1. Background
Sesame oil (SO) is one of the most widely used vegetable oils, and it is very beneficial to health due to its unsaturated fatty acids, antioxidant compounds, and vitamins, reducing cholesterol levels. This is why it is used in the food and pharmaceutical industries (1).
In recent years, Nanotechnology-based techniques such as nanoemulsion have not only provided the ability to produce functional foods but have also succeeded in protecting food nutrients from harmful factors during the production process and storage (2). The use of Nanoemulsions increases bioavailability, maintains lipophilic compounds in a continuous aqueous phase, provides a controlled release of hydrophobic components, and improves the flavor, taste, and texture of the product. They have high kinetic stability and are more resistant to gravitational separation and accumulation of particles. They can be used in the encapsulation of lipophilic compounds such as essential fatty acids, fat-soluble vitamins, flavors, preservatives, and drugs (3).
Encapsulation is a very useful technique in protecting the bioactive substances and functional food compounds such as probiotics, flavors, and vitamins. This technique is used to improve the shelf life and solubility of active compounds. In some cases, it can be used to mask undesirable taste, odor, and color (4).
spray drying in the food and pharmaceutical industries is a method for producing a dry powder from a liquid or slurry by rapid drying with hot gas (5). It is the most commonly used method for drying nanoemulsions due to its flexibility, low cost, efficiency, ease of scale-up, and high-quality powder production (6). The success of encapsulation via spray drying is contingent upon the high retention of core substances, especially volatiles, and the reduction of oil presence on powder particles during the production process and storage (7). Sometimes, nanoemulsions are coated with stabilizing materials such as chitosan (8) and maltodextrin (6) to increase the efficiency of spray drying.
2. Objectives
We used spray drying to prepare dry powder from coated maltodextrin/acacia gum-nanoemulsions containing SO. Due to the high content of antioxidants and unsaturated fatty acids in the SO, the product of this study can be used in the pharmaceutical industry and enrichment of food, especially dairy products.
3. Methods
Acacia gum was purchased from a local market. Lecithin and sodium dihydrogen phosphate were purchased from Merck (Germany). Whey protein concentrate (WPC, protein 10.85%, ash 7.8%, and moisture 4.4%) was obtained from Ramak (Shiraz, Iran). Maltodextrin (with a high degree of dextrose, 16 - 20) was procured from Zarfructose (Karaj, Iran), and polyglycerol polyricinoleate (PGPR) was provided by Domino Food Industry (Tehran, Iran). Commercial SO was purchased from the market and analyzed for active ingredients using an Agilent 7890 gas chromatograph connected to an Agilent 5975 mass spectrometer (GC-MS).
3.1. Nanoemulsion Preparation
The oil-in-water nanoemulsion was prepared by mixing aqueous and oil phases in the presence of a surfactant (9). To prepare the aqueous phase, 0.5 g of acacia gum was dissolved in 50 mL of 5 mM phosphate buffer with pH 7 (6) at 50°C. In another container, 1 g of WPC was dissolved in 50 mL of 5 mM phosphate buffer. The WPC solution was added to the acacia gum container and mixed with a mechanical stirrer until uniform. To prepare the oil phase, 0.2 g lecithin, 4 g SO, and 1 g PGPR were mixed and homogenized with a mechanical stirrer at 50°C. The oil phase was then added drop by drop to the aqueous phase with strong stirring on a magnetic stirrer. The resulting mixture was homogenized at 15,000 rpm and passed through a high-pressure homogenizer (HPH, EF-C3/HE, AVESTIN, Canada) three times at 10,000 psi (6). For comparison, the blank nanoemulsion (B-NE) was prepared by the above method, with the difference that the SO was removed from the oil phase.
