1. Background
Preeclampsia is one of the most serious complications of pregnancy, affecting 3 - 8% of pregnancies and causing more than 60,000 maternal deaths annually (1, 2). It leads to various maternal and fetal complications. Maternal complications include eclampsia, liver, kidney, cardiovascular, and cerebrovascular disorders, cesarean section, labor induction, placental abruption, and maternal death. Fetal complications include low Apgar scores, decreased heart rate, cerebral palsy, intracerebral hemorrhage, retinopathy of prematurity, chronic lung disease, and death (3). Recent studies also suggest that oxidative stress plays a significant role in the pathogenesis of preeclampsia, highlighting the importance of antioxidant therapies in mitigating these complications (4).
Currently, the only effective treatment that can prevent the progression of preeclampsia is the termination of pregnancy (5). If a low-cost and safe treatment were available to control the progression of preeclampsia, doctors could safely delay delivery and allow the pregnancy to continue, providing time for fetal recovery. This could save the lives of many babies and reduce the hospital burden caused by prematurity. Such an approach would align with the United Nations Millennium Development Goals of reducing child mortality and improving maternal health (6). Additionally, advancements in pharmacological interventions targeting specific pathways involved in preeclampsia could potentially transform the management of this condition, offering new hope for affected mothers and their babies (7).
Esomeprazole, a proton pump inhibitor, is widely used to treat women with gastric reflux during pregnancy. Studies have shown that the administration of this drug in the first, second, and third trimesters has not been linked to adverse pregnancy outcomes, including teratogenesis (8-10). Esomeprazole significantly reduces the secretion of sFlt-1 and sEng from placental and endothelial cells, has a potent action in reducing endothelial dysfunction, and possesses antioxidant properties (11). Proton pump inhibitors reduce the adhesion of vascular cells caused by TNF-α molecule-1 (VCAM-1) and decrease the expression of endothelin-1 (ET-1), which constricts endothelial vessels (12).
Cluver et al. showed that daily esomeprazole (40 mg) did not prolong pregnancy in early preeclampsia and did not reduce sFlt-1 concentrations (13). However, Atwa et al. concluded that esomeprazole is associated with prolonging pregnancy in women with premature preeclampsia (14). The above data suggest that esomeprazole may be effective in the treatment of preeclampsia.
2. Objectives
Since there have not been enough clinical studies on this subject, this study was conducted to determine the effect of oral esomeprazole in the treatment of preeclampsia in pregnant mothers.
3. Methods
Pharmacokinetic data indicated that a daily dosage of 40 mg was selected, as it has no adverse effects when administered during pregnancy. This study has been registered in Iran's clinical trial system under the code IRCT20220928056050n1 and was approved by the Ethics Committee of Kurdistan University of Medical Sciences, with ID IR.MUK.REC.1401.194. Informed consent was obtained from all participating women.
In 2023, expectant women with preeclampsia who were referred to Ba'ath Sanandaj Hospital comprised the study's statistical population. The inclusion criteria were: Age between 18 and 45 years, gestational age between 26 and 32 weeks, fetal weight between 500 and 1800 grams, and no fetal abnormalities. The exclusion criteria included the need to terminate pregnancy, eclampsia, chronic hypertension, cerebrovascular problems, kidney disorders, pulmonary edema, severe ascites, heart failure, disseminated intravascular coagulation, fetal distress, prior use of proton pump inhibitors during pregnancy, sensitivity to proton pump inhibitor drugs, and contraindications for proton pump inhibitor use.
3.1. Sample Size and Sampling Method
The sample size for this study was 120 participants (60 in each group), similar to Cluver's study, and the block sampling method was used (13).
3.2. Study Groups
After approval by the ethics committee of Kurdistan University of Medical Sciences, participants were randomly allocated into two groups esomeprazole and placebo using random blocks of four.
