follows: 100 µL of 2.500 mg/mL in row C, 1.250 mg/mL in row D, 0.625 mg/mL in row E, 0.312 mg/mL in row F, 0.156 mg/mL in row G, and 0.087 mg/mL in row H. The final volume in each well was 200 µL (Figure 2B).
After preparing the dilutions, 20 µL of a 1.5 × 106 CFU/mL suspension of Streptococcusmutans was inoculated into all the wells. Table 2 shows the concentrations of the compounds used in the synergism test. The results of the Checkerboard Titration test were analyzed using the fractional inhibitory concentration (FIC) formula. According to the FIC analysis, if the value is less than 0.5, the two compounds exhibit a synergistic effect. If the FIC is greater than 4, the compounds have an antagonistic effect. Fractional inhibitory concentration values between 0.5 and 4 indicate an additive or increasing effect. Based on the results of the FIC Index calculation, most of the concentrations demonstrated an increasing effect of Mentha extract and alum.
| Samples | Concentrations of Mentha Extract and Alum (mg. mL-1) | |||||||
|---|---|---|---|---|---|---|---|---|
| A | ||||||||
| Mentha extract (mg. mL-1) | 80 | 40 | 20 | 10 | 5 | 2.500 | 1.250 | 0.625 |
| Alum (mg. mL-1) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| B | ||||||||
| Mentha extract (mg. mL-1) | 80 | 40 | 20 | 10 | 5 | 2.500 | 1.250 | 0.625 |
| Alum (mg. mL-1) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| C | ||||||||
| Mentha extract (mg. mL-1) | 80 | 40 | 20 | 10 | 5 | 2.500 | 1.250 | 0.625 |
| Alum (mg. mL-1) | 2.500 | 2.500 | 2.500 | 2.500 | 2.500 | 2.500 | 2.500 | 2.500 |
| D | ||||||||
| Mentha extract (mg. mL-1) | 80 | 40 | 20 | 10 | 5 | 2.500 | 1.250 | 0.625 |
| Alum (mg. mL-1) | 1.250 | 1.250 | 1.250 | 1.250 | 1.250 | 1.250 | 1.250 | 1.250 |
| E | ||||||||
| Mentha extract (mg. mL-1) | 80 | 40 | 20 | 10 | 5 | 2.500 | 1.250 | 0.625 |
| Alum (mg. mL-1) | 0.625 | 0.625 | 0.625 | 0.625 | 0.625 | 0.625 | 0.625 | 0.625 |
| F | ||||||||
| Mentha extract (mg. mL-1) | 80 | 40 | 20 | 10 | 5 | 2.500 | 1.250 | 0.625 |
| Alum (mg. mL-1) | 0.312 | 0.312 | 0.312 | 0.312 | 0.312 | 0.312 | 0.312 | 0.312 |
| G | ||||||||
| Mentha extract (mg. mL-1) | 80 | 40 | 20 | 10 | 5 | 2.500 | 1.250 | 0.625 |
| Alum (mg. mL-1) | 0.156 | 0.156 | 0.156 | 0.156 | 0.156 | 0.156 | 0.156 | 0.156 |
| H | ||||||||
| Mentha extract (mg. mL-1) | 80 | 40 | 20 | 10 | 5 | 2.500 | 1.250 | 0.625 |
| Alum (mg. mL-1) | 0.078 | 0.078 | 0.078 | 0.078 | 0.078 | 0.078 | 0.078 | 0.078 |
Checkerboard Titration Assay of mutans Mentha extract and Alum Against Streptococcusmutans
5. Discussion
This study's key finding is the demonstration of a synergistic antibacterial effect between the hydroalcoholic Mentha extract and alum against Streptococcus mutans. This suggests that the combination may be more effective than either agent alone. The combination of these two substances showed enhanced antimicrobial activity compared to their individual effects, indicating a potential novel approach for combating Streptococcusmutans infections.
The inhibition zone diameter results revealed that alum exhibited a larger zone of inhibition (12.04 ± 2.01 mm) compared to the hydroalcoholic Mentha extract (10.33 ± 1.53 mm). However, when examining the MIC and MBC values, the hydroalcoholic Mentha extract showed comparable potency (MIC: 8.20 ± 5.57 mg/mL, MBC: 10 mg/mL) to alum (MIC: 0.35 ± 0.20 mg/mL, MBC: 0.63 ± 0.02 mg/mL). The differences in the inhibition zone diameter and MIC/MBC values suggest that the mode of action and antimicrobial mechanisms of these substances might differ.