3.2. Sesame Oil-Nanoemulsion Coating
To prepare the coating solution for encapsulation of SO-nanoemulsion, 0.5 g acacia gum and 15 g maltodextrin were mixed in 100 mL of 5 mM phosphate buffer (pH 7) at 70°C for 30 min (10). While homogenizing, the encapsulation solution was added to the nanoemulsion container and passed through the HPH at 10,000 psi three times, then sent for spray drying (6). For comparison, a nanoemulsion with sesame oil (FSO) and a nanoemulsion without sesame oil (FBlank) were encapsulated.
3.3. Spray Drying Process
A Spray Dryer (DSD-02, Dorsa, Iran) was used to convert the encapsulated nanoemulsion into powder. An inlet temperature of 140 ± 0.1°C, an outlet temperature of less than 85°C, a feed rate of 29%, a flow rate of air dried at 45%, and a pressure of 4 bar were applied. The prepared powders were stored at room temperature prior to analysis.
3.4. Evaluation of Physicochemical Properties
3.4.1. Particle Size
The mean diameter and size distribution of FBlank and FSO were measured using a particle size Analyzer (Scatteroscope 1 Qudix model, Korea) based on the dynamic light scattering technique. The samples were dispersed in distilled water before measurement. Each sample was measured in triplicate and the average size was reported (11).
3.4.2. Field Emission Scanning Electron Microscope Imaging
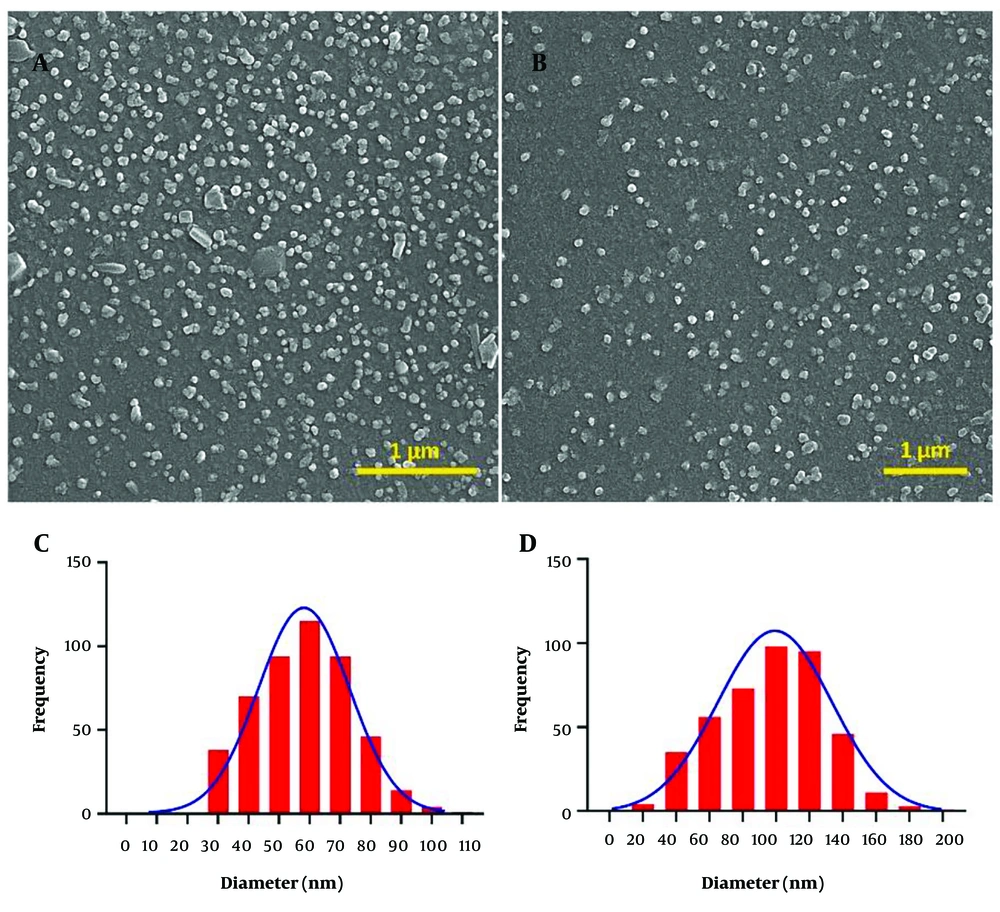
FESEM (MIRA3 TESCAN) technique was used to confirm the shape and size of the FBlank and FSO (12). The sample was covered with a layer of gold and then photographed at a voltage of 15 kV with magnifications of 35,000 and 50,000 times. 450 particles in each image were processed by ImageJ 1.52v and the data were reported as mean ± SD.
