1. Background
Pseudomonas aeruginosa is a gram-negative bacillus found in nearly all human- and animal-impacted environments, as well as in water and soil. This bacterium is a major cause of hospital infections, particularly in patients with compromised immune systems, such as those with malignancies, cystic fibrosis, or burns (1). Although the bacterium is resistant to many antibiotics, this resistance is often associated with biofilm formation (2).
One of the most important roles of quorum sensing (QS) in P. aeruginosa is biofilm production. Specific genes involved in the QS systems of P. aeruginosa play a key role in its pathogenicity and can be useful markers for detecting these organisms (3). Biofilm formation contributes to recurrent infections, chronic conditions, reduced drug penetration into the extracellular matrix, extended hospital stays, higher treatment costs, and potential treatment failure (4). Research has shown that bacteria utilizing QS mechanisms can increase virulence by enhancing traits such as motility, biofilm formation, and the secretion of virulence factors (5).
Flavonoids are a large group of phenolic compounds with a benzo-gamma pyrone structure, synthesized through the phenylpropanoid pathway, and they exhibit various medicinal properties. The activity of flavonoids depends on their chemical structure, degree of hydroxylation, and polymerization. Studies have highlighted their health benefits due to their antioxidant properties (6). Flavonoids protect human enzyme systems and are effective against a range of bacterial and viral infections, heart disease, cancer, and age-related conditions. Recent research indicates that some flavonoids can interfere with QS mechanisms, potentially reducing bacterial pathogenicity (7).
2. Objectives
This study aimed to evaluate the antibacterial effects of certain native plant extracts rich in flavonoids, focusing on their ability to inhibit QS, biofilm formation, and the production of virulence factors in P. aeruginosa, a major pathogenic bacterium.
3. Methods
3.1. Sample Collection and Bacterial Screening
For this study, 384 samples were initially collected with a 95% confidence level using the specified formula below. Of these, 180 samples were identified as P. aeruginosa. The samples (from urine, skin, wound, abscess, and sputum) were collected from Al-Zahra Hospital (Isfahan, Iran) and transferred to the research laboratory at Sinafravar Pharmaceutical Company. These samples were then cultured on cetrimide agar and incubated at 37°C for 24 hours. Suspected colonies were subsequently identified using molecular techniques. Pseudomonas aeruginosa strain ATCC 15692 was used as a standard reference.
3.2. Phenetic Characterization of the Isolates
The purified colonies were evaluated using gram staining, followed by specific biochemical tests including Catalase, MR, VP, OF, oxidase, indole, citrate, and growth on triple sugar iron agar and urease tests (8, 9). Antibiotic susceptibility testing was conducted according to the CLSI 2021 protocol (M100) (10, 11).
3.3. Plant Extract Preparation
The entire tested plants (Thymus vulgaris, Matricaria chamomilla, Melissa officinalis, Zingiber officinale, Echinacea purpurea, Rhus coriaria, and Althaea officinalis) were collected from the laboratory of Sinafravar Pharmaceutical Company. The plants were air-dried in an oven at 40°C for 48 hours. A portion of each plant powder (50 g) was weighed and placed in a flask containing 250 mL of ethanol and methanol (75% concentration for each solvent). The mixture was shaken at room temperature for 48 to 72 hours, after which the extract was filtered through Whatman filter paper. To remove the alcohol, the filtered extract was poured onto glass plates and left to evaporate at room temperature for 24 to 48 hours. The resulting extracts were stored at 4°C in a refrigerator until further use (12).
3.4. Phenolic Compounds
Total polyphenol content (TPC) was determined using a spectrophotometer with gallic acid as the standard, following the method outlined by the International Organization for Standardization (ISO 14502-1, ISO14502, 2005). In this procedure, 0.200 g of each sample was weighed, and 5 mL of 70% methanol (at 70°C) was added. The extract was then mixed and heated at 70°C on a vortex for 10 minutes. After cooling to room temperature, the extract was centrifuged at 200 g for 10 minutes. The supernatant was decanted into a graduated tube, and the extraction step was repeated twice. Next, 4.0 mL of a sodium carbonate solution (7.5% w/v) was added, and the absorbance was measured at 765 nm. Total polyphenol content was expressed as gallic acid equivalents (GAE) in g/100 g of material. The polyphenol concentration in the samples was calculated from a standard curve of gallic acid, ranging from 10 to 50 μg/mL (13).