3.3. Participants
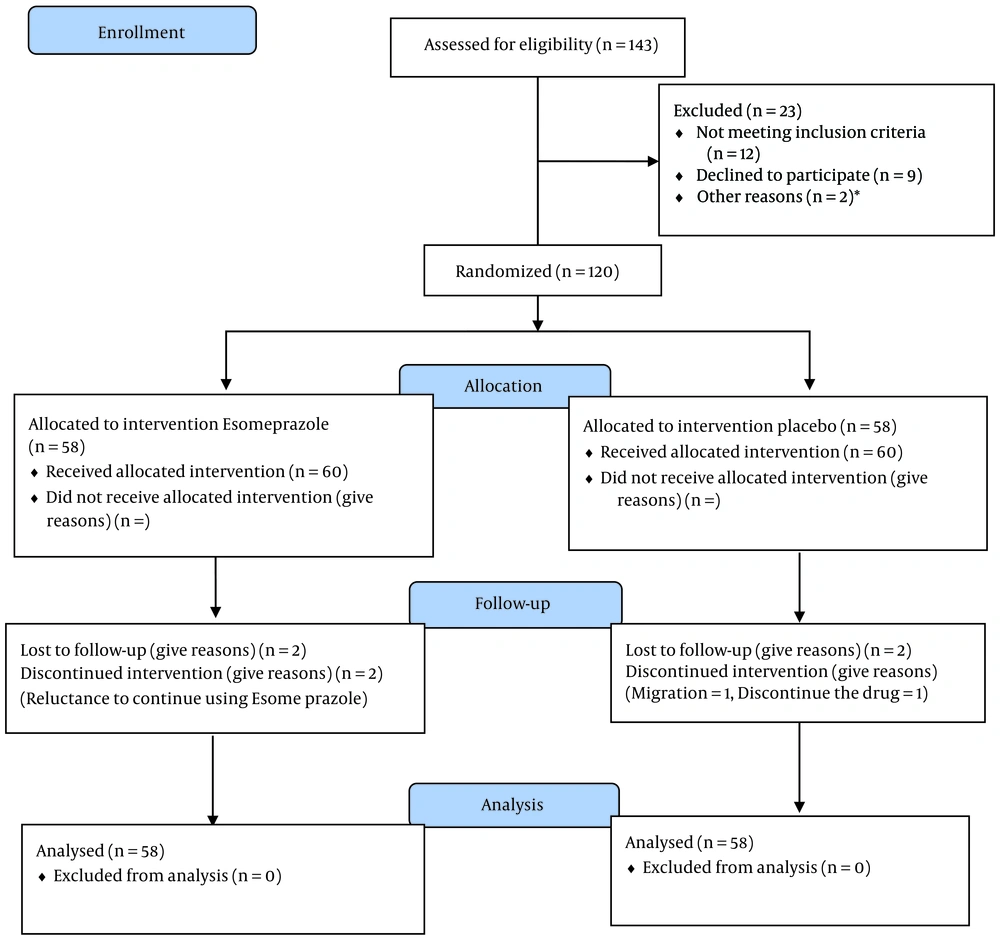
Participants were enrolled from January 2023 to December 2023. Out of the 120 women considered eligible, 4 refused to participate. Sixty women were assigned to the placebo group; one participant dropped out due to migration, and another dropped out due to unwillingness to take the drug. In the esomeprazole group, 2 participants stopped taking the drug, leaving 58 participants remaining (Figure 1).
Other reasons could involve personal or logistical factors that were not explicitly documented but did not meet the primary exclusion criteria.
3.4. Follow-up Period
Patients were followed from the time pregnant mothers with preeclampsia were admitted until the delivery and the final condition of the baby.
3.5. Primary outcome
The primary outcome was the investigation of the prolongation of pregnancy duration associated with the use of esomeprazole (40 mg daily).
3.6. Secondary outcomes
- Maternal outcomes: Maternal death, eclampsia, pulmonary edema, renal failure, liver hematoma, proteinuria (more than 3 g/24 hours), creatinine levels above 125 micromole/L, placental abruption, severe hypertension, use of blood pressure medication, excessive bleeding during delivery, thromboembolism, moderate or severe ascites, and type of delivery.
- Fetal outcomes: Fetal death, fetal growth restriction, changes in fetal heart rate.
- Infant outcomes: Infant death, intraventricular hemorrhage, necrotizing enterocolitis, pulmonary dysplasia, Apgar score, infant weight, neonatal intensive care, and neonatal sepsis.
3.7. Blinding
This study was conducted in a double-blind manner, ensuring that neither the patients nor the presenter knew the type of treatment received in the groups. Both drugs were placed in identical packages, and neither the patients nor the evaluating doctor were aware of the contents of packages A and B.
