1. Background
Following stomach and intestinal cancer, colorectal cancer (CRC) is identified as the third most prevalent gastrointestinal cancer. In 2023, it is projected that approximately 153,020 people will receive a diagnosis of CRC, while 52,550 individuals will succumb to this disease. Among these cases, around 19,550 individuals under the age of 50 will be affected, resulting in 3,750 deaths within this age group (1). Chemotherapy is a significant treatment method for cancer, working by administering cytotoxic agents to cancer cells. However, conventional chemotherapy techniques have a significant drawback: They cannot directly transport the necessary medication to the tumor cells without harming the patient's health (2).
Studies conducted in recent years have revealed the essential roles that bioactive peptides and proteins play in the metabolic functions of organisms, which have implications for human health. These peptides specifically target cancer cells without harming normal tissues, making them a promising therapeutic option (3). Proteolytic enzymes break down proteins into smaller peptides with functional characteristics, including antihypertensive, antidepressant, antimicrobial, antioxidant, and anticancer effects (4). The functionality of these peptides is influenced by their sequence, type of amino acids, and overall length (5).
The potential anticancer properties of these peptides can be utilized in cancer treatment. Some studies have found that fish bioactive peptides, such as those from blue whiting (6), tuna dark muscle (7), and rainbow trout (8), can inhibit the growth of MCF7/6, MDA-MB-231, MCF-7, and HCT-116 cell lines. Numerous protein hydrolysates or oligopeptides with antiproliferative activities have been identified from diverse food sources. Anti-proliferative peptides demonstrate various mechanisms to inhibit cancer cell growth, including the disruption of the cytoplasmic membrane via micellization or pore formation, engagement with cell surface gangliosides, and the promotion of apoptosis (9).
Lanternfish are often wasted despite their high protein and nutrient content, as they have little economic value and are commonly used for low-value fish products like fishmeal, fish oil, or fish silage. Currently, no studies have been carried out regarding the impact of bioactive peptides derived from lanternfish on CRC, specifically on cell line models. Although a variety of myctophid species show a substantial accumulation of wax esters, certain edible species, notably Benthosema pterotum, are devoid of this particular compound. Consequently, these species can be used to create valuable, value-added products (10). A considerable proportion of lanternfish is captured each year in the Makran Sea, although these catches are not intended for consumption.
2. Objectives
This study examines the antiproliferative and pro-apoptotic properties of protein hydrolysates originating from B. pterotum (BPHA and BPHF), with a molecular weight under 3 kDa, on CRC cell line models.
3. Methods
3.1. Experimental Design
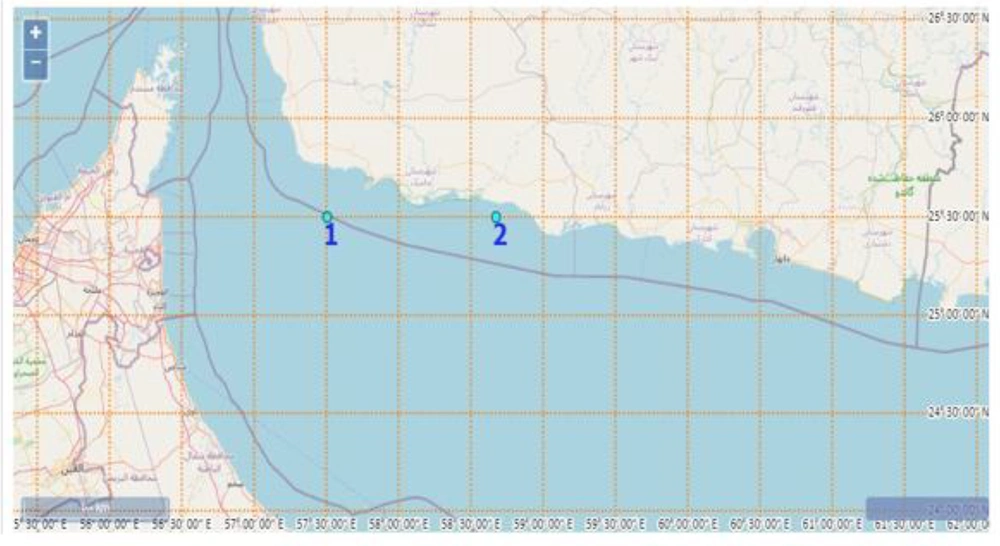
Lanternfish were obtained in the summer from the Makran Sea, with an average weight of 2 - 10 g. The collection locations were 57°00'E/25°10'N and 61°25'E/24°4'N (Figure 1). The fish were carefully preserved by enveloping them with ice in a weight-to-weight ratio of 1:2 and were expeditiously conveyed to the Laboratory of Caspian Sea Ecology Research Center located in Mazandaran, Iran, within 2 hours. The fish were kept at -70°C until ready for use. Novosim Company, based in Denmark, supplied the Alcalase (HA) enzyme, derived from Bacillus licheniformis, and Flavourzyme (HF), extracted from Aspergillus oryzae, through its representation in Iran. These enzymes were stored at 4°C until use. All stages of this study have been reported per the ARRIVE guidelines (11).