3.5. Flavonoid Compounds
The total flavonoid content in the samples was measured using the aluminum chloride method. Briefly, 1 mL of plant extract was transferred into a flask containing 5 mL of double-distilled water. Then, 0.3 mL of 5% sodium nitrite was added. After 5 minutes, 0.3 mL of 10% aluminum chloride was added to the solution. After another 6 minutes, 2 mL of 1 M sodium hydroxide was added, and the solution was brought to volume with double-distilled water. The absorbance of the resulting solution was immediately measured at 515 nm. Total flavonoid content was expressed as catechin equivalents per 100 grams of dry weight of the extract (mg/100 g dry weight) using a catechin standard curve (14).
3.6. High-Performance Liquid Chromatography (HPLC) of Plant Phytochemicals
An Agilent 1100 HPLC system with a UV detector was used in this experiment, employing a liquid phase of 90% water containing 2% acetic acid and 10% acetonitrile (flow rate: 1 mL/min). The analysis utilized a C-18 reversed-phase column (Phenomenex, Gemini 5 μm, 150 mm length × 4.6 mm internal diameter). A 100-microliter aliquot of 0.5 mL of the alcoholic extract was injected into the column at 30°C. Detection wavelengths were set to 280 nm for phenolic compounds and 370 nm for flavonoids (15). Initial standards (flavonoids, quercetin, catechin) were prepared at a concentration of 1000 mg/kg in a 10 mL acetonitrile solution, and the standard graph was used to identify active substances in the extracts.
3.7. Anti-biofilm Properties
Biofilm formation by P. aeruginosa isolates was assessed using the Microtiter Plate Assay method. Fresh broth cultures of the isolated bacteria were calibrated to approximately 0.5 McFarland turbidity standards, diluted in LB broth (1: 100), and then inoculated into a microtiter plate. The plate was incubated at 37°C for 24 hours. Subsequently, 200 μL of 0.1% crystal violet (CV) solution was added to each well and allowed to stain for 15 minutes. Excess dye was washed away with distilled water, and then 200 μL of 33% acetic acid was added to each well for 10 - 15 minutes to dissolve the biofilm-bound dye. Optical density (OD) was measured at 492 nm using a plate reader, with acetic acid serving as the blank. Each strain was tested in triplicate. Biofilm production was assessed based on the methodology established by Stepanovic et al., 2012, using the following formula:
Percentage of inhibition = [(Control OD492 - Treated OD492)/Control OD492] × 100
To examine the effect of herbal extracts on biofilm formation, 10, 30, and 50 μL (25% concentration) of each plant extract were added to each well in triplicate. The results were then evaluated and recorded (16).
3.8. Identification of Quorum Sensing Genes
3.8.1. DNA Extraction
DNA was extracted from selected bacteria using the boiling method (17). For this procedure, overnight growth from 3 to 5 P. aeruginosa colonies on Brain Heart Infusion agar was suspended in 300 μL of deionized distilled water. The suspension was then boiled at 95°C for 10 minutes. Cell debris was removed by centrifugation at 13 000 rpm for 10 minutes, and the supernatant was collected in a microtube. An additional centrifugation at 12,000 rpm for 10 minutes was performed. The purity and concentration of the collected supernatant were analyzed using a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA), with DNA purity determined by the 260/280 absorbance ratio.