3.8. Intervention
First, women's pregnancy information, including age, body mass index, and number of pregnancies, was recorded. Based on the sampling blocks, participants were placed in either the intervention (esomeprazole) or control (placebo) group.
In the intervention group, women were administered 40 mg of esomeprazole capsules (manufactured by Iran's Hakim Pharmaceutical Company) once daily, while the placebo group received 40 mg of placebo capsules once daily.
Management of preeclampsia included hospitalization and careful maternal and fetal care. Maternal care involved measuring blood pressure every four hours, conducting clinical evaluations twice daily, performing daily urinalysis, and evaluating blood tests twice weekly. These tests included a full blood count, kidney function tests, and liver enzymes, especially if there was suspicion of hemolysis, elevated liver enzymes, and low platelet syndrome (HELLP). Additionally, 24-hour urine protein levels were monitored until delivery.
During hospitalization, fetal care included ultrasound assessments to evaluate fetal growth, amniotic fluid index, and overall fetal health, which involved Doppler studies of the umbilical artery, vena cava, and middle cerebral artery. If there were no signs of fetal growth restriction or fetal distress, ultrasound was repeated twice weekly to monitor fetal growth. If signs of growth restriction or fetal distress were observed, ultrasound monitoring was conducted more frequently.
To reduce the risks of neonatal respiratory distress syndrome, intracranial hemorrhage, and necrotizing enterocolitis, all participants received two doses of betamethasone 24 hours apart. A repeat dose was administered one week later. Most participants were treated for hypertension until systolic blood pressure was stabilized between 140 and 150 mmHg, and diastolic blood pressure between 90 and 100 mmHg.
Clinical care was left to the discretion of the clinical team. The indication for delivery was based on clinical judgment. Reasons for delivery included uncontrolled blood pressure, the occurrence of significant maternal or fetal complications, or intrauterine fetal death.
3.9. Statistical Analysis
After collecting and entering the data into SPSS software version 23, frequency and percentage tables, along with graphs, were used to describe qualitative variables, while the mean and standard deviation were used for quantitative variables. For qualitative analyses, the chi-square test and Fisher's exact test were employed, while the independent t-test (or Mann-Whitney U test) was used to compare quantitative variables between the two groups. The level of significance in this study was set at P < 0.05.
4. Results
The mean age, Body Mass Index, and gestational age of mothers in the two groups, esomeprazole and placebo, showed no statistically significant differences (P > 0.05). Similarly, systolic and diastolic blood pressure, hemoglobin levels, platelet count, urea and creatinine levels, and the protein-to-creatinine ratio measured 24 hours before the start of the intervention were not significantly different between the esomeprazole and placebo groups (P > 0.05). The two groups also showed no statistically significant difference in terms of a history of high blood pressure in previous pregnancies or lack of blood flow in the umbilical artery. The average fetal weight in the esomeprazole group was 1644 ± 148 grams, while in the placebo group, it was 1666 ± 113 grams, which was not a statistically significant difference (Table 1).
| Characteristics | Esomeprazole (n = 59) | Placebo (n = 60) | P-Value |
|---|---|---|---|
| Gestation at randomization (week) | 29.4 ± 2.0 | 29.8 ± 1.9 | 0.24 |
| Maternal age (y) | 29.7 ± 7.4 | 30.1 ± 5.9 | 0.26 |
| Body Mass Index (kg/m2) | 27.1 ± 4.9 | 26.6 ± 5.2 | 0.67 |
| Systolic blood pressure (mmHg) | 129.7 ± 10.8 | 131.1 ± 5.9 | 0.73 |
| Diastolic blood pressure (mmHg) | 85.1 ± 8.6 | 85.9 ± 6.3 | 0.54 |
| Hemoglobin (g/dL) | 12.4 ± 1.1 | 12.6 ± 1.0 | 0.42 |
| Platelet count (109/L) | 173.5 ± 49.3 | 166.5 ± 55.8 | 0.48 |
| Urea (mmol/L) | 18.8 ± 4.3 | 18.6 ± 4.1 | 0.81 |
| Creatinine (mg/dL) | 0.88 ± 0.15 | 0.91 ± 0.18 | 0.32 |
| 24-Hour protein creatinine ratio (g/24 hr) | |||
| Estimated fetal weight (g) | 1644 ± 148 | 1666 ± 113 | 0.37 |
| Nulliparous | 17 (29.3) | 18 (31.0) | 0.78 |
| History of hypertension in previous pregnancy | 15 (25.9) | 14 (24.1) | 0.83 |
| Absent blood flow on umbilical artery Doppler | 1 (1.7) | 2 (3.4) | - |
a Values are expressed as mean ± SD or No. (%).