3.2. Preparations of Lanternfish Protein Hydrolysate
First, whole lanternfish were defrosted at room temperature. Subsequently, 50 g of the fish were measured and carefully deposited into a 250 mL Erlenmeyer flask. To each flask, 100 mL of distilled water was added, maintaining a weight-to-volume ratio of 1:2. The samples were subjected to heat treatment at 85°C for 20 minutes to deactivate internal enzymes (12). The HA enzyme, with a protein content of 1%, was used for hydrolysis, conducted for 90 minutes at pH 8.5 and 58°C. The slurry was then cooled following enzyme deactivation at 90°C for 10 minutes. The HF enzyme, also containing 1% protein content, was introduced into the second Erlenmeyer flask. The pH and temperature were adjusted to 7 and 50°C, respectively, before the enzyme's addition. This procedure was maintained for 90 minutes. The hydrolysis process was concluded by placing the samples in a water bath at 90°C for 10 minutes. After cooling, the samples were centrifuged at 12,500 rpm at 4°C for 10 minutes. The supernatants were then filtered, lyophilized with Dura-stop (NY, USA), and preserved at -20°C until prepared for additional analyses (13). The protein hydrolysate powder, produced using HA and HF enzymes, was stored in a polyethylene bag under vacuum conditions at room temperature within a desiccator until required (Table 1).
| Enzymes | pH | Temperature (°C) | Time (min) |
|---|---|---|---|
| HA | 8.5 | 58 | 90 |
| HF | 7 | 50 | 90 |
Abbreviations: HA, Alcalase; HF, Flavourzym.
3.3. Ultrafiltration
The hydrolyzed protein powder was dissolved in a 10% (w/v) solution and then filtered through 3 kDa and 10 kDa Amicon filters (Amicon Ultra-15; Millipore Co., Billerica, MA, USA) at 25°C for 30 minutes under a centrifugal force of 13,850 rpm (Sigma 2-16KL, Spain). Subsequently, the hydrolyzed protein solution and its fractions were collected, lyophilized, and later analyzed to evaluate their anticancer efficacy.
3.4. Composition of Chemicals in Lanternfish
The procedure established by the Association of Official Analytical Chemists (AOAC) was used to determine the estimated composition of the samples. A consistent sample weight was employed to assess the moisture content by drying in an oven at 105°C. The ash content of the sample was determined by placing the wet specimen into a crucible and subjecting it to combustion in a furnace at 550°C for 5 hours. The protein content was quantified using the Kjeldahl method, while the total fat was extracted using the Soxhlet extraction technique (14).
3.5. Degree of Hydrolysis
The degree of hydrolysis (DH) was measured using 20% (v/v) trichloroacetic acid (TCA). For this purpose, an equal volume of the protein solution was dissolved in a TCA solution, blended, and centrifuged at 5°C at 7,200 rpm after stirring at 20°C. The amount of protein in the solution phase was then measured using the Lowry method, and the DH was calculated using the following equation (15).