For rapid identification of the bacterium, LasI, LasR, rhlI, and rhlR genes were targeted. These genes were amplified by PCR using a specific set of primers listed in Table 1 (18).
| Primer Type and Primer Sequence (5' - 3') | Amplicon Size, bp |
|---|---|
| LasI | 295 |
| 5'-CGTGCTCAAGTGTTCAAGG-3' | |
| 5'-TACAGTCGGAAAAGCCCAG-3' | |
| LasR | 130 |
| 5'-AAGTGGAAAATTGGAGTGGAG-3' | |
| 5'-GTAGTTGCCGACGACGATGAAG-3' | |
| rhII | 155 |
| 5'-TTCATCCTCCTTTAGTCTTCCC-3' | |
| 5'-TTCCAGCGATTCAGAGAGC-3' | |
| rhIR | 133 |
| 5'-TGCATTTTATCGATCAGGGC-3' | |
| 5'-CACTTCCTTTTCCAGGACG-3' |
The amplification of genes was performed in a final volume of 25 µL (for two separate PCR reactions), containing 12.5 µL of Master Mix (Amplicon, Denmark), 20 pmol of each primer, and 5 µL of DNA template. The PCR protocol was carried out on a thermal cycler (BioRad, T100, USA) with an initial denaturation at 94°C for 5 minutes, followed by 34 cycles of denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 1 minute. This was followed by a final extension cycle at 72°C for 1 minute. Gel electrophoresis with 1% agarose gel was used to separate the PCR products and DNA bands were visualized under UV light using a gel documentation system (Major Sciences, Taiwan) (18).
3.9. Gene Expression
Gene expression was evaluated using a one-step quantitative RT-PCR TaqMan system (Applied Biosystems, Madrid, Spain) with the ABI 7500 system, following the manufacturer’s conditions. Reactions were performed in a total volume of 25 μL in a MicroAmp Optical 96-well plate, sealed with optical adhesive covers, and used TaqMan Universal Master Mix (Applied Biosystems, Madrid, Spain). Expression levels of each gene were normalized to the housekeeping gene 16S rRNA, using a standard curve for relative quantification. Real-time PCR analysis focused on the 16S rRNA and LasI genes, with a set temperature of 60ºC. After RNA extraction, DNA contamination was detected in the RNA samples. To ensure RNA purity, DNase treatment was applied before cDNA synthesis (19). The RNA sample quality was evaluated using a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA), and the A260/A280 absorbance ratios confirmed the RNA's validity.
3.10. Anti-quorum Sensing Activity
The anti-QS activity of the methanolic extract was assessed by quantifying violacein production. A 1 mL aliquot of freshly grown Chromobacterium violaceum (CV026) reference strain (OD 600 nm = 0.7) was added to 20 mL of nutrient broth (NB, Hi-media, India) containing hexonyl homoserine lactone (0.0625 μg/mL) and varying concentrations of plant extract (0.01, 0.02, 0.03, 0.04, 0.05, 0.075, or 0.1 mg/mL). Cultures without the plant extract served as controls. All cultures were incubated for 24 hours at 30°C with shaking at 180 rpm. After incubation, 1 mL of overnight culture from each flask was centrifuged at 16 000 × g for 10 minutes. The pellet, containing violacein produced by CV026, was resuspended in 1 mL of dimethyl sulfoxide (DMSO). The solution was then centrifuged again at 16 000 × g for 10 minutes to remove cell debris, and the absorbance was measured at 585 nm using a microplate reader (Spectra Max Plus, USA).
3.11. Swarming Test Evaluation
The extract (50 microliters) was mixed with 10 mL of Müller-Hinton agar, and then fresh bacteria were cultured on the medium. The plates were placed at 37°C for 3 days. Finally, the swarming measurement was determined by assessing the progress of bacteria on the surface using an overnight culture of bacterial suspension with a McFarland turbidity of 0.5. After incubation, both treated plates (plant extract (50 μL) + bacterial suspension (20 μL) and untreated plates were observed to measure the diameters of the motility zone (20).
