1. Background
One of the most important plant genera in Iran's northern and central regions is the oak genus (Quercus), which consists of 45 different species (1). The formation of a gall on the Oak tree is a natural phenomenon following the sting of various insects, especially bees. The extract obtained from oak galls contains phenolic compounds such as tannic acid and gallic acid. These compounds have antibacterial, antiviral, antifungal, and insect pest effects (2, 3). Acid, the primary cause of tooth decay, is secreted by bacteria. Gram-positive bacteria, such as Streptococci mutans, Sanguis, Mitis, Oralis, and intermedius make up around 56% of the bacteria that induce gingivitis brought on by dental plaque (4). Conversely, another opportunistic infection (OI) is Candida infection. Nowadays, oral nystatin and other systemic and local therapies are utilized to treat Candida infections. The primary drawbacks of this treatment are its bitter taste, frequent ingestion necessity, and preparation requirements (5). Therefore, a different approach without the aforementioned restrictions is required.
Mouthwashes are essential in plaque control and gingivitis. Along with having a broad antibacterial range, the ideal mouthwash should also have little drug resistance and a lower propensity to eradicate the mouth's natural microbiota (6). Chlorhexidine and other chemical mouthwashes are commonly used. Chlorhexidine mouthwashes have three primary benefits: Non-toxicity, long-term effects, and favorable antibacterial activity. However, some of its drawbacks include tooth discoloration, altered taste perception, gingivitis, dry mouth, and unpleasant effects after swallowing. Researchers have increasingly focused on herbal mouthwashes as a way to get around these restrictions. Herbal mouthwashes are preferable to chlorhexidine because they include natural ingredients and are less harmful (7).
Given the importance of the matter and the possibility of the use of antimicrobial effects of oak gall extract, and also given the lack of studies in this regard, this exploratory in vitro study was conducted.
2. Objectives
Its purpose was to prepare a novel herbal mouthwash from Iranian oak gall extract and investigate its antimicrobial effects on five oral pathogens: Candida tropicalis, C. albicans, Streptococcus sanguis, S. mutans, and S. salivarius. As clinical implications, if proven effective, such an herbal mouthwash may replace chemical mouthwashes in some scenarios.
3. Methods
This preliminary explorative in vitro study was performed on five oral pathogens. The study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (ethics code: IR.AJUMS.REC.1400.490).
3.1. Sample Collection
First, library studies on plant distribution in Iran were carried out with the assistance of flora books and research studies. The harvested plant was stored out of direct sunlight at around 20°C to 28°C (8). Quercus brantii galls were gathered from Yasuj, Iran. Pharmacologists identified and verified the plant; it was assigned the herbarium specimen number A2411111GP. The specimen has been placed in the Medicinal Plant Research Center's herbarium at the Ahvaz Jundishapur University of Medical Sciences' Faculty of Pharmacy in Ahvaz, Iran.
3.2. Extraction
The plant was dried and pulverized at lab temperature in order to obtain its aerial portion (8). The extract was extracted from the ground plant by maceration method with 70% ethanol thrice for two days. Then the extract was passed through a double-layer cloth and filtered using Whatman No. 1 filter paper with a vacuum pump device. At 40°C, an extract was concentrated using a vacuum distillation process. The extract was then put into 300 mL glass tubes and freeze-dried for 24 hours to turn it into a powder. A container was used to store the dried powder at a temperature of 4°C (8).
3.3. Preparation of Oak Gall Mouthwash
To prepare the experimental mouthwash, oak gall extract, distilled water, glycerin (1%), phosphoric acid (0.01%), ethanol (8%), sodium benzoate (0.1%), and sodium (0.1%) were used. The extraction yield was 14%. The produced mouthwash was examined for its pH and viscosity (8). In the first investigation, the change in color of the pH paper indicated that the pH was 4 (acidic); hence, the mouthwash was gradually mixed with sodium bicarbonate until the pH reached seven. Next, a DV-II+ Pro viscometer (Brookfield Engineering, Middleboro, Massachusetts, USA) was used to evaluate the viscosity (8). At 5% torque and 100 RPM, the viscosity of the prepared mouthwash was 30 CP (centipoise).