The average delivery time in the esomeprazole group was 37.3 ± 1.2 weeks, compared to 37.5 ± 1.7 weeks in the placebo group, and this difference was not statistically significant (P = 0.75). However, the frequency of delivery between weeks 37 - 38 was 44.9% in the esomeprazole group and 62.1% in the placebo group, while for weeks 39 - 41, it was 22.4% in the esomeprazole group and 12.1% in the placebo group. The frequency of all maternal outcomes examined in the esomeprazole group did not show statistically significant differences from the placebo group (P > 0.05). Additionally, there were no cases of eclampsia, pulmonary edema, hemolysis, placental abruption, renal failure, liver hematoma, moderate or severe ascites, thromboembolism, or maternal death in either group (Table 2).
| Outcome | Esomeprazole (n = 59) | Placebo (n = 60) | P-Value |
|---|---|---|---|
| Primary maternal outcomes | |||
| Gestation at delivery (week) | 0.38 | ||
| 32 - 34 | 2 (3.4) | 0 | |
| 35 - 36 | 17 (29.3) | 15 (35.8) | |
| 37 - 38 | 26 (44.9) | 36 (62.1) | |
| 39 - 41 | 13 (22.4) | 7 (12.1) | |
| Gestation at delivery (week) | 37.3 ± 1.2 | 37.5 ± 1.7 | 0.75 |
| Secondary maternal outcomes | |||
| Admission to intensive care unit | 3 (5.2) | 4 (6.9) | 0.99 |
| blood pressure > 160/110 mmHg | 9 (15.5) | 12 (20.7) | 0.47 |
| Proteinuria ≥ 3 g/24 h | 8 (13.8) | 13 (22.4) | 0.23 |
| Platelet count < 50 × 109 | 0 | 2 (3.4) | 0.49 |
| Use of blood pressure medicine | 16 (27.6) | 13 (22.4) | 0.52 |
| Major postpartum hemorrhage | 6 (10.3) | 7 (12.1) | 0.84 |
| Cesarean delivery | 23 (39.6) | 25 (43.1) | 0.71 |
| Fetal outcomes | |||
| IUGR | 1 (1.7) | 4 (6.9) | 0.36 |
| Changes in fetal heart rate pattern | 7 (12.1) | 6 (10.3) | 0.77 |
| Fetal death | 1 (1.7) | 0 | 0.99 |
| Neonatal outcomes | |||
| Neonatal death | 0 | 2 (3.4) | 0.49 |
| Apgar score < 7 at 5 minutes | 1 (1.7) | 1 (1.7) | 0.99 |
| Neonatal intensive care unit admission | 20 (34.5) | 21 (36.2) | 0.85 |
| Intubation and mechanical ventilation | 9 (15.5) | 11 (19.0) | 0.62 |
| Birthweight (g) | 2439 ± 283 | 2405 ± 298 | 0.54 |
a Values are expressed as mean ± SD or No. (%).
b No participant had any of the following maternal outcomes: Maternal death, severe renal impairment, eclampsia, pulmonary edema, hemolysis, placental abruption, thromboembolic disease, and moderate-to-severe ascites.
c No participant had any of the following neonatal outcomes: Bronchopulmonary dysplasia, umbilical artery pH < 7.05, surfactant use, grade III or IV hyaline membrane disease, retinopathy of prematurity, neonatal sepsis, grade III or IV intraventricular hemorrhage, necrotizing enterocolitis.
There were no significant differences in fetal outcomes, including intrauterine growth restriction (IUGR), changes in fetal heart rate patterns, and fetal death, between the two groups (P > 0.05). Neonatal outcomes, such as infant death, Apgar scores below 7, admission to the intensive care unit, and mechanical ventilation, also did not show statistically significant differences between the two groups (P > 0.05). Furthermore, the average weight of babies in the two groups did not differ significantly (P = 0.54). Neonatal outcomes, including intraventricular hemorrhage, necrotizing enterocolitis, pulmonary dysplasia, neonatal sepsis, retinopathy of prematurity, hyaline membrane disease, use of surfactant, and umbilical artery pH < 7.05, were not observed in either group (Table 2).