3.12. Effect of Extracts on Pyocyanin Production
A McFarland turbidity of 0.5 was prepared and then diluted 1: 10 in tubes containing 5 ml of liquid LB. To these tubes, 1.8, 3.75, and 7.5 microliters of the extracts were added, resulting in concentrations of 0.037%, 0.075%, and 0.15% with 1% DMSO. The tubes were placed in a shaker incubator for 72 hours at 37°C. After 72 hours, the tubes were vortexed for 20 seconds, and 2 mL of the suspension was transferred to a sterile microtube and centrifuged for 10 minutes at 4°C with 1100 rpm. Then, 1 milliliter of the supernatant was transferred to 1 milliliter of chloroform and vortexed until a color change from green to blue occurred. The suspension was centrifuged, and 500 microliters of 0.1 N HCl were added. Subsequently, 200 microliters from each microtube were transferred to the wells of a 96-well plate, and optical absorption was measured at 545 nm (21).
3.13. Data Analysis Method
Data were statistically analyzed using SPSS 18 software, employing one-way analysis of variance (ANOVA). The post hoc test (LSD) was used to examine significant differences between groups. Additionally, the significance level of the tests was set at P < 0.05, and all tests were conducted in triplicate.
4. Results
In this study, 180 P. aeruginosa isolates were identified from a total of 348 clinical samples. Urine samples had the highest isolation frequency, while skin samples had the lowest. The distribution of isolates was as follows: Urine (62 isolates, 34%), trachea (41 isolates, 22%), sputum (32 isolates, 16%), blood (22 isolates, 12%), wounds (12 isolates, 11%), and skin (11 isolates, 5%). Additionally, 94% of the isolates exhibited beta-hemolytic activity, while only 6% showed no hemolytic activity (gamma hemolytic). A prior report indicated that 83% of isolates were resistant to at least one antibiotic, while 17% were susceptible or semi-susceptible. Among these, 68% of the isolates were classified as multidrug-resistant (MDR), with the highest resistance found in isolates from burn, lung, and ICU samples (22).
4.1. Presence of Quorum-Sensing Genes
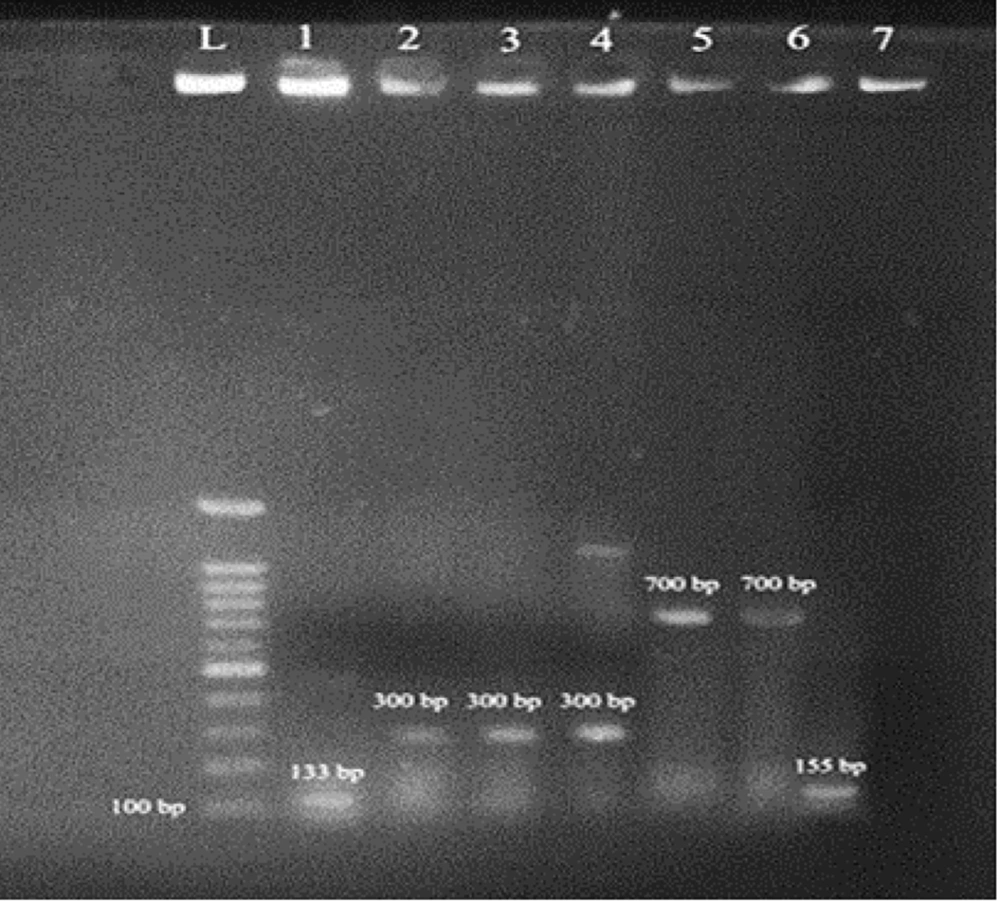
Molecular methods were applied to confirm the presence of quorum-sensing genes, using the 260/280 nm absorbance ratio as an indicator of DNA purity, which was found to be 1.8. The P. aeruginosa strain (ATCC 15692) served as a positive control. PCR was performed to detect the rhlI, LasR, LasI, and rhlR quorum-sensing genes in P. aeruginosa isolates. As shown in Figure 1, the band for LasR gene amplification was approximately 700 bp. The band lengths for the amplified regions of LasI, LasR, and rhlR were approximately 300 bp, 133 bp, and 135 bp, respectively. The findings revealed that the LasI gene had the highest frequency (54%), followed by lasR (34%), rhlR (12%), and rhlI (4%). Additional details on changes in LasI gene expression are presented in Appendices 1 - 4 in Supplementary File.