3.4.3. Zeta Potential Measurement
The FBlank and FSO were dispersed in distilled water and the zeta potential of the nanoparticles was measured using a Zeta Sizer (Malvern, England).
3.4.4. Differential Scanning Calorimetry Analysis
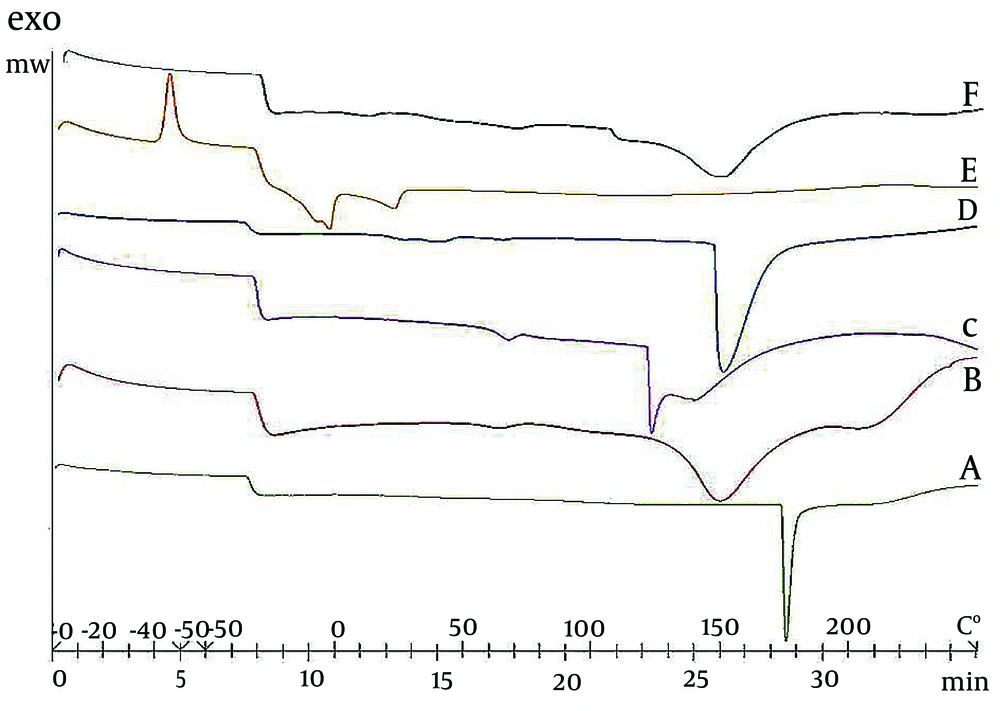
The thermal behavior of maltodextrin, WPC, gum acacia, FBlank, and FSO was determined using a DSC instrument (Mettler Toledo, Switzerland) with a heating program in the temperature range of - 50 to + 250°C using a sealed empty aluminum pan as a reference (9). Thermograms were obtained and compared in terms of peak position and size.
3.4.5. Fourier Transform Infrared Spectroscopy
FTIR spectroscopy was used to investigate the interaction of oil with formulation components in the range of 4000 - 400 cm-1. FTIR spectra of dry powders produced by the spray dryer in KBr were recorded on a Tensor 27 spectrophotometer (Bruker, Germany).
