1. Background
Nanotechnology involves the manipulation of materials at the atomic, molecular, and supramolecular levels. The initial, widely recognized definition of nanotechnology (1) focused on the specific goal of precisely controlling atoms and molecules to create products on a larger scale. Nanoparticles are synthesized using chemical processes that often involve hazardous chemicals (2) and reducing agents. By reducing the size of materials to the nanoscale, various properties such as melting point, chemical properties, boiling temperature, magnetic properties, light absorption, catalytic activity, and thermal conductivity are altered. When the particle size of a material is reduced to a specific dimension, its properties are influenced not only by its structure (3) and composition but also by its dimensions. Metal nanoparticles (MNPs) are enveloped by a self-generated monolayer (4), which has led to their expanded utilization in fields such as adjustable optical devices, sensor technologies, and drug delivery methods (5).
Nanoparticle drug delivery systems (DDSs) are engineering technologies that use nanoparticles for targeted delivery and production control of therapeutic drugs. A modern DDS should minimize side effects and reduce drug dosage. Currently, nanoparticles are extensively utilized in drug delivery applications. Drug delivery systems are employed to enhance the therapeutic properties encapsulated within a drug as a reservoir. These structures enable controlled and sustained drug release, protection of the drug molecule, particle sizes smaller than cells, the capability to traverse biological barriers for targeted drug delivery to specific sites, improved drug stability in the bloodstream, targeted drug delivery, and enhanced bioavailability. Compatibility plays a crucial role in determining the effectiveness of a drug delivery system, thereby influencing pharmacokinetics and drug distribution within the body.
Over the past fifty years, advancements in polymer science, chemistry, biology, as well as mechanical and physical sciences have introduced various categories of carriers to the field of medical sciences, each possessing unique characteristics and performance capabilities (6, 7). For instance, eco-friendly materials can be employed to enhance molecule dissolution by creating a specific pH, while certain polymers can boost water solubility by absorbing water. Liposomes, for example, are utilized in drug delivery for individuals with HIV, delivering substances like siRNA to human T-cells (8), as well as in the treatment and administration of anticancer drugs (9), fungicides (10), antiparasitic drugs (11), antibacterial drugs (12), and antiviral drugs (13).
The most common applications of nanocrystalline silver particles (AgNPs) are in photography, catalysis, and as antibacterial agents. The first precursor for the synthesis of silver crystals is silver nitrate (AgNO3), which has good stability in polar solvents. Light sensitivity is important in experiments with AgNO3, and neglecting it can cause changes in the structure of silver nanocrystals. Therefore, storage and maintenance of AgNO3 should be conducted under controlled conditions. The purpose of this study is to investigate and synthesize medicine with the help of AgNPs to achieve better therapeutic effects. Additionally, with the progress of science, the use of various drugs to treat diseases has decreased due to side effects, and medical science is seeking new solutions such as drug carriers.
2. Objectives
The green synthesis of reactive AgNPs was conducted with omeprazole (OMP) and omeprazole sulfide (OMPS). The synthesized AgNPs were characterized using various analytical tools, and their antibacterial properties were tested under different conditions.
3. Methods
3.1. Materials
Pure OMP and OMPS, along with sodium borohydride (NaBH4, 99%), AgNO3 (99.9%), and polyvinylpyrrolidone (PVP), were procured from Merck. The Fourier transform infrared spectroscopy (FT-IR) spectra of the samples were acquired using a spectrum two spectrophotometer. The morphology and particle sizes of the synthesized powder were analyzed through transmission electron microscopy (TEM) images captured on a Zeiss EM10C instrument operating at an accelerating voltage of 100 kV. X-ray diffraction (XRD) patterns were recorded using an X'Pert Pro X-ray diffractometer with Cu-Kα radiation. UV-Visible (UV-Vis) absorption spectra in the range of 200 - 500 nm in ethanol were measured using a Shimadzu UV-2550 spectrophotometer.
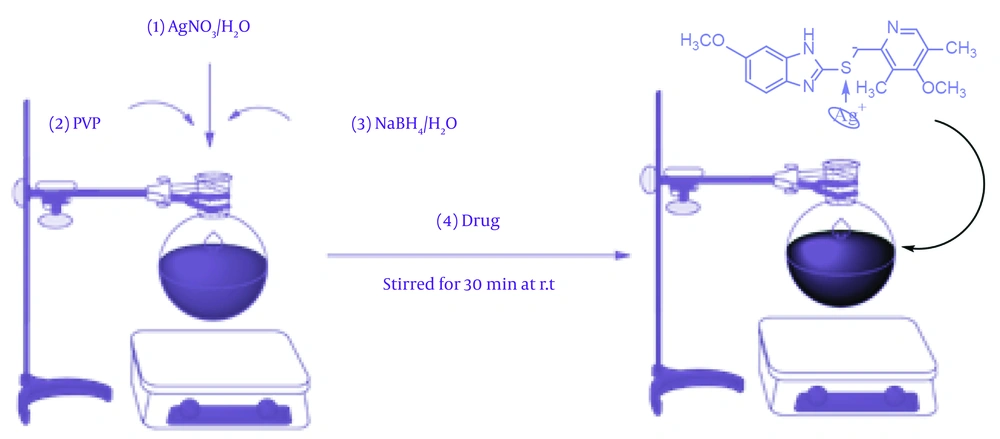
3.2. Synthesis of Nanoparticles with Omeprazole and Omeprazole Sulfide (One-Pot)
A solution containing 0.082 g of AgNO₃ in 10 mL of water was combined with 0.03 g of PVP in 5 mL of water. This mixture was then placed in an ice bath for 30 minutes. Subsequently, the solution was removed from the ice bath, and 0.018 g of NaBH4 was added dropwise over a period of 30 minutes, resulting in a sudden color change from yellow to brown and black. After the complete addition of NaBH4, 0.331 mmol of OMP in 10 mL of ethanol was introduced to the solution and allowed to react for 4 hours. Following the completion of the reaction time, the solution was centrifuged and washed with distilled water to remove impurities and excess ethanol. The resulting precipitate was separated, dried, and prepared for spectral analysis. The suspension was centrifuged at 10,000 rpm for 15 minutes, and the resulting precipitate was washed three times with double-distilled water to eliminate any water-soluble impurities. Subsequently, the precipitate underwent three washes through dispersion and centrifugation using ethanol to remove any excess OMP and reducing agent. The precipitate was then dried in an oven at 60°C for 10 hours, resulting in the acquisition of a pale grey powder with a yield of 92% (14, 15).