3.4. Microbial Culture
We followed Iran's Health Ministry's established procedures for autoclaving all media and other materials in order to sterilize the culture media and other materials utilized in this investigation. We also worked close to the flame, wore disposable gloves, and utilized sampling tips to reduce any potential contamination. Standard strains of the American type culture collection (ATCC) were used in this study. The Scientific and Industrial Research Center (Iran) provided the samples as lyophilized vials. These vials were injected into the sterile trypticase soy broth (TSB) medium obtained from Merck, Germany. Then they were incubated at 37°C for 48 hours. Afterwards, a solid brain heart infusion agar (BHIA) medium obtained from Merck (Germany) was used to culture the fungal and bacterial suspensions C. tropicalis (ATCC20336), C. albicans (ATCC10231), S. Sanguis (ATCC10556), S. mutans (ATCC35668), and S. salivarius (ATCC 9222) for 48 hours in order to assess the formation of microbial colonies (8).
3.5. Trypticase Soy Broth Culture Medium
Trypticase soy broth solution was prepared by dissolving 30 grams of powder (TSB, Merck) in distilled water (1 liter). Afterwards, 1 minute of boiling was done to complete the dissolution of the powder. The TBS solution was poured into tubes. An autoclave was used to sterilize the tubes and TBS solutions for 15 min at 121°C. After sterilization, the tubes were stored in a fridge (8). To standardize the broth media, we used a pH meter (pH: 7.5 ± 0.2 at 25°C).
3.6. Minimum Inhibitory Concentration
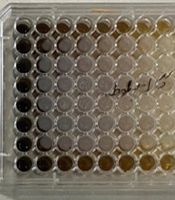
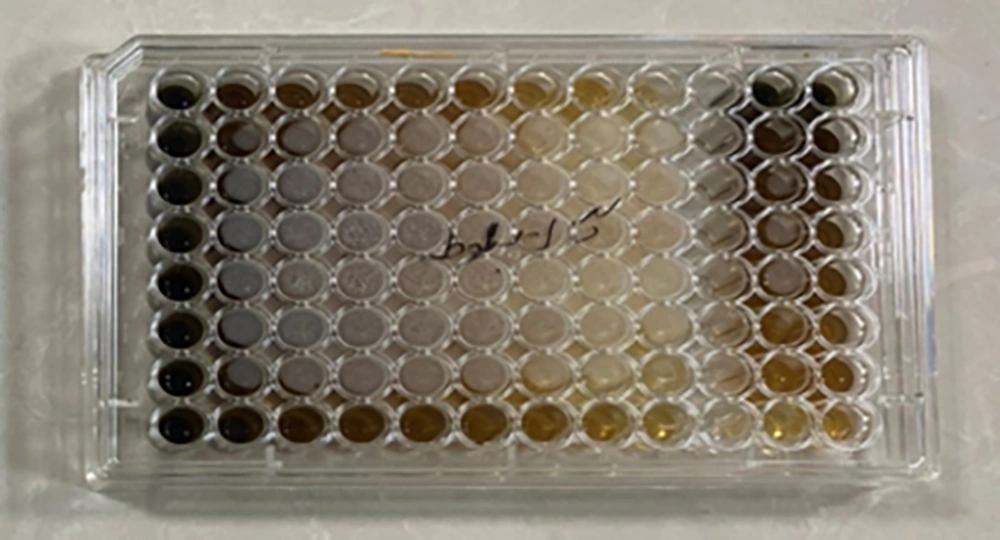
In this research, the microbroth dilution method was used to determine the minimum inhibitory concentration (MIC) of the herbal mouthwash of oak gall in mg/mL. For this purpose, 200 mg/mL oak gall mouthwash (OGM) solution was readied. Afterwards, sterile microplates were used to dilute the mouthrinse in a serial concentration using sterile distilled water. This reduced the concentration of the mouthwash 2 to 1. A 0.5 McFarland TSB culture medium of 1.5 × 108 with OD = 0.08 to 0.1 at absorbance wavelength of 600 nm was used for microbial suspension. Next, a TSB medium (~106 CFU/mL) was used to dilute it 1:100. Finally, in each of the wells, 100 µL of the mouthwash were added with twofold dilutions and 100 µL of microbial suspension. This made the concentration of the final microbial suspension of each of the wells ~5 × 105 CFU/mL. Of the wells, one was reserved as the inoculation negative control: This well did not include any microorganisms but contained the mouthwash and TSB culture medium (8). Another well was prepared as a positive control for treatment, which only included microbial suspension without mouthwash. The microplate was placed in a greenhouse at 37°C for 18 - 24 hours. Lastly, MIC or Minimum Inhibitory Concentration was defined as the smallest mouthrinse concentration where no microbial growth would be detectable visually (8) (Figure 1). In addition, for the quality control of the culture media, the standard species such as Escherichia coli ATCC 25922 and Staphylococcus aureus (ATCC25923) were used. For examining the validity of the experiments, the assay was performed in triplicate.