3.6. Preparation of Cell Culture
The HT-29 cell line, a human colorectal adenocarcinoma cell line, was acquired from the cellular bank of the Iran Pasteur Institute. The cells were transferred to the lab and cultivated in a DMEM/F12 medium supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum (Sigma Aldrich, Germany). They were then placed in a 75 cm3 flask and allowed to enter the logarithmic growth phase through incubation. To achieve complete separation of the cells, trypsin-EDTA (Sigma Aldrich, Germany) was introduced into the flask. Subsequently, 100 µL of a cell suspension containing 5 × 104 cells was meticulously added to each of the 96 wells on the plate. The plate was then incubated for 24 hours (16).
3.7. Calculation of Cell Viability Percentage
The cell suspension was centrifuged at 25°C for 5 minutes at 6,000 rpm, and the supernatant was removed. An appropriate volume of PBS was used to resuspend the cell pellet. The cell suspension was then mixed with an equal volume of 0.4% trypan blue solution and incubated at room temperature for 3 minutes. The mixture was applied to a hemocytometer slide and placed on the microscope stage. Unstained (viable) and stained (non-viable) cells were counted in several grid squares, and the average number of cells in each square was calculated. The average number of cells per square was multiplied by the dilution factor and the conversion factor (10,000 for a standard hemocytometer) to obtain the number of cells per milliliter of the original suspension. To determine the proportion of viable cells, the total number of unstained cells was divided by the total cell count, and the resulting value was multiplied by 100 (16).
3.8. Anticancer Activity of Lanternfish Protein
After 24 hours, the cells were washed with phosphate buffer and exposed to different concentrations of BPHA and BPHF ≤ 3 kDa fractions (10, 50, 100, 250, 500, 1000, 1500 μg/mL). The untreated cells, which were not subjected to BPHA or BPHF solutions, served as the blank control, while cisplatin (6.8 μg/mL; Sigma Aldrich, Germany) was used as the positive control. After a 48-hour incubation period, the MTT solution [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma Aldrich, Germany] was added to the plate at a concentration of 0.5 mg/mL. The plate was then incubated for 4 hours at 37°C. Following this incubation, the medium was carefully removed and replaced with an equal volume of DMSO to dissolve the purple formazan crystals. The absorbance of the solution was measured using a spectrophotometer equipped with a microplate reader (Dynex Opsys MR 24100) at a wavelength of 570 nm and compared to the control sample. The IC50 concentration for each treatment was determined by analyzing the cell viability graph (17).
3.9. Flow Cytometry
The evaluation of cell apoptosis through flow cytometry was conducted using the Annexin V-FITC apoptosis detection kit (BD Pharmingen, USA) (16). In summary, following 24-hour treatments involving incubation of the cells with BPHA, BPHF, and cisplatin at the IC50 concentration in a CO2 incubator at 37°C, the cells were collected and rinsed with binding buffer. The cells were then counted to achieve a final concentration of 1 × 106 cells/mL at 37°C in the CO2 incubator. Annexin V and propidium iodide (PI) were subsequently added and incubated in a light-restricted environment for 15 minutes. Following the washing procedure, the cell suspension was treated with a 1% formaldehyde solution for 10 minutes while being maintained at a low temperature on ice. The washing step was repeated twice using binding buffer, and the RNase enzyme (EMD Biosciences, USA) was introduced into the system. The mixture was then incubated at 37°C for 15 minutes. Finally, the samples underwent another round of washing before being analyzed using the FACSCalibur flow cytometer (BD Biosciences, USA) in conjunction with CellQuest Pro software (16).
3.9. Statistical Analysis
Statistical analyses were performed using Prism software, version 8. To assess the differences between groups, a one-way analysis of variance (ANOVA) was conducted, followed by Tukey's test. The significance level was set at P < 0.05.