3.4.6. Extraction of Derivatized Unsaturated Fatty Acids
We used the alkaline methanolysis method with some modifications for the derivatization and extraction of fatty acids (13). Briefly, a specified amount of sample was transferred to a glass tube with a screw cap. 0.7 mL of 10 M KOH aqueous solution and 5.3 mL of methanol (GC grade) were added, gently mixed, and incubated for 1.5 hours at 55°C. The tube was shaken vigorously every twenty minutes for 5 s. The tube was cooled and 0.58 mL of sulfuric acid was added, mixed, and incubated for 1.5 hours at 55°C. After cooling, 3 mL of n-hexane was added and vortexed to extract the FAMEs. It was then centrifuged for 5 minutes (3000 rpm) to separate the n-hexane layer. Finally, the n-hexane phase was separated and, after absorbing its remaining water by adding anhydrous sodium sulfate, transferred into a dark vial for GC-MS analysis.
3.4.7. Encapsulation Efficiency
To calculate the efficiency of the Encapsulation, the peak area of unsaturated fatty acids (oleic acid) in GC-MS chromatograms of SO and FSO were compared.
4. Results
4.1. Particle Size
The mean particle sizes for FBlank and FSO are shown in Table 1. As shown, in both the DLS and FESEM results, the particle size of the FBlank is smaller than that of the FSO. FESEM images of FBlank and FSO are shown in Figure 1. The average particle size of FBlank and FSO was estimated to be 58.18 nm and 96.5 nm, respectively (Table 1). It appears that the particles are similar in shape and size, and no aggregation of particles has occurred.
| Samples | Particle Size (nm) | Zeta Potential | EE% b | ||||
|---|---|---|---|---|---|---|---|
| DLS c | FESEM d | 0 | 90 | ||||
| 0 | 30 | 90 | |||||
| B-NE | 415.7 (62.9) | 875.3 (117.2) | - | - | - | - | - |
| FBlank before spray drying | 135.7 (16.8) | 158.6 (26.3) | - | - | - | - | - |
| Spray dried FBlank | 138.2 (6.0) | 135.3 (7.0) | 142.0 (6.5) | 58.2 (0.7) | - 40.80 | - | - |
| SO-NE | 144.0 (7.9) | 299.7 (61.9) | - | - | - | - | - |
| FSO before spray drying | 163.6 (14.7) | 169.5 (8.6) | 165.0 (8.7) | - | - | - | - |
| Spray dried FSO | 166.5 (11.2) | 168.3 (9.5) | 164.7 (15.3) | 96.5 (1.5) | - 48.10 | 93.2 (3.6) | 92.9 (4.6) |
a Blank nanoemulsion (B-NE), So-nanoemulsion (SO-NE) and encapsulated SO-nanoemulsion (FSO) and blank (FBlank).
b According to GC-MS analysis.
c n = 3, mean ± SD.
d n = 450, mean (std. error of mean).
4.2. Zeta Potential
zeta potential is an important factor in the stability of nanoparticles. As shown in Table 1, the zeta potential of the FBlank is - 40.8 mV and that of the FSO is - 48.1 mV, which is in the proper range to be effective.
4.3. Differential Scanning Calorimetry
Differential scanning calorimetry, which is the most widely used technique to determine the phase transition characteristics of materials during temperature changes, was used to study the thermal behavior of pure materials and encapsulated Nanoemulsions (Figure 2). As seen, the thermograms of the materials show that lecithin, WPC, maltodextrin, and acacia gum have endothermic peaks at temperatures of about 40°C, 70°C, 110 - 130°C, and 150°C, respectively.