Figure 1 shows a proposed mechanism for the synthesis of Ag@OMPS, which begins with an electrophilic reaction involving Ag⁺ and the oxygen of PVP, leading to the generation of a zwitterionic intermediate. Sodium borohydride acts as a blocking agent to prevent silver accumulation. This zwitterionic intermediate is prepared to deposit its silver ion in a one-way reaction as an electrophile against the sulfur of OMP. Finally, the silver-sulfur bond is formed in the final product.
4. Results
In continuation of our focus on the design and synthesis of chemically and biologically active heterocycles (14, 16), this study aimed to covalently functionalize AgNPs with OMP. UV-visible spectroscopy, XRD, and TEM analyses confirmed the connection of silver with the drug. Both OMP and OMPS, as well as a combination of the two drugs, were applied to AgNPs. The preparation of Ag@OMP involved the addition of AgNO3 in ultrapure water and starch to a round-bottom flask. Subsequently, an excess amount of NaBH4 was added as a reducing agent. The molar ratio of metal to reducing agent was chosen as 1:2 to reduce all Ag⁺ particles to metallic silver. An ethanol solution of OMP was then introduced into the reaction vessel, with the sulfur of OMP strongly binding to the surface of AgNPs. The formation of Ag@OMP was verified through analysis to confirm the association of silver with the drug. Both OMP and OMPS, along with a combination of the two drugs, were incorporated onto AgNPs in this mixture.
4.1. Fourier Transform Infrared Spectrometer
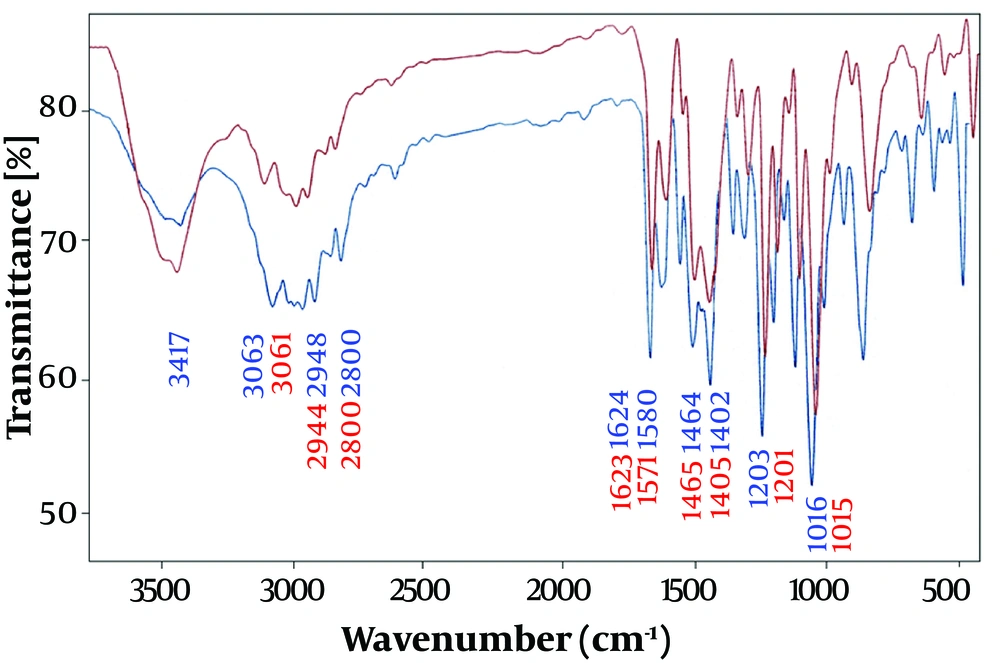
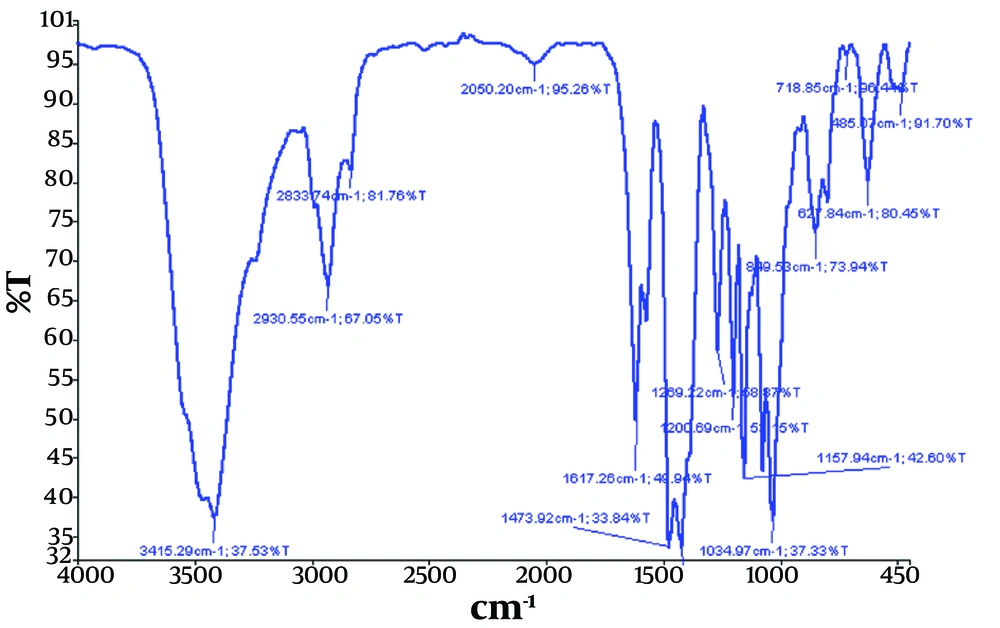
The interaction of the drug OMP with the surface of AgNPs and the synthesis of Ag@OMP nano drug were investigated by recording and comparing the Fourier transform infrared spectra of OMP and Ag@OMP. Figure 2 shows the infrared spectrum of OMP (lower-blue band) and Ag@OMP (upper-red band).