3.7. Minimum Bactericidal Concentration
The minimum bactericidal concentration (MBC) was determined in mg/ml using the broth microdilution method: An MHA plate was used to culture, at 37°C for 24 hours, 20 µL of the contents of the MIC well as well as two wells with concentrations greater than that of MIC. Then, any colonies grown on the plates were counted. The MBC was defined as the smallest concentration that could reduce growth for at least log 3 of the original concentration (5 × 105 CFU/mL). Each experiment was repeated six times in order to ensure the high accuracy of the MIC and MBC findings for each of the five oral pathogens (8).
3.8. Statistical Analysis
Descriptive statistics were computed. Kolmogorov-Smirnov and Shapiro-Wilk tests were used for normality assessment. Kruskal-Wallis and Bonferroni adjusted post hoc test. One-sample Wilcoxon tests of SPSS 27 (IBM, Armonk, NY, USA) was used for comparing MIC and MBC values of different microorganisms with the value zero. The level of significance was predetermined as 0.05.
4. Results
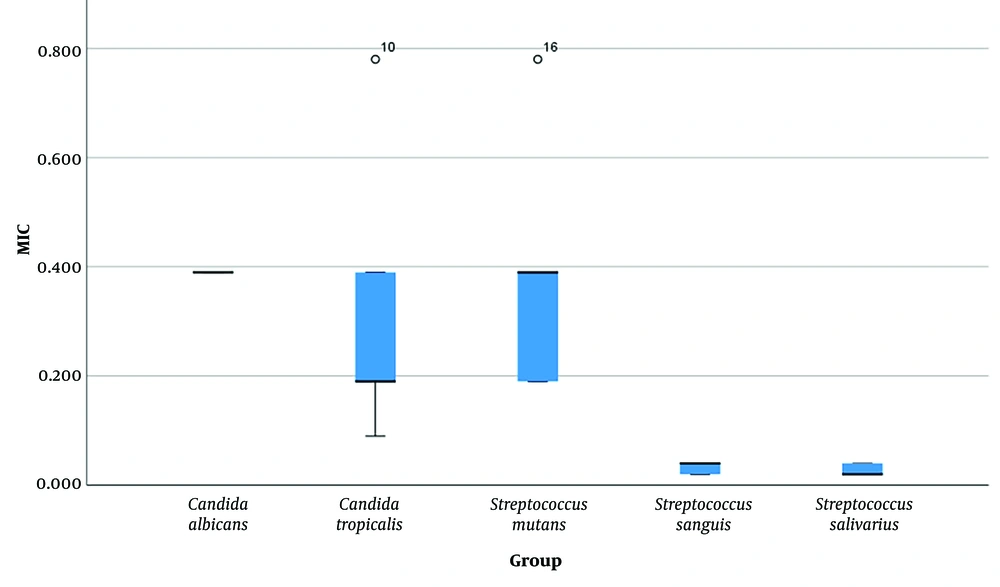
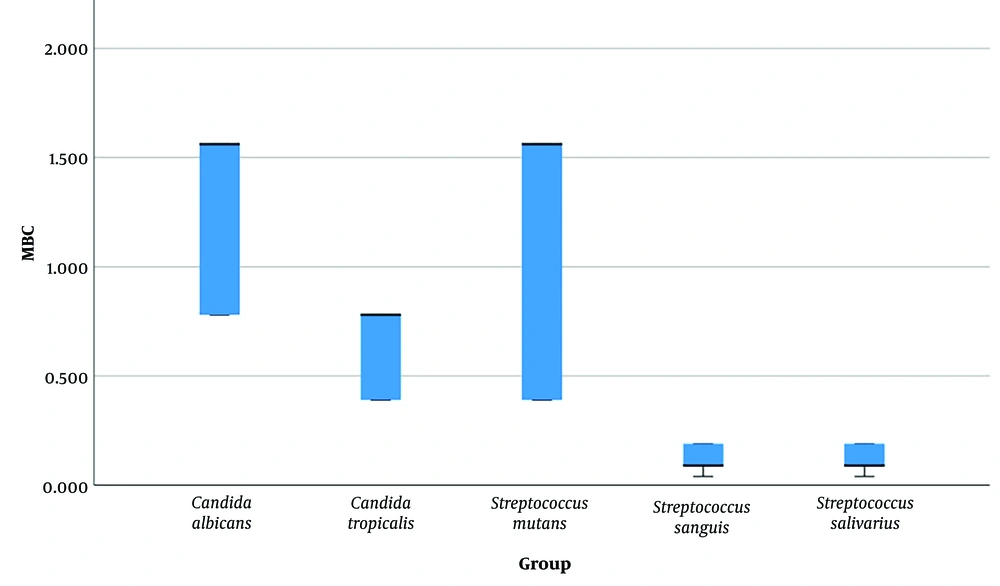
Table 1 shows descriptive statistics for all MIC and MBC measurements pertaining to all microorganisms evaluated. Figures 2 and 3 show descriptive statistics for MIC and MBC, respectively. The one-sample Wilcoxon test showed that MICs and MBCs of all microorganisms were above zero (Table 2).
| Variables and Organism | Mean ± SD | Median (Min - Max) | Q1, Q3 |
|---|---|---|---|
| MIC (mg/mL) | |||