Our study aligns with previous research reporting the antimicrobial activity of Mentha extract and alum against various bacterial strains. However, there is limited literature on the specific combination of hydroalcoholic Mentha extract with alum against Streptococcus mutans. This study provides novel evidence in the field, highlighting the potential of combining these natural substances to combat this specific pathogen.
The observed synergistic effect of the hydroalcoholic Mentha extract with alum on Streptococcus mutans growth inhibition may be attributed to their distinct mechanisms of action. Mentha extract contains various bioactive compounds, including polyphenols and flavonoids, which have been reported to disrupt bacterial cell membranes, inhibit vital enzymes, and interfere with cellular processes. Meanwhile, alum's antimicrobial activity is attributed to its ability to induce protein denaturation and disrupt the bacterial cell wall. The combination of these agents likely targets multiple bacterial pathways, leading to enhanced bacterial growth inhibition.
It is plausible that the hydroalcoholic Mentha extract and alum act through complementary pathways, enhancing bacterial growth inhibition. The combination of multiple bioactive compounds in Mentha extract and alum may result in greater disruption of bacterial cell structures, metabolic pathways, or vital enzymes, ultimately contributing to the observed synergistic effect.
The synergistic combination of hydroalcoholic Mentha extract with alum holds promise for potential applications in dentistry, particularly in the prevention and treatment of dental caries caused by Streptococcus mutans. Dental caries, a prevalent oral health issue, is primarily associated with Streptococcus mutans biofilm formation and acid production. The demonstrated antimicrobial efficacy of this combination against Streptococcusmutans suggests its possible utility as a natural adjunctive therapy or as a component of oral care products aimed at reducing bacterial load and promoting oral health.
The increasing prevalence of antimicrobial resistance has underscored the need for alternative antimicrobial agents. While conventional antibiotics have been used extensively for bacterial infections, their overuse and misuse have contributed to the development of resistant strains. The combination of hydroalcoholic Mentha extract with alum offers a potentially safer and more sustainable alternative due to the lower risk of resistance development associated with natural plant-derived compounds. Moreover, the synergistic effect may allow for lower effective concentrations of both substances, reducing the likelihood of adverse effects and promoting safer treatment options.
While this study demonstrated promising results, several limitations need to be considered. The in vitro nature of the study may not fully capture the complexity of interactions in a biological system. Therefore, future research should include in vivo studies and, if feasible, clinical trials to evaluate the safety and efficacy of this combination in living organisms. Additionally, assessing the potential cytotoxicity of the combination on human cells and its effects on other commensal oral bacteria is essential to determine its selectivity and safety for oral applications.
This study lays a foundation for future research on natural antimicrobial agents. Further studies should focus on identifying and characterizing the active compounds in the hydroalcoholic Mentha extract and their interactions with alum. Exploring the potential of this combination against other pathogenic bacteria and multidrug-resistant strains could further validate its broad-spectrum antimicrobial potential. Additionally, investigating different delivery methods or formulations to optimize the antimicrobial effects and improve the stability and shelf life of this combination would be beneficial.
The use of natural plant-derived compounds like Mentha extract and alum may positively impact the environment, as their production generally involves fewer synthetic chemicals and reduces the ecological footprint compared to conventional pharmaceuticals. Moreover, the potential cost-effectiveness of these natural substances may provide affordable alternatives for antimicrobial therapy, particularly in resource-limited settings.
5.1. Conclusions
The present study demonstrated a synergistic antibacterial effect of hydroalcoholic Mentha extract combined with alum against Streptococcusmutans. While alum exhibited a larger inhibition zone diameter, the hydroalcoholic Mentha extract showed comparable MIC and MBC values. This finding holds promise for the development of natural and effective antimicrobial agents to combat bacterial infections. The synergistic combination may have potential applications in dentistry and beyond, offering a safer and more sustainable approach to addressing antimicrobial resistance. However, further investigations are necessary to elucidate the precise mechanisms of action, explore clinical applications, and ensure the safety and efficacy of this combination for future therapeutic use. Overall, this research provides valuable insights into the field of antimicrobial research and may pave the way for developing alternative and effective treatment strategies.