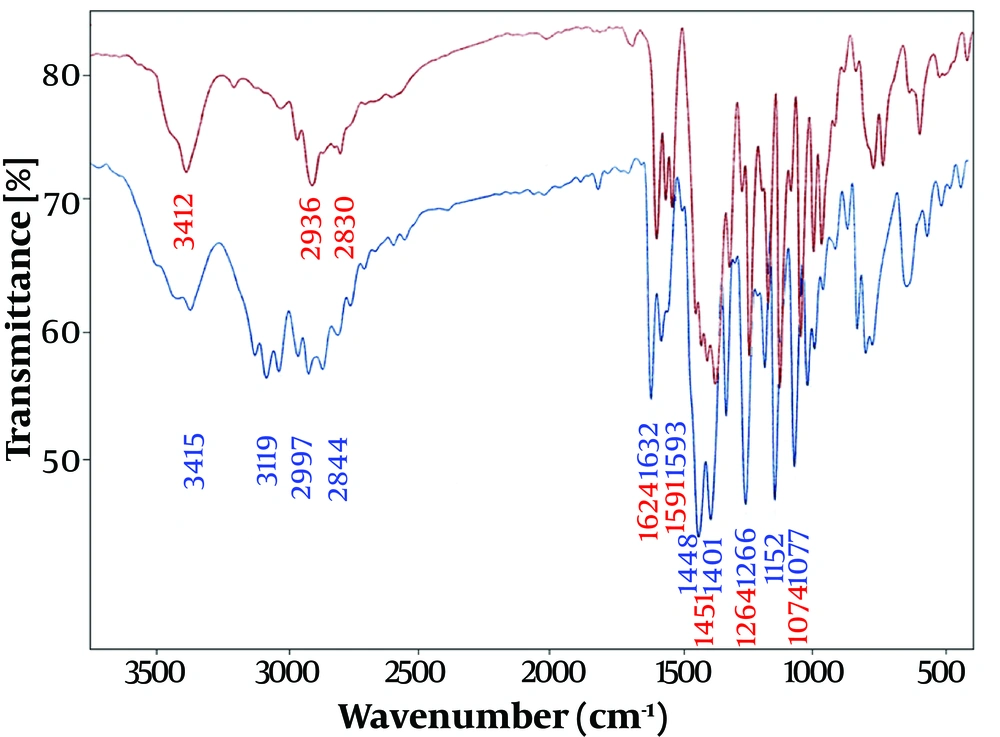
The interaction of the drug OMPS with the surface of AgNPs and the synthesis of the nano drug Ag@OMPS are illustrated in Figure 3.
Additionally, the interaction of the two drugs, OMP and OMPS, with the surface of AgNPs and the synthesis of the Ag@omeprazole-omeprazole sulfide (Ag@OMP-OMPS) nanodrug were investigated by recording the Fourier transform infrared spectrum (Figure 4).
Therefore, in Figures 2, 3, and 4, the appearance of similar signals with changes in their position and intensity confirms the successful binding of drugs to the surface of AgNPs, as recorded in Table 1 below.
| FT-IR (cm-1) | CH-Aromatic Stretching Vibrations | CH2 Aliphatic Stretching Vibrations | C=C Symmetric Stretching Vibrations | C=C Asymmetric Stretching Vibrations | C-H Aliphatic Stretching Vibrations | N-H Stretching Vibrations | S=O Stretching Vibrations | C-O Stretching Vibrations | C=N Stretching Vibrations |
|---|---|---|---|---|---|---|---|---|---|
| OMP | 1402 | 1464 | 1508 | 1580 | 2800 - 2948 | 3417 | 1075 | 1015 | 1624 |
| Ag@OMP | 1405 | 1465 | 1509 | 1571 | 2800 - 2944 | 3417 | 1074 | 1016 | 1623 |
| OMPS | 1401 | 1448 | - | 1591 | 2844 - 2997 | 3415 | - | 1264 | 1623 |
| Ag@OMPS | 1401 | 1451 | - | 1593 | 2830 - 2936 | 3412 | - | 1266 | 1624 |
| Ag@OMP-OMPS | 1424 | 1473 | - | 1598 | 2833 - 2930 | 3415 | 1200 | 1269 | 1617 |
Abbreviations: FT-IR, Fourier transform infrared spectroscopy; OMP, omeprazole; AgNPs, nanocrystalline silver particles; Ag@OMP, omeprazole@AgNPs; OMPS, omeprazole sulfide; Ag@OMPS, omeprazol sulfide@AgNPs; Ag@OMP-OMPS, Ag@omeprazole-omeprazole sulfide.