5. Discussion
Preeclampsia is a pregnancy complication in which placental dysfunction leads to maternal hypertension. It is estimated that preeclampsia causes 60,000 maternal deaths and 500,000 fetal deaths annually, with more than 95% occurring in middle-income countries (1). There is an urgent need to find a treatment for early-onset preeclampsia that slows disease progression and prevents preterm delivery to improve neonatal outcomes (2). Esomeprazole has been introduced as a potential therapeutic drug for preeclampsia (3).
Gu et al. showed that esomeprazole treatment inhibits AMPKα and activates mTOR, thereby inhibiting autophagy in the placenta and reducing preeclampsia symptoms (5). Kaitu'u-Lin et al. demonstrated that the combination of metformin and esomeprazole was effective in reducing the secretion of sFlt-1 and the expression of the sFlt-1 isoform e15a mRNA in primary cytotrophoblasts, placentas, and endothelial cells (6). Binder et al. showed that the combination of esomeprazole and sulfasalazine decreased the secretion of sFlt-1 and markers of endothelial dysfunction (8). However, in the study by Abbas et al., esomeprazole at a dose of 40 mg did not effectively reduce serum levels of sFlt-1 and sEng in patients with early preeclampsia (9).
In the laboratory study by de Alwis et al., which evaluated proton pump inhibitors esomeprazole magnesium hydrate and trihydrate on the pathophysiological markers of preeclampsia in preclinical human models using primary human tissues and cells, esomeprazole was shown to increase vascular relaxation and reduce key factors associated with preeclampsia, including sFLT-1 and endothelial dysfunction. Esomeprazole magnesium hydrate was more effective than esomeprazole magnesium trihydrate (10). Meanwhile, studies by Choi et al. in South Korea and Bello et al. also indicated that the use of PPIs during pregnancy is not associated with a reduction in the risk of preeclampsia (11, 12).
In our study, the duration of pregnancy in the esomeprazole group did not differ statistically from the placebo group (approximately 2 days). However, the frequency of women with pregnancies lasting between 39 and 41 weeks was higher than in the placebo group. Similarly, in the study by Cluver et al., which examined 119 women with early preeclampsia in South Africa, esomeprazole did not prolong pregnancy (13). Additionally, in the study by Abbas et al., the average prolongation of pregnancy in the esomeprazole group was slightly higher than in the placebo group (11.4 ±9.4 vs. 10.3 ± 6.3), but this difference was not statistically significant (9).
In the study by Atwa et al. in Egypt, conducted on 160 pregnant mothers divided into two groups of 80, esomeprazole prolonged pregnancy in women with preeclampsia by 1.7 weeks (14). In another study comparing the effects of magnesium sulfate and esomeprazole in rats with preeclampsia, Shafik et al. showed that esomeprazole extended the pregnancy period beyond term, thus improving fertility (15). Esomeprazole may have beneficial effects, but the relatively low dose used in these studies (40 mg daily) might play a role in the varied results. It is noteworthy that the delivery time for mothers using esomeprazole in our study was 37.3 ± 1.2 weeks, which was higher than the gestational ages at delivery in the studies by Cluver et al. (31.2 weeks) and Atwa et al. (34.5 weeks) (13, 14).
In this study, no maternal outcomes such as eclampsia, pulmonary edema, hemolysis, placental abruption, renal failure, hepatic hematoma, ascites, thromboembolism, or maternal death were observed in either group. Similarly, no maternal complications were reported in the study by Abbas et al. (9). However, in the study by Cluver et al., cases of eclampsia and placental abruption were observed in the placebo group, while pulmonary edema, ascites, and thromboembolism occurred in both groups, findings that differ from ours (13).
Other maternal outcomes, such as admission to the intensive care unit, severe hypertension (160/110 mmHg), platelet count below 50 × 109, proteinuria (greater than 3 g per 24 hours), heavy bleeding during labor, and cesarean delivery, occurred more frequently in the placebo group compared to the esomeprazole group, although the differences were not statistically significant. This is consistent with the findings of Cluver et al. (13). In Atwa et al.'s study, postpartum bleeding showed no difference between the two groups (14), but the frequency of blood pressure above 160/110 mmHg in our study was much lower than in similar studies.