4. Results
4.1. Physicochemical Properties
Table 2 presents the chemical composition and DH for BPHA and BPHF, based on wet weight. The results indicate that BPHA has a significantly higher DH (42.35%) compared to BPHF (27.37%) (P < 0.05). The wet raw materials for all three samples included fat, protein, ash, and moisture. BPHA and BPHF exhibited higher protein levels at 80.26% and 79.45%, respectively. The presence of the same letters above the numbers indicates no significant difference (P < 0.05). The mean ± SD values are derived from triplicate determinations (n = 3). Benthosema pterotum was observed on the wet substrate, with BPHA and BPHF analyzed in terms of dry substrate content. BPHA underwent hydrolysis with HA, while BPHF was subjected to hydrolysis using HF.
a Values are given as mean ± SD from triplicate determinations (n = 3).
b Lanternfish based on the wet matter, BPHA and BPHF based on the dry matter.
c P < 0.05 was considered statistically significant.
4.2. MTT Assay
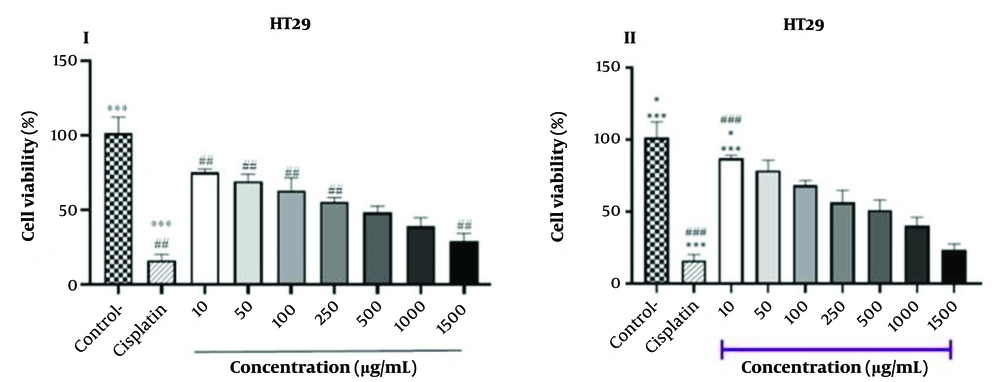
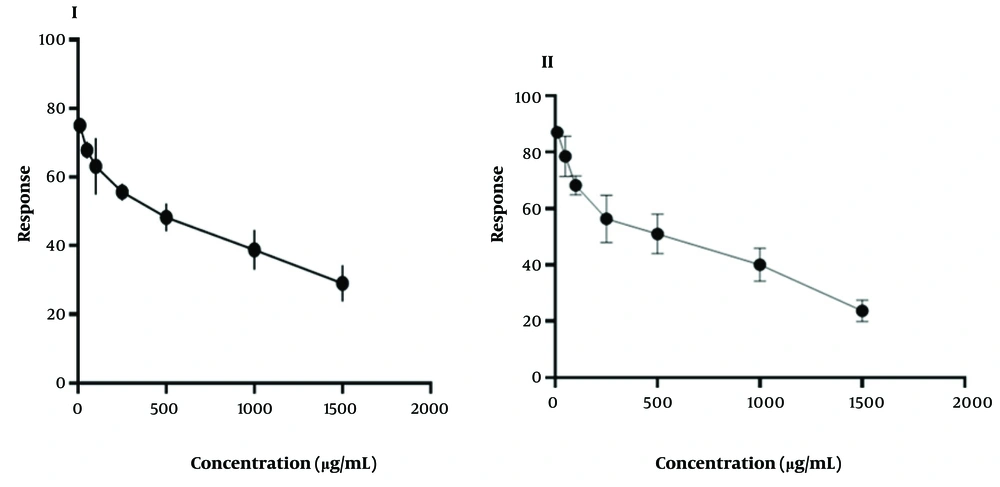
The results of the experiment on HT-29 cancer cell lines treated with different concentrations of hydrolyzed protein using HA and HF enzymes are presented in Figure 2. The study found a reduction in cell viability (%) with an increase in peptide concentration (ranging from 10 to 1500 µg/mL), which was more effective for peptides with a molecular weight under 3 kDa. Notably, peptides isolated with the HA enzyme at concentrations of 250 - 500 µg/mL and the HF enzyme at concentrations of 500 - 1000 µg/mL were found to be comparable to the effects of cisplatin (6.8 µg/mL), a known cancer treatment drug (as shown in Table 3). Figure 3 illustrates the dose-response relationship of BPHA and BPHF, demonstrating their inhibitory effect on cancer cell growth. As the concentration of BPHA and BPHF increases, cancer cell growth decreases linearly, indicating a dose-dependent inhibition.