4.4. FTIR
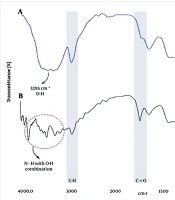
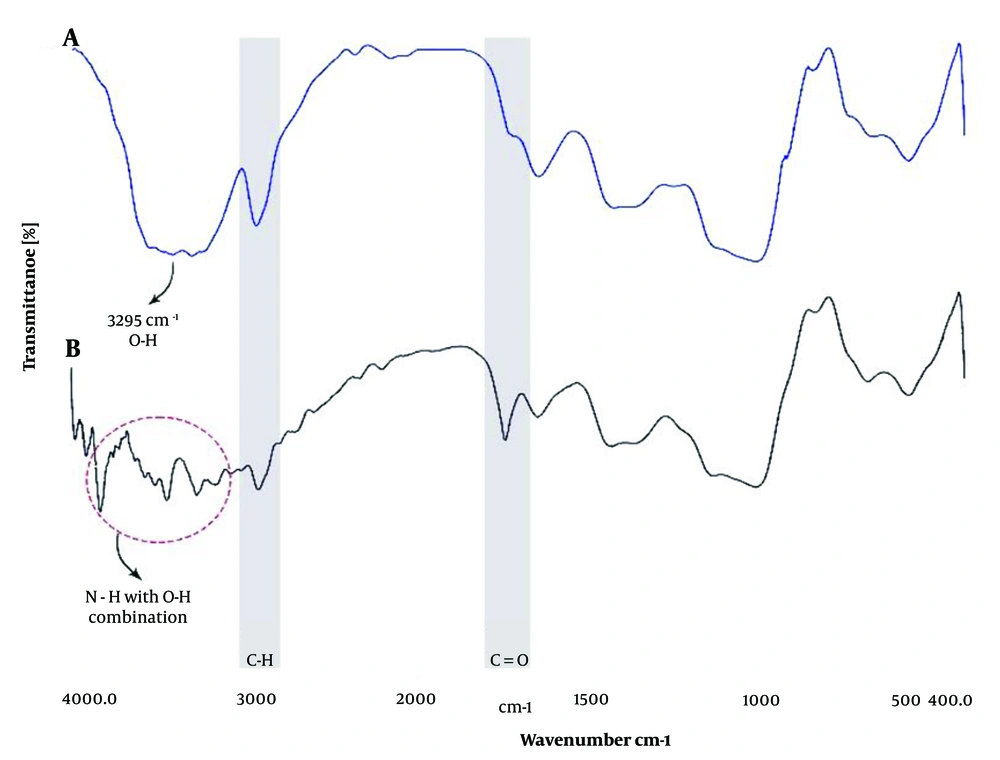
The FTIR spectra of FBlank and FSO are shown in Figure 3A. The absorption peak at about 3300 cm-1 is observed in both spectra and is the broadest in the FBlank spectrum. This may be caused by hydroxyls (OH) present in water and formulation components. The IR spectrum of FSO in Figure 3B shows C=O groups peaking around 1742 cm-1. The wide and strong absorption band around 3500 cm-1 ascribes to stretching vibrations of N-H and N-H with O-H combination in FSO. The absorption peak at about 2900 cm-1 belongs to the C-H band.