| Candida albicans | 0.390 ± 0.000 | 0.390 (0.390 - 0.390) | 0.390, 0.390 |
| Candida tropicalis | 0.305 ± 0.252 | 0.190 (0.090 - 0.780) | 0.190, 0.340 |
| Streptococcus mutans | 0.388 ± 0.215 | 0.390 (0.190 - 0.780) | 0.240, 0.390 |
| Streptococcus sanguis | 0.033 ± 0.010 | 0.040 (0.020 - 0.040) | 0.025, 0.040 |
| Streptococcus salivarius | 0.027 ± 0.010 | 0.020 (0.020 - 0.040) | 0.020, 0.035 |
| MBC (mg/mL) | |||
| Candida albicans | 1.301 ± 0.404 | 1.562 (0.780 - 1.562) | 0.976, 1.562 |
| Candida tropicalis | 0.650 ± 0.201 | 0.780 (0.390 - 0.780) | 0.488, 0.780 |
| Streptococcus mutans | 1.171 ± 0.605 | 1.562 (0.390 - 1.562) | 0.683, 1.562 |
| Streptococcus sanguis | 0.115 ± 0.061 | 0.090 (0.040 - 0.190) | 0.090, 0.165 |
| Streptococcus salivarius | 0.115 ± 0.061 | 0.090 (0.040 - 0.190) | 0.090, 0.165 |
Descriptive Statistics Pertaining to Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of Each of the Five Microorganisms a
| Variables | Candida albicans | Candida tropicalis | Streptococcus mutans | Streptococcus sanguis | Streptococcus salivarius |
|---|---|---|---|---|---|
| MIC | 0.014 | 0.026 | 0.026 | 0.023 | 0.023 |
| MBC | 0.023 | 0.023 | 0.023 | 0.026 | 0.026 |
P-Values Calculated Using the One-Sample Wilcoxon Test
4.1. Minimum Inhibitory Concentration
The Kruskal-Wallis test showed a significant overall difference among the MIC values of the 5 microorganisms (P = 0.000, Table 1 and Figure 2). The results of the Bonferroni-adjusted post hoc test showed 4 pairwise comparisons (Table 3).