The survival percentages of HT-29 cells were assessed over a 48-hour period against varying concentrations of BPHA; I, protein hydrolyzed with Alcalase (HA) (3 < kDa) and BPHF; II, protein hydrolyzed with Flavourzyme (HF) (3 < kDa); the viability (%) of cells was determined by copmparing the results to an untrated sample (control) and the cisplatin group, which serves as the (positive control) (* P < 0.05, *** P < 0.001).The # symbol denotes a notable comparision between cisplatin and various concentarions of hydrolyzed lanternfish protein (## P < 0.01 and ### P < 0.001).
| HT-29 Cell Line | IC50 (µg/mL) b |
|---|---|
| Cisplatin | 6.8 ± 0.16 |
| BPHA | 313.7 ± 80.0 |
| BPHF | 392.8 ± 82.8 |
a Values are expressed as mean ± SD.
bIC50: Half maximal inhibitory concentration is a measure of the potency of a substance in inhibiting a specific biological or biochemical function.
As shown in Table 3, the calculated Log IC50 value of BPHF is 2.594, corresponding to an IC50 value of 392.8 µg/mL. This signifies that a concentration of 392.8 µg/mL of BPHF is required to inhibit 50% of cancer cell growth. Furthermore, the 95% confidence intervals for Log IC50 and IC50 are (2.494 - 2.699) and (311.6 - 499.7), respectively, indicating a high degree of precision in the measurements. The calculated Log IC50 value of BPHA is 2.497, corresponding to an IC50 value of 313.7 µg/mL. This signifies that a concentration of 313.7 µg/mL of BPHA is required to inhibit 50% of cancer cell growth. Furthermore, the 95% confidence intervals for Log IC50 and IC50 are (2.403 - 2.594) and (252.7 - 392.5), respectively, indicating a high degree of precision in the measurements.
4.3. Apoptosis
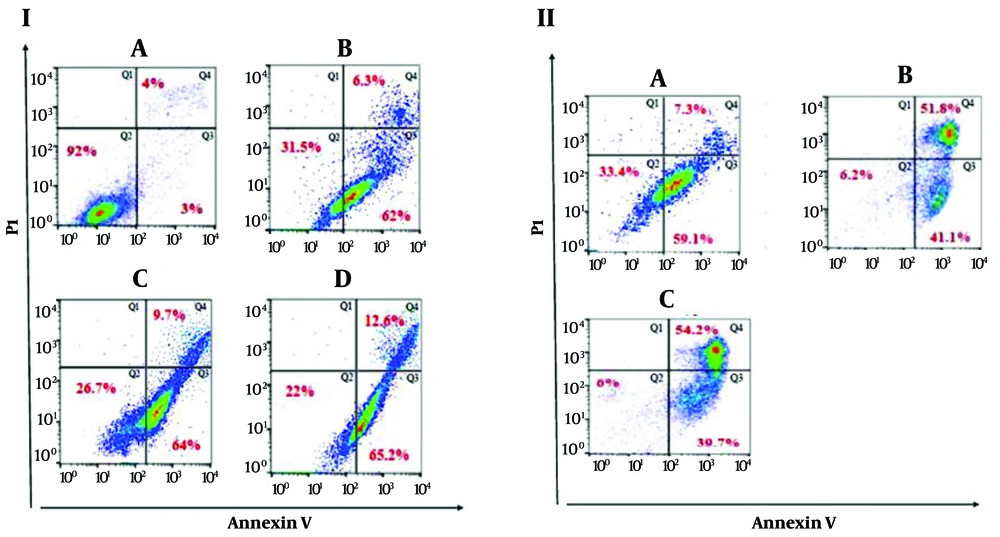
As shown in Figure 4 (I), the following treatments were applied: (A) Control, untreated sample; (B) treatment with BPHA 200 µg/mL; (C) treatment with BPHA 400 µg/mL; (D) treatment with BPHA 600 µg/mL after 24 hours. Figure 4 (I) illustrates that BPHA at 200 µg/mL (Figure 4B) caused 31.5% primary apoptosis, 62% secondary apoptosis, and 6.3% necrosis. In contrast, BPHA at 400 µg/mL (Figure 4C) resulted in 26.7% primary apoptosis, 64% secondary apoptosis, and 9.7% necrosis in cancer cells. BPHA at 600 µg/mL (Figure 4D) induced 22% primary apoptosis, 65.2% secondary apoptosis, and 12.6% necrosis in cancer cells. Overall, BPHA induced a noticeable change in apoptosis at different concentrations, with all concentrations causing approximately 60% secondary apoptosis.