4.2. X-ray Diffraction
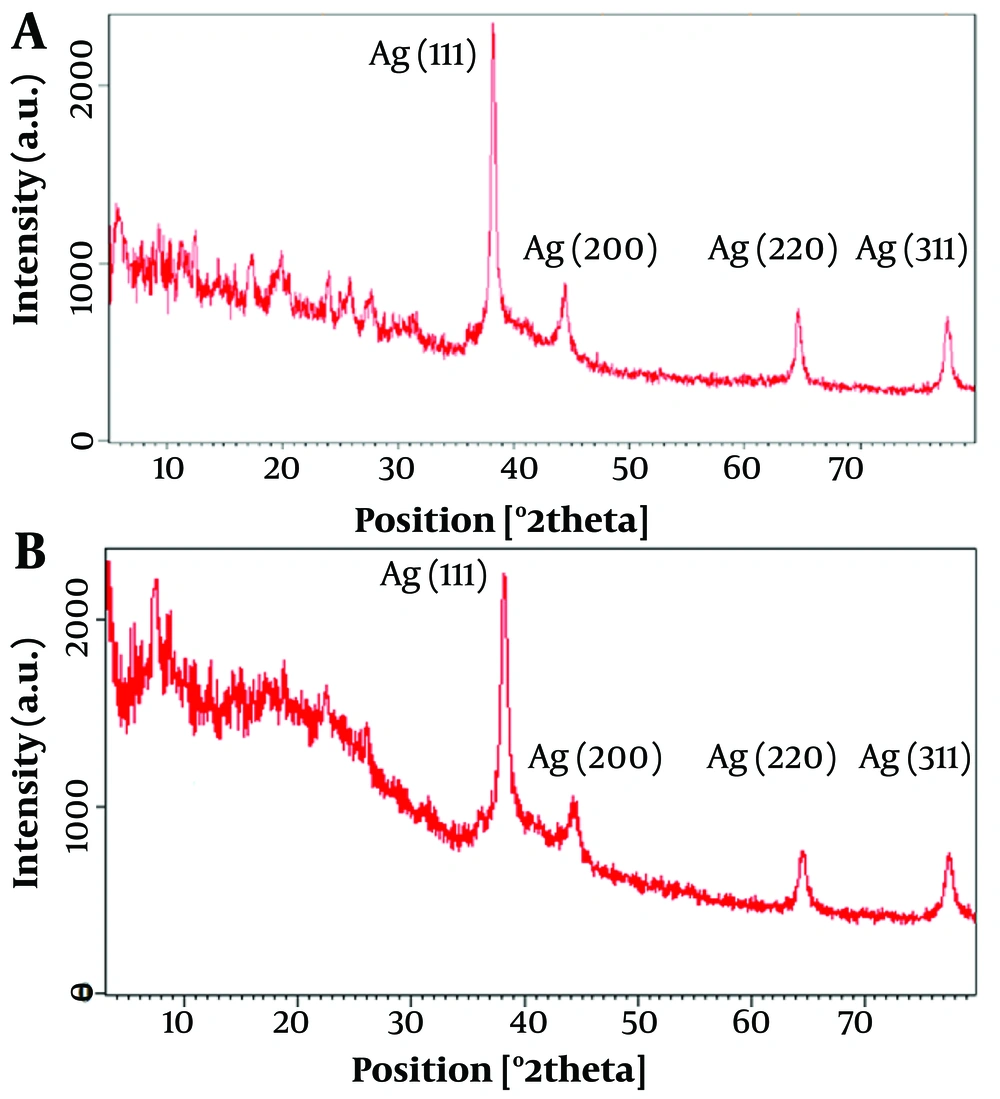
The powder XRD patterns of Ag@OMP (A) and Ag@OMPS (B) are depicted in Figure 5. In Figure 5A, the peak position at 2θ = 38° - 80° corresponds well with Ag@omeprazole. In Figure 5B, the distinctive peaks of AgNPs were observed at 2θ = 38° - 80°, identified as (111), (200), and (220) reflection lines of the face-centered cubic (FCC) structure of metallic silver. The average crystallite size, D, was determined using Scherrer's equation to be 58 nm in Ag@OMP (Figure 5A) and 28 nm in Ag@OMPS (Figure 5B), where D = Kλ/(β cosθ), with λ representing the wavelength of Cu-Kα radiation used (λ = 1.54 Å), β as the full width at half maximum (0.31) of the diffraction peak, θ as the Bragg angle for the measured peak, and K as a constant equal to 0.94. This value aligns well with the TEM image. The intensity of peaks indicates the high degree of crystallinity of the AgNPs.
