1. Background
Neonatal jaundice is a widespread issue and, in severe cases, can cause brain damage in newborns (1). Increased bilirubin production, reduced hepatic clearance, and enhanced enterohepatic circulation are key contributors to unconjugated hyperbilirubinemia (2). Phototherapy is the global standard for managing this condition, with blood transfusion considered a last-resort method for reducing exceptionally high bilirubin levels (3). Potential serious side effects of phototherapy include transient erythematous rashes, loose stools, and high fever (4). Consequently, researchers have explored alternative treatment options, including traditional medicine and, in particular, medicinal plants (5). Among various medicinal plants in Asian medicine, Cotoneaster has long been used to treat neonatal jaundice. Numerous Cotoneaster Medikusa species, traditionally used for jaundice treatment, belong to the Rosaceae family, which comprises approximately 500 species distributed across Europe and Asia (6). Five of the 28 Cotoneaster species are native to Iran (7). Among the medicinal plants available for treating neonatal jaundice, Cotoneaster discolor Pojark, Cotoneaster nummularioides Pojark, and Cotoneaster nummularius are particularly important. This plant yields a yellowish-white substance with a sweet taste known as Purgative manna, a primary component of the Rosaceae family. The key ingredients of purgative manna include mannitol, fructose, glucose, and sucrose, with mannitol comprising approximately 40 - 60 percent of its composition (8). Manna is the plant’s most crucial ingredient. Research on different Cotoneaster species in Iran indicates that manna contains various sugars and phytochemicals, with High-Pressure Liquid Chromatography (HPLC) tests confirming mannitol as the most abundant carbohydrate (9). Due to its laxative properties, mannitol effectively treats jaundice by helping to expel meconium from the intestines (10).
In Iranian medicine, Cotoneaster is prescribed using two distinct methods. The first is a traditional form obtained from herbal drug stores, locally referred to as the Cotoneaster plant or manna in Iran (11). The recommended consumption method is as follows: Five grams of Cotoneaster are mixed with 30 cc of boiling water, and after cooling and complete dissolution, the insoluble particles and impurities are filtered out; the 30 cc mixture is then divided into three portions, with 10 cc given to the baby every 8 hours (12). The second form of Cotoneaster available in pharmacies is an industrial version (billineaster) produced in factories. Traditional medicine employs these properties for treating neonatal jaundice, often accompanied by complications.
2. Objectives
This study aims to investigate the effects of Cotoneaster treatment on jaundiced newborns.
3. Methods
The research design for this study was an observational, cross-sectional study examining newborns admitted to the neonatal intensive care unit and neonatal ward of the Children's Medical Center in Tehran from 2021 to 2022. The survey focused on healthy neonates who had consumed Cotoneaster following treatment for neonatal jaundice and experienced complications. In this study, we investigated the impact of these substances on the neonates. Demographic data of neonates, complications from herbal drug consumption, and bilirubin levels at admission were collected in pre-prepared questionnaires. We then compared complications in the two groups of plant-based and industrial forms of Cotoneaster.
Cotoneaster consumption complications were defined as any clinical signs and/or symptoms that necessitated neonatal admission, including dehydration, electrolyte abnormalities, exaggerated hyperbilirubinemia, and NEC. Exaggerated hyperbilirubinemia was classified as a bilirubin level above 17 mg/dL (13). Necrotizing enterocolitis was defined using modified Bell's criteria: In stage 1, there are no definitive radiologic signs but gastric residuals and abdominal distention may be observed; in stage 2, pneumatosis intestinalis and/or hepato-biliary gas are present; and stage 3 involves bowel perforation, pneumoperitoneum, and/or peritonitis (14).
3.1. Inclusion Criteria
Neonates younger than 28 days old who were admitted to our hospital with neonatal jaundice and had a history of Cotoneaster consumption, either traditional or factory-produced, for jaundice treatment that resulted in complications were included.
3.2. Exclusion Criteria
Neonates with gastrointestinal obstruction, metabolic disease, congenital diarrhea, or those who had consumed anything other than Cotoneaster or other herbal plants were excluded. Additionally, neonates with positive blood or urine cultures or those who consumed Cotoneaster for neonatal jaundice treatment without experiencing complications were excluded.