The data presented in this figure displays the results of the FACS scan examination of the cells subsequent to flow cytometry in HT29 cells. In the first set of experiments I, the cells were categorized as follows: A, control, untreated sample; B, treated with BPHA 200 μg/mL; C, treated with BPHA 400 μg/mL; D, treated with BPHA 600 ug/mL after 24 hours. In the second set of experiments II, the cells were treated with varying concentrations of BPHF: A, 250 μg/mL; B, 500 μg/mL; C, 750 μg/mL. II: A, administration of BPHF at a concentration of 250 μg/mL; B, administration of BPHF at a concentration of 500 μg/mL; C, administration of BPHF at a concentration of 750 pg/mL.
Figure 4 (II) presents the following treatments: (A) Treatment with BPHF 250 µg/mL; (B) treatment with BPHF 500 µg/mL; (C) treatment with BPHF 750 µg/mL after 24 hours. As shown in Figure 4A, BPHF at 250 µg/mL caused 33.4% primary apoptosis, 59.1% secondary apoptosis, and 7.3% necrosis. On the other hand, BPHF at 500 µg/mL (Figure 4B) resulted in 6.2% primary apoptosis, 41.1% secondary apoptosis, and 51.8% necrosis in cancer cells. BPHF at 750 µg/mL (Figure 4C) induced 6% primary apoptosis, 39.7% secondary apoptosis, and 54.2% necrosis in cancer cells. Overall, BPHF induced a noticeable change in apoptosis at different concentrations, with all concentrations causing approximately 50% secondary apoptosis.
5. Discussion
The current research, utilizing an in vitro model of colon adenocarcinoma, demonstrates that bioactive peptides derived from lanternfish can hinder cell proliferation and increase apoptosis. While some mechanisms related to the anticancer properties of fish-derived peptides have been identified, additional research is needed to explore internal signaling pathways. Fish protein hydrolysates and peptides have demonstrated the capacity to positively impact human health due to their impressive antioxidant, anti-inflammatory, antiproliferative, antihypertensive, and cardioprotective characteristics (18).
This study indicated that the amount of protein increased following hydrolysis using HA and HF compared to the initial raw sample. In line with this investigation, Chai et al. recorded increasing levels of protein, free amino acids, and nitrogen compounds for hydrolyzed proteins of lanternfish, with hydrolyzed proteins reported to contain 53.6% peptides (19). Benthosema pterotum demonstrates a higher total amino acid content than deep-sea lobster and Indian white shrimp, while it shares a similar level with oil sardine and blue crab (20).