4.3. UV-Visible Absorption Spectra
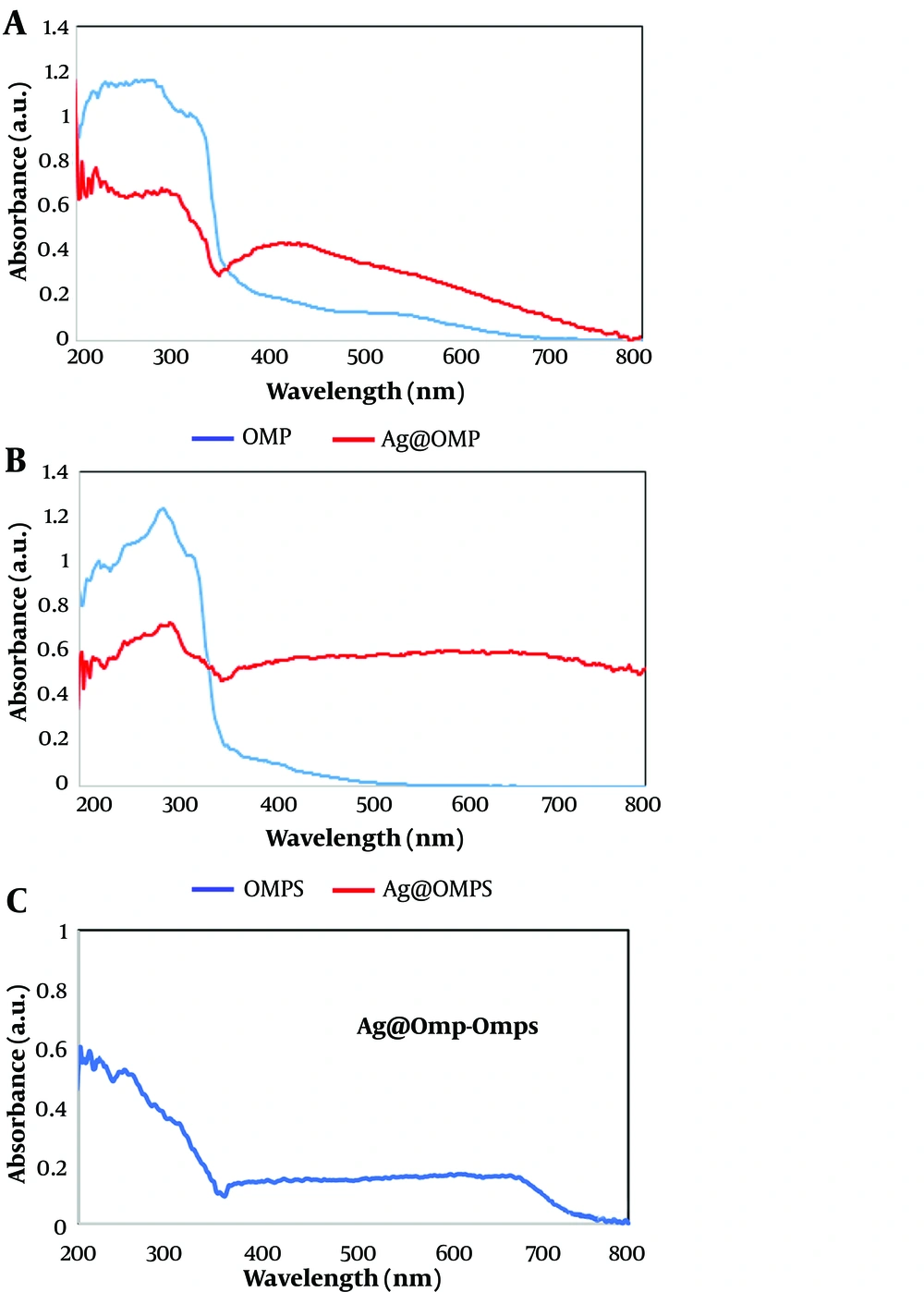
Figure 6 outlines the UV-Vis absorption spectra of Ag@OMP and Ag@OMPS in an ethanol solution. The pure OMP appears with a λmax at 400 nm, corresponding to the n→π* transition of the O-OMP molecule, while a solution of Ag@OMP shows a λmax at 300 nm, indicating the surface plasmon resonance absorption band (SPRAB) of AgNPs. Similarly, the pure OMPS appears with a λmax at 400 nm, corresponding to the n→π* transition of the S-OMPS molecule, whereas a solution of Ag@OMPS shows a λmax at 300 nm.
In the final shape, the UV-Vis absorption spectra of Ag@OMP-OMPS in an ethanol solution show a λmax at 400 nm, corresponding to the n→π* transition of the O-OMP and S-OMPS molecules and the SPRAB of AgNPs. Compared to the absorption band of the free drugs at 400 nm, this indicates a lower concentration of drugs on AgNPs. The appearance of two new peaks at 300 nm is related to the Ag-drugs complex.