3.3. Data Collection
The study's objectives were clearly defined, along with the specific data points required from patient records, such as demographics, treatment information, and complications. A standardized protocol was developed to specify the data collection process, including precise definitions for each variable to eliminate potential ambiguities. Checklists were provided to aid data collectors in gathering all necessary information. Each patient file was systematically reviewed to ensure no relevant information was overlooked. Following data collection, a thorough data-cleaning process was undertaken to identify any outliers, inconsistencies, or errors in the recorded data.
3.4. Statistical Analysis
Quantitative data were measured using the mean ± SD, while frequency was used for qualitative data. Categorical variables were compared using the Chi-Square test. Statistical analysis was conducted using SPSS software, version 23.0 for Windows (IBM, Armonk, New York).
4. Results
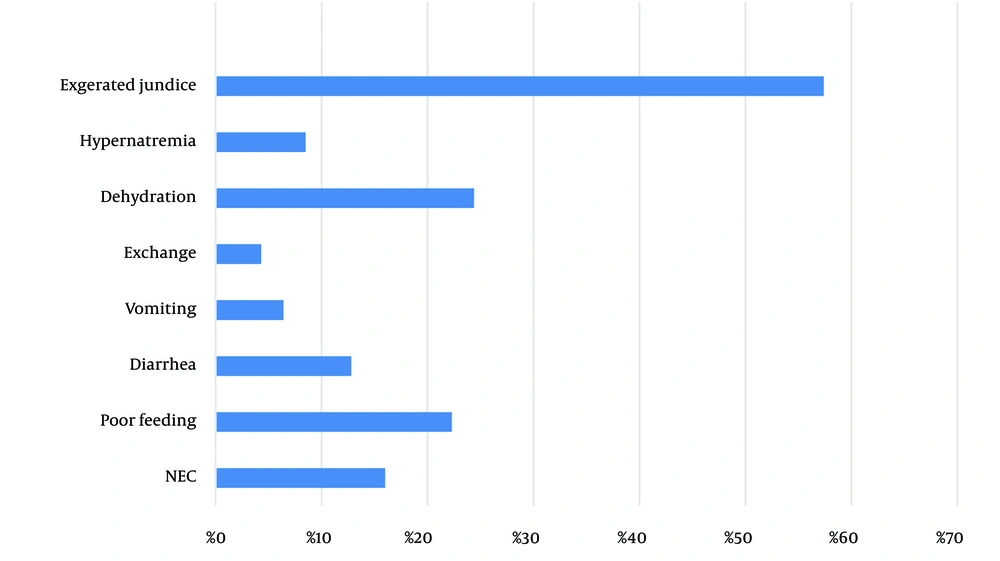
From a total of 1,200 neonates admitted to the Neonatal Intensive Care Unit and neonatal ward from 2021 to 2022, ninety-four neonates were admitted due to complications from Cotoneaster, representing 7.8% of the cases. Table 1 shows the demographic data of the neonates included in the study. The most common complication observed was exaggerated hyperbilirubinemia, which occurred in 58.5% of the cases. Necrotizing enterocolitis (NEC) was reported in 16.0% of the complications following Cotoneaster consumption (Figure 1). Nine neonates exhibited signs of NEC stage 2, and six were admitted with NEC stage 1. Poor feeding had a significant relationship with the Cotoneaster plant group (P-value = 0.024) as shown in Table 2.