4.5. GC-MS Analysis and Encapsulation Efficiency
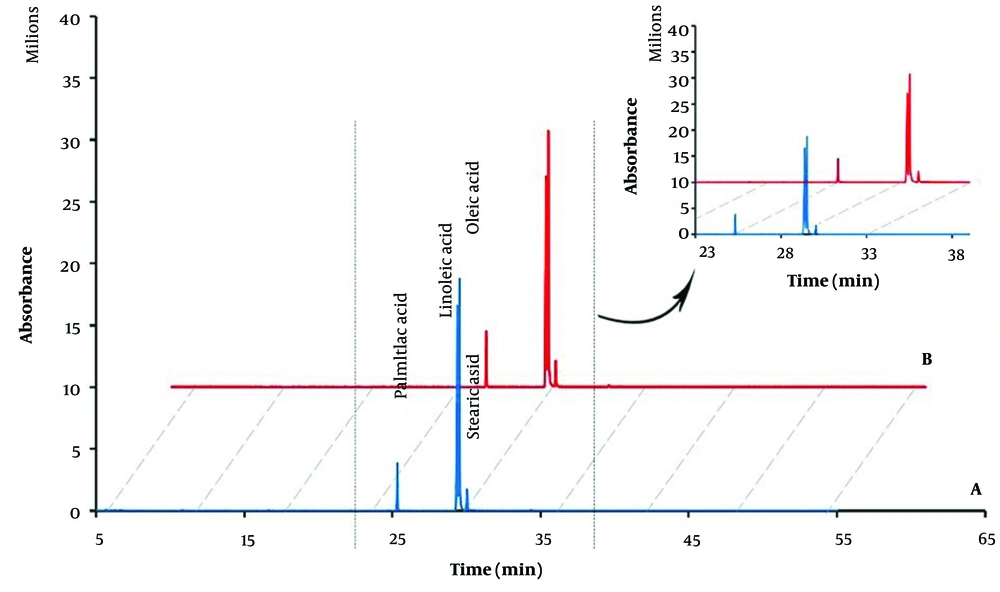
The chromatograms of SO and FSO is shown in Figure 4. In the chromatogram of SO, there are four peaks corresponding to palmitic acid, linoleic acid, oleic acid and stearic acid with retention times of 25.3, 29.3, 29.5 and 30.0; and the percentages of area under the peak are 7.12, 39.84, 47.75 and 4.06, respectively (Table 2). The maximum amount of area under the peak for SO is related to oleic acid. Corresponding peaks observed in SO were also observed in FSO. By comparing the level under the peak of oleic acid in SO and FSO, the EE% of the final formulation was estimated to be 92.93%.
| Samples | Area% | |||
|---|---|---|---|---|
| Oleic Acid | Linoleic Acid | Palmitic Acid | Stearic Acid | |
| SO | 47.75 | 39.84 | 7.12 | 4.06 |
| FSO | 49.58 | 36.51 | 7.74 | 4.53 |
5. Discussion
Particle size in Nanoemulsions affects their loading and stability. Methods based on light diffraction are used to determine the size of particles between 0.1 and 1000 micrometers. In fact, when a light beam hits a colloidal system, it is scattered by the particles. The image of these optical interactions in the detector is an estimate of particle size (3). We studied the product of each step of synthesis and the stability of final nanoparticles over 90 days by the DLS method (Table 1). Uncoated Nanoemulsions, whether with or without SO, did not have good stability over time and increased in size after thirty days at a temperature of 25°C. The Particle Size of the B-NE is significantly larger than that of the SO-NE. This difference may be due to the non-formation of a stable and uniform nanoemulsion in the absence of SO (as a core in the formation of nanoemulsion). However, maltodextrin/acacia gum coating has helped the stability of Nanoemulsions and reduced the size change during storage. The observed difference between Particle Size measured by the PSA and FESEM may be related to the loss of moisture and greater density of the particles during the drying process or to the difference in the mechanism of size determination between the two methods. The FESEM images show that the particles have almost spherical and uniform structures. In addition, the average Particle Size of FBlank and FSO was estimated to be 58.58 nm and 96.5 nm, respectively. An increase in the particle size of the sample containing SO may be due to the entrapment of the SO in the particles.
The zeta potential indicates the type and amount of charge accumulation in the immobile layer, as well as the intensity of adsorption of opposite ions on the particle surface. The higher the zeta potential of colloidal particles, the higher the electrostatic repulsive force and, consequently, the higher the physical stability. Nanoparticles with zeta potential greater than ± 30 mV are considered highly charged, while nanoparticles with zeta potential less than ± 10 mV are considered slightly charged. If the value of the zeta potential is higher than ± 30 mV, the dispersed system is more stable, and the accumulation of particles does not occur, and vice versa (14). According to the results, although the values of zeta potential of both formulations are sufficient to ensure their physical stability, the value of zeta potential is higher for the FSO, which is due to the presence of oil in this formulation. This further increased stability can be attributed to the molecular interactions between the oil and the formulation components. This level of stability makes the industrial application of the produced nanoparticles more likely.