4.4. Transmission Electron Microscopy
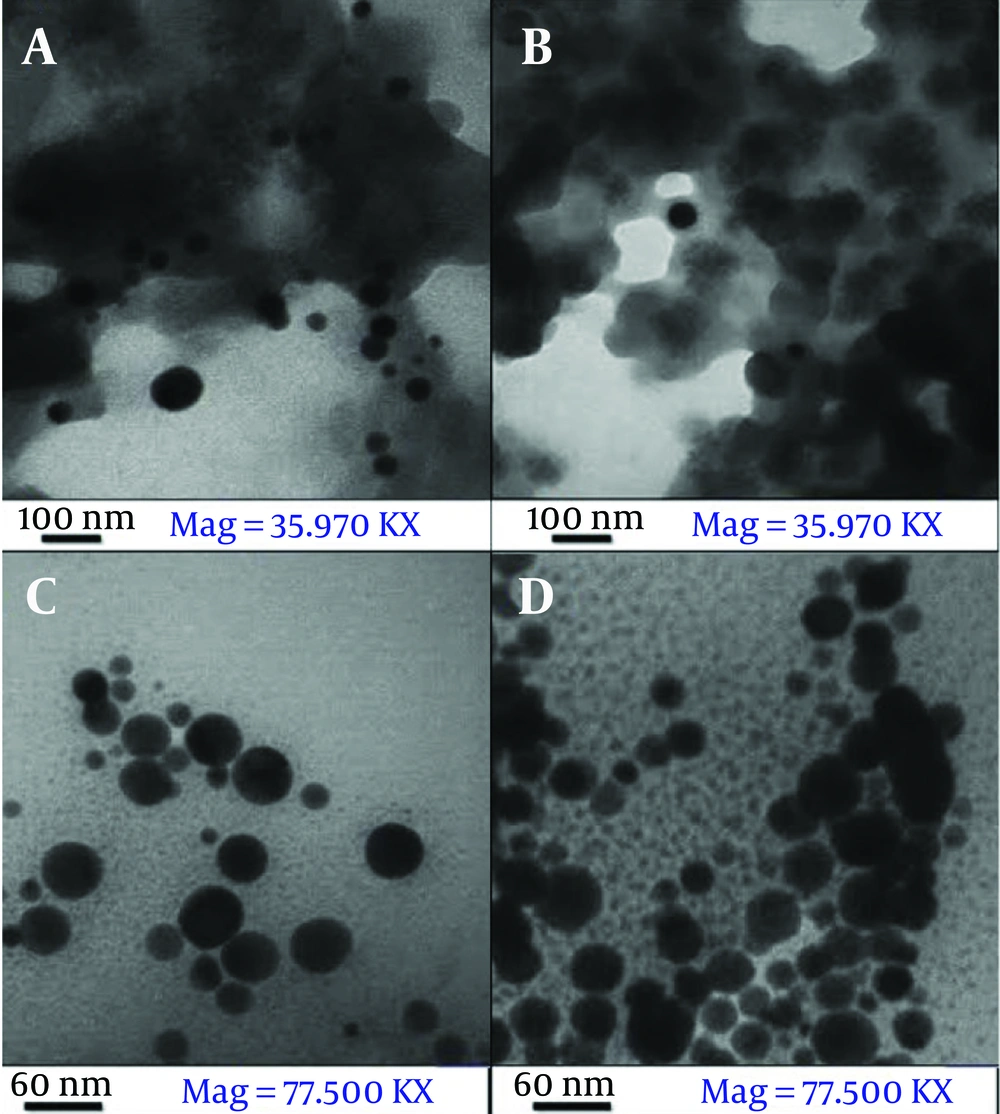
The TEM images of Ag@OMP (Figure 7A and B) reveal that the particles are spherical in shape with an approximate size of less than 40 nm; Scherrer's calculation indicates a size of 28 nm.
The TEM images of Ag@OMPS (Figure 7C and D) reveal that the particles are spherical in shape with an approximate size of less than 60 nm; Scherrer's calculation indicates a size of 58 nm.
4.5. Antibacterial Properties
In the experiment, Mueller Hinton agar, tubes containing half McFarland standard, tubes containing DMSO, antibiogram discs, and pure bacteria in a suitable culture medium were utilized. The process involved using a swab, loop, flame, hood, and lamp. The bacterial strains Pseudomonas, Staphylococcus, Escherichia coli, Bacillus, Enterococcus, and Micrococcus were employed. Various studies have investigated the therapeutic effects of OMP in combination with antibiotics for patients with duodenal ulcers and Helicobacter pylori infection (17). In this particular study, the antibacterial activities of Ag@OMP, Ag@OMPS, and Ag@omeprazole- Ag@OMP-OMPS were assessed using the minimum inhibitory concentration (MIC) and disc diffusion methods.
4.5. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
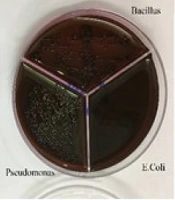
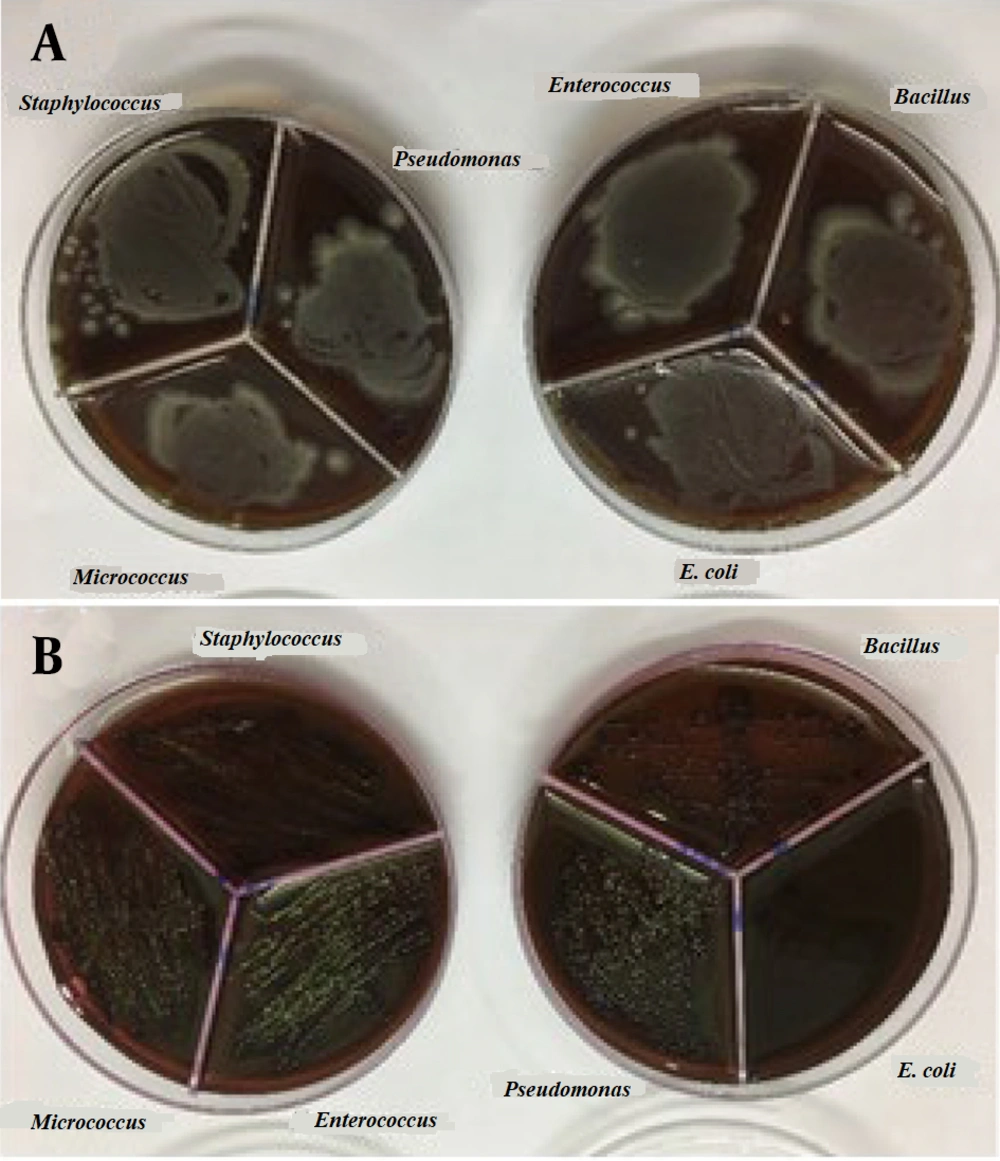