In our study, systolic and diastolic blood pressure did not differ significantly after the intervention. However, in Atwa et al.’s study, blood pressure in the esomeprazole group was significantly lower than in the control group (14).
Regarding fetal outcomes, there was one case of fetal death in the esomeprazole group, and the frequency of fetal growth restriction was higher in the placebo group. The occurrence of significant changes in fetal heart rate patterns was similar between the two groups. In the study by Cluver et al., one case of fetal death occurred in both groups (13). The frequency of fetal growth restriction and significant changes in fetal heart rate in our study was lower than in similar studies. Considering that esomeprazole is not found in umbilical cord blood, it is unlikely to have an adverse effect on the fetus (16). In a case report, Oriaifo et al. stated that esomeprazole could reduce the risks associated with preeclampsia in both the fetus and mother, and prevent progression to eclampsia (17).
In our study, neonatal outcomes such as intraventricular hemorrhage, necrotizing enterocolitis, pulmonary dysplasia, neonatal sepsis, retinopathy, hyaline membrane disease, and the use of surfactant were not observed in the infants of mothers in either study group. However, in Cluver et al.'s study, all of these outcomes were observed in both groups (13).
The frequency of newborns treated with oxygen was consistent with a similar study, but the frequency of neonatal special care in our study was much higher than in Cluver et al. and Atwa et al.'s studies (13, 14). This discrepancy could be due to the availability of newborn care equipment and the preference of specialist doctors to admit newborns to the NICU.
The Apgar score was less than 7 in two infants in our study (1.7% in each group), whereas in Cluver et al.'s study, the frequency of Apgar scores below 7 in the placebo group was 11.7%, higher than in our study (13).
We observed only two neonatal deaths in the placebo group, while Cluver et al. reported 9 cases in the placebo group and 7 cases in the esomeprazole group (13). In Atwa et al.'s study, infant mortality was significantly high in both groups (14). The lower neonatal death rate in our study may be due to the availability of specialized neonatal care in the hospital where our study was conducted.
In this study, the average weight of babies in both groups did not differ significantly. In Atwa et al.'s study, the average weight of infants in the intervention group was higher than in the control group. The average weight of infants in our study was significantly higher than in the two studies by Cluver et al. and Atwa et al. (13, 14), which can be attributed to the higher gestational age at delivery in our study.
Although the differences in maternal, fetal, and neonatal outcomes between the esomeprazole and placebo groups were not statistically significant, there were notable differences in the frequency of outcomes compared to the two similar clinical studies conducted in South Africa and Egypt. Factors such as genetic characteristics, socioeconomic differences, and access to healthcare services may have contributed to these observed differences.
A meta-analysis conducted by Salman Hussain et al. in 2022 investigated the relationship between PPI use and the risk of preeclampsia. The findings indicated that the use of proton pump inhibitors may be associated with a slight increase in the risk of preeclampsia in pregnant women, with no evidence to suggest that PPI use reduces the risk of preeclampsia or early-onset preeclampsia (18). A prospective cohort study of 430 women supported these findings, showing that sFLT-1 and sEng levels were lower among women with preeclampsia who were randomized to PPIs than among those who were not (19). Sandrim et al. mentioned that PPI use is associated with an increased risk of adverse cardiovascular events due to interference in nitric oxide (NO) homeostasis (20). Several studies confirm that NO is reduced during preeclampsia, and most importantly, preeclampsia causes subclinical hemolysis, leading to the elimination of NO (21, 22).
The clinical trial nature of this study is one of its strengths, and given the very limited research conducted on the effect of esomeprazole on preeclampsia, the results are significant. One limitation of this study was the lack of determination of sFLT-1 and sEng levels.
5.1. Conclusions
Although the length of pregnancy termination in our study was not significantly different, it was at least 1.7 weeks longer compared to similar studies. Additionally, while the frequency of adverse maternal, fetal, and neonatal outcomes in pregnant mothers in the esomeprazole group was lower than in the placebo group for most variables, these differences were not statistically significant. Therefore, it cannot be concluded with certainty that esomeprazole is ineffective in preventing or controlling preeclampsia. This uncertainty is partly due to the fact that different doses of esomeprazole have not been extensively studied, and clinical trials on this topic remain very limited.