| Sample 1-Sample 2 | Test Statistic | SE | Std. Test Statistic | P-Value |
|---|---|---|---|---|
| Streptococcus salivarius-Streptococcus sanguis | 2.000 | 4.936 | 0.405 | 1.000 |
| Streptococcus salivarius-Candida tropicalis | 13.500 | 4.936 | 2.735 | 0.062 |
| Streptococcus salivarius-Streptococcus mutans | 16.500 | 4.936 | 3.343 | 0.008 |
| Streptococcus salivarius-Candida albicans | 18.000 | 4.936 | 3.647 | 0.003 |
| Streptococcus sanguis-Candida tropicalis | 11.500 | 4.936 | 2.330 | 0.198 |
| Streptococcus sanguis-Streptococcus mutans | 14.500 | 4.936 | 2.938 | 0.033 |
| Streptococcus sanguis-Candida albicans | 16.000 | 4.936 | 3.242 | 0.012 |
| Candida tropicalis-Streptococcus mutans | -3.000 | 4.936 | -0.608 | 1.000 |
| Candida tropicalis-Candida albicans | 4.500 | 4.936 | 0.912 | 1.000 |
| Streptococcus mutans-Candida albicans | 1.500 | 4.936 | 0.304 | 1.000 |
Bonferroni-Adjusted P-Values Calculated for Pairwise Comparisons Between the Minimum Inhibitory Concentration Values of 5 Microorganisms
4.2. Minimum Bactericidal Concentration
The Kruskal-Wallis test showed a significant overall difference among the MBC values of the 5 microorganisms (P = 0.000, Table 1, Figure 3). The results of the Bonferroni-adjusted post hoc test showed 4 pairwise comparisons (Table 4).
| Sample 1-sample 2 | Test Statistic | SE | Std. Test Statistic | P-Value |
|---|---|---|---|---|
| Streptococcus sanguis-Candida albicans | 17.667 | 4.983 | 3.546 | 0.004 |
| Streptococcus salivarius-Candida albicans | 17.667 | 4.983 | 3.546 | 0.004 |
| Streptococcus sanguis-Candida tropicalis | 11.333 | 4.983 | 2.275 | 0.229 |
| Streptococcus salivarius-Candida tropicalis | 11.333 | 4.983 | 2.275 | 0.229 |
| Streptococcus sanguis-Streptococcus mutans | 16.000 | 4.983 | 3.211 | 0.013 |
| Streptococcus salivarius-Streptococcus mutans | 16.000 | 4.983 | 3.211 | 0.013 |
| Streptococcus sanguis-Streptococcus salivarius | 0.000 | 4.983 | 0.000 | 1.000 |
| Candida tropicalis-Streptococcus mutans | -4.667 | 4.983 | -0.937 | 1.000 |
| Candida tropicalis-Candida albicans | 6.333 | 4.983 | 1.271 | 1.000 |
| Streptococcus mutans-Candida albicans | 1.667 | 4.983 | 0.334 | 1.000 |
Bonferroni-Adjusted P-Values Calculated for Pairwise Comparisons Between the Minimum Bactericidal Concentration Values of 5 Microorganisms
5. Discussion
Because their natural constituents are less harmful and more compatible with the body's physiology, herbal mouthwashes are more appropriate (9, 10). Previous research has shown that the extract from oak galls contains phenolic components such as gallic acid and tannic acid. These substances contain antifungal, antibacterial, antiviral, and insect pesticide properties (3). Each Gall has a variable concentration of these substances and their antibacterial compounds. Tannic acid, which comprises 50 - 70% of the components in Mazo Gall, is the primary, effective substance. Galls from Mazo and Qalqaf have a higher tannin concentration than the other galls, at around 52% (11, 12). Hence, in the present investigation, the inhibition efficacy of OGM was evaluated against common mouth microorganisms: Candida tropicalis, C. albicans, S. sanguis, S. mutans, and S. salivarius (8).