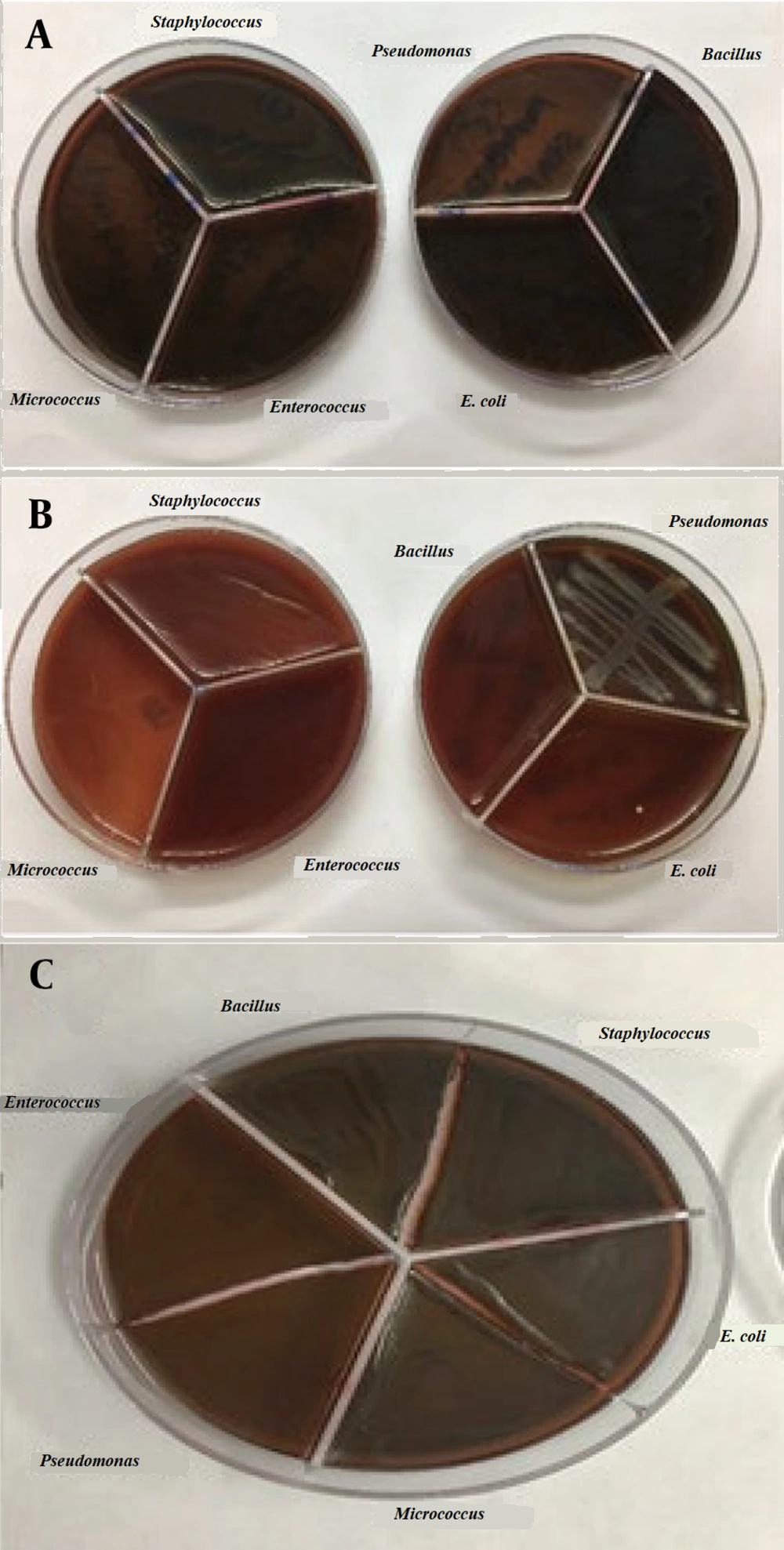
To prepare the drug reference tube, 1 g of Ag@omeprazole (Ag@OMP) was dispersed in 5 mL of DMSO. For determining the minimum MIC, ten tubes containing 1 mL of trypticase soy broth (TSB) were used. Initially, 1 mL of the drug reference tube was added to the first tube. Subsequently, 1 mL from the first tube was transferred to the second tube, and this process was repeated until the tenth tube, from which the final 1 mL was selected. Each tube was then inoculated with 5 × 10⁵ to 10⁶ colony-forming units (CFU)/mL of bacteria and incubated at 37°C for 18 - 24 hours (18). Gram-positive bacteria (Staphylococcus aureus ATCC 27853, Bacillus subtilis ATCC 1715, Micrococcus luteus ATCC 1408) and gram-negative bacteria (Pseudomonas aeruginosa ATCC 1310, E. coli ATCC 25922, and Enterococcus faecalis ATCC 29212) were used in this experiment. Subsequently, each tube was streaked and incubated on an agar plate at 37°C for 18 - 24 hours. Due to the turbidity observed in the test tubes during the MIC and minimum bactericidal concentration (MBC) methods, which made it difficult to determine the lowest drug concentration inhibiting bacterial growth, the results obtained from this test were presented in the form of a graph or report. The results of these tests can be seen in Figure 8A and B.