| Variables (N = 94) | Mean (95%CI) or No. (%) |
|---|---|
| Birth weight (g) | 3161 (3063, 3258) |
| Gestational age (week) IQR | 38 (37, 39) |
| Sex (male) | 55 (58.8) |
| Delivery route (C/S) | 66 (70.2) |
| Current weight (g) | 3480 (2696, 4262) |
| Admit ion age (day) | 8.7 (7.5, 9.9) |
| Complications (N = 94) | Total | Confidence Interval%95 | Industrial Cotoneaster Usage (N = 51) | Cotoneaster Plant Usage (N = 43) | P-Value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
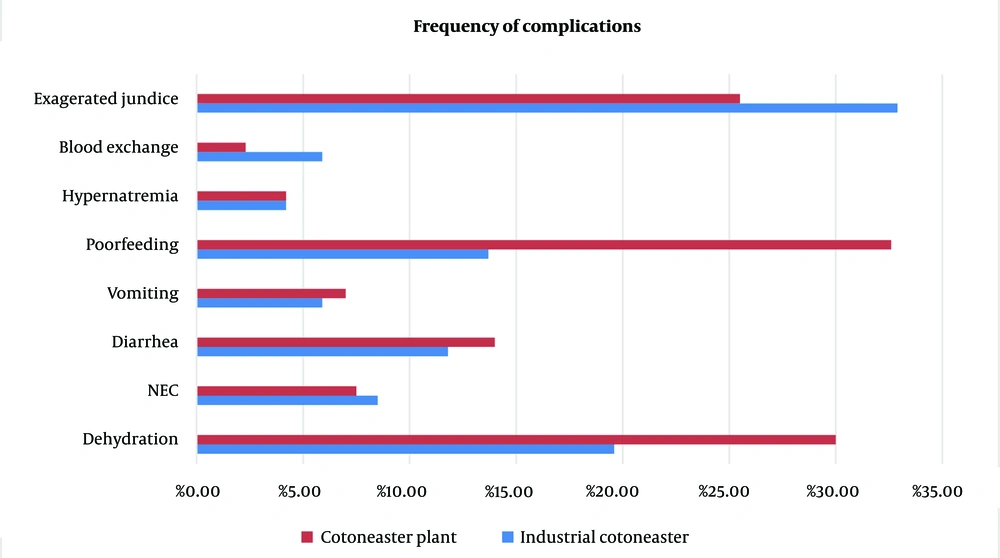
| Dehydration | 23 (24.4) | -0.137 | 3.279 | 10 (19.6) | 13 (30) | 0.071 |
| Hypernatremia | 8 (8.5) | -1.709 | 1.282 | 4 (4.2) | 4 (4.2) | 0.779 |
| NEC | 15 (16) | -1.449 | 1.546 | 8 (8.5) | 7 (7.5) | 0.949 |
| Vomiting | 6 (6.4) | -1.929 | 2.098 | 3 (5.9) | 3 (7) | 0.935 |
| Diarrhea | 12 (12.8) | -1.342 | 1.304 | 6 (11.8) | 6 (14) | 0.978 |
| Poor feeding | 21 (22.3) | -2.316 | -0.162 | 7 (13.7) | 14 (32.6) | 0.024 |
| Blood exchange | 4 (4.3) | -1.621 | 3.066 | 3 (5.9) | 1 (2.3) | 0.3 |
| Exaggerated jaundice | 55 (58.5) | -1.90 | 0.991 | 31 (32.9) | 24 (25.5) | 0.531 |
Abbreviation: NEC, necrotizing enterocolitis.
a Values are expressed as No. (%) unless otherwise indicated.
Despite the use of Cotoneaster, the mean total serum bilirubin (TSB) during admission was 17.6 ± 3.7 mg/dL. The mean bilirubin level in the industrial Cotoneaster group was 17.3 ± 3.0 mg/dL, while in the Cotoneaster plant group, it was 19.2 ± 3.0 mg/dL. The average duration of Cotoneaster usage was 2.49 days, with a 95% confidence interval of (1.9, 3). Fifty-four cases exhibited a median weight loss of -240.8 g, with a range of (-125, -306.2) grams (Table 3).
| Variables | OR* | 95%CI | P-Value |
|---|---|---|---|
| Abdominal distention | 1.20 | 0.22 - 6.27 | 0.577 |
| Poor feeding | 3.03 | 1.09 - 8.42 | 0.029 |
| Diarrhea | 1.21 | 0.36 - 4.08 | 0.751 |
| Low urine | 3.65 | 0.90 - 14.78 | 0.056 |
| Vomiting | 1.20 | 0.22 - 6.27 | 0.829 |
| NEC | 0.94 | 0.23 - 3.75 | 0.934 |
| Blood exchange | 0.38 | 0.03 - 3.80 | 0.622 |
| Sepsis | 0.98 | 0.94 - 1.01 | 1.00 |
| UTI | 1.02 | 0.97 - 1.07 | 0.457 |
| Hypernatremia | 1.20 | 0.28 - 5.13 | 0.801 |
| Dehydration | 0.26 | 0.05 - 1.30 | 0.103 |
Abbreviation: NEC, necrotizing enterocolitis.