In this study, DSC was used to investigate the melting and crystallization behavior of the components, any interactions between the components, and to determine if these properties were altered by other materials in the encapsulated nanoemulsion powder (15). The peak of WPC represents the denaturation process of its protein structure, and the peak of lecithin represents its layered structure in the gel phase. Similarly, the peaks of acacia gum and maltodextrin are caused by the removal of water from their structure by heat. By comparing these peaks with those observed in the thermogram of the FBlank, it can be concluded that the process of denaturation of WPC and the process of removal of water from the structure of acacia gum were not affected by the formulation. Furthermore, the absence of the peak related to the removal of water from the maltodextrin structure and the peak related to the lecithin gel structure in the thermogram of the blank formulation indicates physical interactions between the components as well as physical changes in the structure of the ingredients. Also, based on the similarity of the thermograms of the FBlank and the FSO, it can be concluded that the addition of SO did not change the thermal behavior of the materials.
As a result, the endothermic peak of acacia gum at over 140°C made it the right choice for coating and protecting the nanoemulsion. In spray drying, the inlet temperature was about 140°C and the outlet temperature was always below 85°C throughout the process. As a result, the stability of the raw materials used in the synthesis is guaranteed. This process is also confirmed in the GC-MS results, where the peaks of unsaturated fatty acids of SO are well seen in the final sample (Figure 4).
As can be seen in FTIR investigations (Figure 3), the peaks of both samples are almost similar in pattern. But in FSO spectra, the observed absorption band at 1742 cm⁻¹ and the changes in the range of 3000 to 3600 cm⁻¹, respectively, related to the carbonyl group and the interactions of O-H with N-H, confirm the presence of SO in the coated nanoemulsion (16). Sesame oil contains fatty acids and antioxidant compounds. These compounds have the ability to create hydrogen bonds with the ingredients of the formulation, and two of the absorption peaks mentioned above can confirm these interactions.
Based on the results of the GC-MS, the maximum area under the peak for SO is attributed to oleic acid, which is consistent with the results of Heydari Gharehcheshmeh and Moghtadaei studies (17, 18). As shown in Table 2, similar peaks are observed in the chromatograms of SO and FSO, which are related to palmitic acid, linoleic acid, oleic acid, and stearic acid. This confirms the correct performance of the nanoemulsion production step and the process of encapsulation and spray drying.
Determination of EE% is one of the main steps for the evaluation of the method. The higher the amount of EE%, the more successful the design of the formulation. The chemical interactions between the encapsulated material and the other components of the nanoemulsion should not cause any loss of the active ingredients. This characteristic was verified by GC-MS analysis. Based on the chromatograms, the presence of SO fatty acids in the final formulation confirmed the accuracy of the Encapsulation process and the absence of destructive chemical interactions between SO and the other components of the designed formulation. The Encapsulation Efficiency of FSO is 92%, and only a 0.3% change has been observed during 90 days of storage at ambient temperature. The observed stability in particle size and EE% of nanoparticles prepared in this project is due to the production of stable nanoemulsion from SO and its proper coating with maltodextrin/acacia gum, which makes it suitable for use in food and pharmaceutical industries.
5.1. Conclusions
In this study, SO loaded to maltodextrin/acacia gum-coated nanoemulsion was prepared by using biocompatible materials that can be used in the pharmaceutical and food industries. The dry encapsulated nanoemulsion had a high amount of oleic acid and linoleic acid as active ingredients, which showed that the materials and preparation method did not have a significant effect on the active ingredients of SO. Maltodextrin/acacia gum coating contributed to the Physicochemical Stability of Nanoemulsions, and the final product did not have any significant change in particle size and EE% during 90 days of storage at ambient temperature. The prepared formulation had reasonable stability, which is required for a regular shelf life. According to the results, it can be concluded that the dried encapsulated Nanoemulsion of SO can be used to enrich foods in the nutraceutical industry and produce super-beneficial foods.