According to our findings, the effectiveness against the microorganisms stated is acceptable. Olivier Gall found the strongest sensitivity to Quercus infectoria anti-caries against Staphylococcus aureus (13). Additionally, the concentration of 0.018 µg/mL showed the greatest percentage of biofilm reduction (13). Quercus infectoria olivier was shown to have antibacterial properties against S. mutans, S. salivarius, Porphyromonas gingivalis, and Fusobacterium nucleatum in a study by Basri et al. using both acetone and methanol extractions (14). In a study, Safarpour et al. assessed the ethanol, acetone, and water extractions of Iranian oak gall. Gram-negative bacteria could be less susceptible than gram-positive bacteria, according to their study's findings (8, 15). According to the observations of Basri et al. (14), water, acetone, and ethanol extractions of Darmazo oak gall were all effective against bacteria. The similar efficacy against oral plaque development in S. mutans, S. salivarius, and S. sanguis was also demonstrated by Babadi et al. using Jaft extraction (16). The results of this investigation show that Iranian OGM has good in vitro effectiveness. Because the environment in the mouth and saliva has a direct impact on how well GSM works, more research involving in vivo experiments is required to determine the most effective treatment plan for GSM.
While this preliminary in vitro study demonstrates promise, further research on herbal mouthwashes could yield significant clinical implications. If found effective and biocompatible in vitro, animal studies followed by human clinical trials can be conducted on such mouthwashes to investigate their safety and efficacy in more relevant situations. If proven useful and safe, they can be used as potential alternatives to the available antimicrobial mouthwashes that have some side effects. There are a number of clinical trial studies on the evaluation of the effect of herbal mouthwash in maintaining oral health. In a 2023 study (17), the effect of herbal mouthwash including Anacyclus pyrethrum, Punica granatum, Capparis spinosa, and Q. infectoria on oral health of school children was evaluated. The results showed that after intervention, the Bleeding on Probing, Plaque Index, and Gingival Index reduced on the 30th day from baseline. Hence, the prepared herbal mouthwash might be safe and significantly effective in maintaining oral health, and it might be used as an adjunct to control oral hygiene (17). In another clinical trial published in 2022 (18), the effect of mouthwash consisting of Myrtus communis, Portulaca oleracea, P. granatum, Boswellia serrata, and Q. brantii in patients with plaque-induced gingivitis was investigated (18). In that study (18), the patients received the prepared herbal mouthwash or chlorhexidine for 14 days (twice a day). At the end of the study, the results showed improvements in the periodontal indices and there were no significant differences between the patients who received herbal or synthetic mouthwash (18).
Plant polyphenols such as tannic acid and gallic acid can act as antimicrobial agents through some mechanisms (19): One of their mechanisms is interaction with proteins and bacterial cell walls. Moreover, polyphenolic compounds can inhibit nucleic acid synthesis and change the membrane permeability and the cytoplasmic functions by microbial cells (19). In the present study, statistically significant differences were found between MICs or MBCs of the tested mouthwash against some microorganisms. Such significant differences mean that the difference was less likely due to chance. In recent years, several herbal mouthwashes have been presented to the global market. Some studies conclude that herbal mouthwashes reduce oral bacteria more effectively than commercial chemical alternatives, suggesting their utility and safety (20, 21). Researchers have evaluated the possible use of herbal extracts in oral hygiene products. One of the herbal mouthwashes is red ginseng mouthwash, which is important because of its abundant medicinal values, including antibacterial effects (20, 21). Some studies showed herbal mouthwashes can reduce dental plaque and consequently prevent dental caries. They may affect microbial strains such as Baccharis dracunculifolia, Q. brantii, and Zataria multiflora (21-23). In a clinical trial, the effect of a mouthwash consisting of Myrtus communis, Portulaca oleracea, P. granatum, B. serrata, and Q. brantii on plaque-induced gingivitis was investigated (18). Their patients received the prepared herbal mouthwash or chlorhexidine for 14 days (twice a day). At the end of their study, they observed improvements in periodontal indices; there were no significant differences between patients who received herbal or synthetic mouthwashes (18). In the study of Alipour et al. (22), the antimicrobial properties of the combination of Oak Husk of Q. brantii and Z. multiflora leaves were investigated. Their results showed antibacterial activity for future clinical studies (22).