The complications included exaggerated hyperbilirubinemia, dehydration, poor feeding, NEC, diarrhea, hypernatremia, and vomiting. Table 4 presents the demographic and laboratory results of the two groups: Cotoneaster plant and industrial form for the treatment of neonatal jaundice, while Figure 2 illustrates the frequency of complications in the two groups.
| Variables | Industrial Cotoneaster (Mean) | Cotoneaster Plant (Mean) | Mean Difference | 95%CI | P-Value |
|---|---|---|---|---|---|
| Gestational age | 38.11 | 37.48 | 0.62 | 0.03, 1.22 | 0.037 |
| Birth weight | 3190 | 3123 | 67.05 | - 129, 263 | 0.499 |
| Current weight | 3145 | 3908 | -762 | -2340, 815 | 0.339 |
| TSB | 17.70 | 17.53 | 0.17 | -1.43, 1.77 | 0.831 |
| WBC | 10.41 | 10.01 | 0.39 | -0.83, 1.62 | 0.522 |
| HB | 16.19 | 15.75 | 0.44 | -0.70, 1.58 | 0.447 |
| BUN | 8.05 | 7.24 | 0.81 | -1.01, 2.64 | 0.379 |
| CR | 0.36 | 0.40 | -0.04 | -0.11, 0.02 | 0.234 |
| PLT | 301.24 | 315.28 | -14.03 | -56.20, 28.12 | 0.510 |
| Neut | 36.21 | 36.04 | 0.17 | -4.91, 5.27 | 0.945 |
| lymph | 49.09 | 49.43 | -0.33 | -5.59, 4.93 | 0.901 |
| NA | 138.48 | 138.32 | 0.16 | -2.37, 2.70 | 0.897 |
| K | 4.90 | 4.72 | 0.18 | -0.13, 0.50 | 0.251 |
Abbreviation: TSB, total serum bilirubin.
5. Discussion
The results of our study demonstrate that despite the use of traditional medicine, 58.5% of cases still refer to exaggerated hyperbilirubinemia. In four cases, blood exchange occurred due to high bilirubin levels, possibly because parents had certain expectations about its effects, resulting in a delayed hospital arrival. Poor feeding was a common complication, observed more frequently in the Cotoneaster plant group than in the industrial Cotoneaster group (P-value < 0.024). Poor feeding can lead to dehydration and hypernatremia as well. On the other hand, this condition may indicate an early symptom of NEC (15). Necrotizing enterocolitis is an ominous sign in the gastrointestinal tract, presenting with vomiting, abdominal distention, poor feeding, and diarrhea. This complication is classified into three stages: Abdominal distention and mucosal thickness are present in the early stages, while intestinal pneumatosis is observed in stage 2 (14).
The complications primarily involve gastrointestinal issues linked to the oral route of drug administration. By understanding the gut physiology of neonates and the ingredients of Cotoneaster, we can explain the mentioned complications. Neonates have different drug absorption, distribution, metabolism, and excretion (ADME) processes compared to adults. Gastric acid production, gastric emptying time, bile salt production, mucosal structure, epithelial permeability, absorptive surface area, intestinal transit time, transporter functionality, biotransformation reactions, digestive enzyme activity, and postnatal microbiome all influence ADME in neonates (16) .
Furthermore, more than 90 compounds have been discovered in Cotoneaster products. These chemical compounds can be classified into flavonoids, procyanidins, phenolic acids, cotonefurans, cyanogenic glycosides, triterpenes, sterols, fatty acids, volatile compounds, and carbohydrates (10). Cotoneasters contain a large number of flavonoids. Anti-cholinesterase activity has been observed in numerous flavonoids. Quercetin and macluraxanthone were found to exhibit concentration-dependent inhibition against acetylcholine esterase (AChE), along with various flavonoids such as rutin and kaempferol 3-O-β-d-galactoside (17). Acetylcholine and nitric oxide produced by the myenteric plexus in neonates regulate intestinal motility by stimulating and inhibiting smooth muscles (18). Disturbances in the balance between nNOS-and acetylcholine esterase-producing neurons can cause changes in bowel motility (19). The anti-cholinesterase activity of flavonoids may explain the occurrence of NEC in our cases.