This study demonstrated the effectiveness of using enzymes to isolate bioactive peptides under 3 kDa from lanternfish. The results indicate that bioactive peptides derived from lanternfish, with a molecular weight under 3 kDa, can reduce the viability of colon adenocarcinoma cells (HT-29) in a dose-dependent manner. Annexin V/PI kit and flow cytometry studies on HT-29 cells using BPHA and BPHF found that after 24 hours of incubation with bioactive peptides, apoptosis increased significantly at all concentrations. The highest total primary and secondary apoptosis was 77.7% and 93.9%, respectively, at concentrations of 600 and 750 µg/mL of peptides with a molecular weight under 3 kDa isolated with HA and HF enzymes. This indicates a significant difference compared to the control group.
Similar studies on hydrolyzed peptides from Rohu roe fish at different concentrations from 50 to 500 µg/mL found significant effects on colon adenocarcinoma cells with 65% early apoptosis (21). Furthermore, peptides extracted from flathead fish hindered the development of colon cancer cells (HT-29) (22). Scholars have also explored apoptosis, necrosis, and disruptions in the cell cycle to comprehend the mechanism of cell death in cancer cells triggered by protein hydrolysates or peptides derived from food (21).
Currently, RP-HPLC fractionation is used to purify bioactive compounds that can inhibit the growth of cancer cells in fish hydrolysates. The antiproliferative properties of 18 fish protein hydrolysates were investigated on two human breast cancer cell lines in vitro. Notably, significant growth inhibition in both cancer cell lines was demonstrated by three blue whiting hydrolysates, three cod fish hydrolysates, three fish hydrolysates, and one salmon hydrolysate. The initial analysis of the hydrolysates' composition demonstrated the existence of a diverse combination of unbound amino acids, peptides of different magnitudes up to 7 kDa, and, to a lesser degree, lipids and sodium chloride (22).
Anti-proliferative peptides exhibiting diminished reactivity towards reactive oxygen species (ROS) have the potential to inhibit cancer development by mitigating oxidative stress. This, in turn, can contribute to the suppression of genetic abnormalities such as mutations and chromosomal rearrangements, which are significant factors in carcinogenesis (23). It is noteworthy that several recent studies have provided evidence to support the notion that fish and its discards possess significant potential as a rich source of anti-carcinogenic peptides (24).
The most significant difficulty associated with the transport of proteins and peptides in vivo lies in their instability. To optimize delivery capacity, it is essential to consider various aspects such as molecular size, molecular charge, protein internal structure, solvent effect, lipid membrane packing and hydration, stability, and affinity towards receptors. The conversion of proteins and peptides into nanoparticles has emerged as a prominent strategy in this regard (25). Therapeutic proteins that exhibit homology to native self-proteins generally do not induce an immune response due to pre-existing immunological tolerance; however, they can trigger an immune reaction by disrupting B-cell tolerance (26).
Additionally, a recent study has indicated that certain fish by-products contain anticancer peptides that can potentially impede the growth of cancer cells in humans. Isolating these bioactive peptides from fish by-products and incorporating them into food supplements may positively impact human health. According to the outcomes of this research, the utilization of bioactive peptides derived from lanternfish may offer a significant enhancement to existing colon cancer chemotherapy treatments. Additionally, this therapy proves to have low toxicity in healthy cells, making it an effective treatment option. Moreover, further in vivo studies or clinical treatments need to be conducted to investigate the effects of the bioactive peptide on other apoptotic or angiogenic proteins, as well as to gain broader insight into its safety.
5.1. Conclusions
The findings of this research suggest that bioactive peptides obtained from lanternfish could serve as a beneficial supplement to current colon cancer chemotherapy regimens. Furthermore, this treatment has demonstrated minimal toxicity in normal cells, underscoring its efficacy as a viable therapeutic approach. Additionally, it is imperative to conduct further in vivo experiments or clinical trials to explore the impact of the bioactive peptide on various apoptotic and angiogenic proteins and to enhance our understanding of its safety profile.