Studies on herbal mouthwashes can bring inventive hygiene control measures to the equation; in this regard, our study suggests and introduces a new mouthwash that can help improve patient oral hygiene hopefully with fewer side effects, although the latter needs its own assessments. Mechanisms for such beneficial effects might be in the high content of polyphenols and tannin present in Galls of Oak. Tannin may be an effective compound responsible for the antimicrobial activity observed in this study. There are a number of mechanisms for antibacterial activity of tannin such as creating a complex between tannin and microbial enzymes (such as cellulase), changing the permeability membrane of microorganism due to the astringent properties of tannin (14, 24). Another antibacterial mechanism of tannin is exerted by its effect on bacterial metabolism through inhibition of oxidative phosphorylation (14, 24). The mechanisms for the antifungal activity of nystatin may be related to its action on the microorganism cell membrane and changing the membrane permeability and the cytoplasmic functions (25). Also, it is suggested that the mechanism for antifungal activities of nystatin is similar to mechanisms of effects of polyphenolic compounds (26).
People are increasingly using herbal medications to prevent or treat illnesses (27). Because the herbal mouthwashes have few or no adverse effects, they are employed in a variety of formulations and in both in vitro and in vivo testing. As additions to regular oral hygiene, natural plant mouthwashes may help reduce plaque and contain antibacterial properties (27).
This study was limited by some factors. The sample size of this preliminary study was not large, therefore larger studies should test and verify our results. Moreover, the results of in vitro studies are not generalizable to clinical situations full of a multitude of known and unknown confounding factors that can affect how the environment responds to the drug. On the other hand, this is at the same time the advantage of in vitro studies, since ruling out such confounding factors is exactly the reason an in vitro study is needed before conducting any more complicated animal and clinical designs. Another limitation of the study was that only the antibacterial efficacy of GSM was examined; expiration and duration of effectiveness were not assessed. Overall, the in vitro nature of the study and its lack of additional confounding variables call for future animal studies to examine our findings in a much more complicated setting. In future studies, more comprehensive evaluation of the effectiveness of the researched herbal mouthwash seems necessary; in such studies, adding positive control (such as synthetic mouthwashes) and negative control groups will strengthen the study. Of course, in the oral environment with the presence of food, drinks, pH fluctuations, and other ever-changing factors, the efficacy of an antimicrobial product may change under some conditions. Therefore, clinical studies are needed to verify in vitro results. Future research should first identify the optimal dosage in vitro, then assess its safety again in vitro; if it was deemed biocompatible and safe, future clinical trials should evaluate its efficacy against placebo.
5.1. Conclusions
The findings demonstrate the antibacterial and antifungal properties of OGM. The highest MIC of the OGM was found against S. salivarius. Also, it showed the highest MBC against S. salivarius and S. sanguis. Furthermore, it was found that S. Salivarius and C. albicans have the highest and lowest sensitivity to the OGM, respectively, which could be due to its concentrated nature. According to this study and the previous clinical trials, the OGM can be considered as a safe and effective oral hygiene aid. In future studies, the researchers can evaluate the clinical trial of the prepared OGM and extrapolate the advantages and disadvantages of OGM.