Cyanogenic glycoside consumption is accompanied by symptoms such as vomiting, nausea, abdominal cramps, and diarrhea (20). This characteristic can explain the vomiting observed in our patients. Cyanogenic glycosides are important plant chemicals that contribute to the potential toxicity of Cotoneaster species. These glycosides can release hydrogen cyanide (HCN) when broken down by enzymes, leading to toxic effects. Hydrogen cyanide has a strong affinity for Fe3+ ions, which can inhibit the cytochrome oxidase enzyme system and disrupt the transfer of oxygen from oxyhemoglobin to body tissues (10, 21). Amygdalin is a notable example of a natural cyanide compound and is classified as a cyanogenic glucoside, which is a sugar molecule linked to cyanide. When amygdalin is ingested, the enzyme β-glucosidase in the gastrointestinal tract metabolizes it into hydrogen cyanide, causing toxicity (22). This mechanism may explain the occurrence of NEC in neonates.
Cotonefurans have antifungal effects that can alter the gut microbiome (23). Carbohydrates are an important component of purgative manna. Mannitol is the main sugar in the Cotoneaster plant. Mannitol, fructose, glucose, and sucrose are the primary substances found in purgative manna, with mannitol accounting for about 40 to 60 percent of its composition (24). Mannitol, a non-absorbable sugar alcohol, functions as an osmotic diuretic and is only minimally absorbed after oral intake. By increasing osmolarity in the gut, it acts as an osmotic laxative. Consequently, there is an increase in fluid retention in the bowel, which may result in the evacuation of the meconium content of the colon (25).
The most commonly reported adverse events (AEs) include mild changes in serum electrolytes, nausea, and abdominal pain, all of which resolve on their own. Other adverse events include vomiting and abdominal distension (26). Intravenous injection of mannitol can worsen electrolyte abnormalities by causing a shift of free water into the intravascular space. These abnormalities may lead to hyponatremia, hypokalemia, and hypocalcemia (27).
The excretion of bilirubin in meconium was measured to be between 29.2 and 90.8 mg per sample (28). Therefore, mannitol decreases the enterohepatic cycle of bilirubin. As previously stated, mannitol can cause dehydration due to increased gut osmolality, particularly in the early days after birth when breastfeeding may be challenging for the newborn. Despite numerous studies supporting the effectiveness of Cotoneaster on neonatal jaundice (29), our research, like that of Boskabadi et al. (30), contradicts this hypothesis. While the mentioned studies only examined mannitol's laxative effects, neonatal jaundice has multiple causes. In contrast, lactose in breast milk acts as a natural laxative in a controlled manner, without complications.
Potential side effects of phototherapy may encompass temporary erythematous rashes, diarrhea, and elevated fever (4); however, when compared to the use of Cotoneaster, these effects result in fewer complications in neonates. Although a case-control study can provide more accurate results, our findings in clinical practice suggest to clinicians that while Cotoneaster may have potential therapeutic benefits, its side effects are not well-characterized compared to traditional phototherapy, which has a well-established safety profile. In clinical practice, this discrepancy emphasizes the need for further research into herbal treatments and careful consideration of patient safety and treatment efficacy, especially in neonates. Integrating such treatments should be done cautiously and based on solid evidence.
5.1. Conclusions
The most common complications following Cotoneaster derivatives are exaggerated hyperbilirubinemia, dehydration, poor feeding, NEC, diarrhea, hypernatremia, and vomiting. Given the sensitivity of neonates, jaundice treatment should be approached with caution and based on extensive studies. The use of herbal medicines in neonates is questionable due to these complications. Future studies with long-term effects, other herbal medicines, and different ages of infants are necessary to obtain more accurate results.