In another experiment, as shown in Figure 9A-C, following the cultivation of bacteria in a pure agar medium, silver OMP and silver OMPS were added to the agar medium. The combination of silver and the drug resulted in limited growth in some cases and no growth in other bacterial media, as depicted in the accompanying images.
4.6. Disc Diffusion
To determine the inhibition zone for the microbes used in this study, the disc diffusion method (19) was employed. Initially, bacterial cultures of 0.5 McFarland units were prepared and spread on agar plates. Subsequently, the discs were loaded with 30, 40, 50, and 60 µL from the reference tube. Finally, the plates were incubated at 37°C for 18 - 24 hours. It was observed that OMP and OMPS alone did not inhibit the tested bacteria; however, their combined effect with silver was noticeable. The larger the diameter of the non-growth halo, the more effective the drug performance. Table 2 indicates that in the Ag@OMP-OMPS test, a dilution of 0.2 is the lowest concentration in the growth halo.
| Drugs | Density | Disk Diffusion Zone Inhibition Produced by Drugs (mm) | |||||
|---|---|---|---|---|---|---|---|
| Pseudomonas | Staphylococcus | Basilus | Enterococcus | Escherichia coli | Micrococcus | ||
| OMP | 0.4 | - | - | 4 | - | - | 3 |
| 0.5 | - | - | - | - | 3 | - | |
| OMPS | 0.4 | - | 3 | 5 | - | - | 4 |
| 0.5 | - | - | - | - | 4 | - | |
| Ag@OMP | 0.4 | - | - | 4 | - | - | 4 |
| 0.5 | - | 4 | - | - | 4 | - | |
| Ag@OMPS | 0.4 | - | - | 5 | - | - | 5 |
| 0.5 | - | 4 | 3 | - | 3 | - | |
| Ag@OMP-OMPS | 0.4 | 4 | 4 | - | - | - | 7 |
| 0.5 | - | 6 | 4 | - | 4 | - | |
Abbreviations: OMP, omeprazole; OMPS, omeprazole sulfide; AgNPs, nanocrystalline silver particles; Ag@OMP, omeprazole@AgNPs; Ag@OMPS, omeprazol sulfide@AgNPs; Ag@OMP-OMPS, Ag@omeprazole-omeprazole sulfide.
The MIC and MBC in this study were not desirable because the solution inside the tubes was turbid, making detection infeasible.
5. Discussion
At a glance, the synthesis of AgNPs with the drugs OMP and OMPS was successful, demonstrating enhanced antibacterial properties and improved drug characteristics. Omeprazole sulfide exhibited superior antibacterial properties compared to OMP silver, with the combination of both drugs showing moderate effectiveness. The study revealed that the drugs alone did not possess antibacterial effects, but when combined with nanosilver, they exhibited enhanced efficacy.
The interaction of the drug OMP with the surface of AgNPs and the synthesis of the Ag@OMP nanodrug were investigated by recording and comparing the Fourier transform infrared spectra of OMP (lower-blue band) and Ag@OMP (upper-red band). Additionally, the interaction of the drug OMPS with the surface of AgNPs and the synthesis of the nanodrug Ag@OMPS were confirmed in Figures 2, 3, and 4. The appearance of similar signals with changes in their position and intensity proves the successful binding of drugs to the surface of AgNPs.
The powder XRD patterns of Ag@OMP (a) and Ag@OMPS (b) depicted peak positions at 2θ = 38° - 80°, which correspond well with Ag@OMP. The distinctive peaks of AgNPs were observed at 2θ = 38° - 80°, identified as (111), (200), and (220) reflection lines of the FCC structure of metallic silver. The TEM images of Ag@OMPS reveal that the particles are spherical in shape with an approximate size of less than 60 nm; Scherrer's calculation indicates a size of 58 nm.
These experiments showcase the potential for developing new nanodrugs and enhancing the antimicrobial properties of existing medications, aligning with advancements in nanomedicine production and aiding in the treatment of various medical conditions.
5.1. Conclusions
The green synthesis of AgNPs with OMP and OMPS has been investigated for their antibacterial properties. The nanoparticles were characterized using various techniques. The results indicate that the size of OMP nanoparticles is less than 40 nm, while the length of OMPS nanoparticles is less than 60 nm. Omeprazole@AgNPs and Ag@OMPS have demonstrated antibacterial properties, with the UV-Vis spectrum showing distinct peaks for the different arrangements. Overall, the study demonstrates the potential of utilizing AgNPs in combination with OMP and OMPS for enhanced antibacterial activities.