PCR reaction for the identification of quorum-sensing genes. L: Marker (bp100); band 1 shows P. aeruginosa with the rhlR gene, with a 133 bp band length; band 2 shows P. aeruginosa with the LasI gene, with a 300 bp band length; band 5 shows P. aeruginosa with the LasR gene, with a 700 bp band length; band 7 shows P. aeruginosa with the rhlR gene, with a 155 bp band length.
RNA treated with DNase demonstrated that DNA contamination was successfully removed after treatment with DNase I (Figure 2).
Gene expression was assessed based on the presence or absence of plant extracts. The ΔCt values and fold changes in gene expression were calculated using the internal control gene (16S rRNA) and the LasI gene, as shown in Table 2. Generally, lower Ct and ΔCt values indicate higher gene expression. The P. aeruginosa isolates showed decreased expression, indicating the positive effect of these extracts in reducing gene expression in P. aeruginosa isolates.
| Plant Extracts | ΔCt of Internal Control Gene | ΔC to LasI Gene | Fold Change |
|---|---|---|---|
| Thymus vulgaris | 17.5 | 11.1 | 2.7 |
| Matricaria chamomilla | 14.23 | 12.3 | 1.4 |
| Rhuscoriaria | 13.1 | 12.8 | 1.7 |
| Echinacea purpureae | 14.3 | 10.5 | 1.9 |
4.2. Biofilm Production
4.2.1. Microtiter Plate Method
The results obtained from the microtiter plate method indicated that 40.42% of isolates were strong biofilm producers, while 13.02% showed no detectable biofilm production. As shown in Table 3, the method successfully discriminated between strong, moderate, and weak biofilm-forming isolates.
| Parameter | Biofilm Formation | |||
|---|---|---|---|---|
| Strong | Moderate | Week | Non | |
| Microtiter plate method (MTP) | 40.42 ± 2.12 | 38.42 ± 1.82 | 8.14 ± 1.30 | 13.02 ± 0.54 |
Abbreviation: MTP, Microtiter plate method.
a Values are expressed as mean ± SD.
4.3. High-performance liquid chromatography Analysis of Studied Plants
The results of the HPLC analysis were determined based on the retention times and peak areas, which showed specific peaks at different points. By comparing these peaks with standard samples, the type of effective substances could be identified. The most important compound in Matricaria chamomilla was apigenin; in Melissa officinalis, it was geraniol; in Thymus vulgaris, thymol; in Echinacea purpurea, chicoric acid; in Zingiber officinale, 6-gingerol; and in Rhus coriaria and Althaea officinalis, the primary compound was quercetin (Appendices 6 - 11 in Supplementary File).
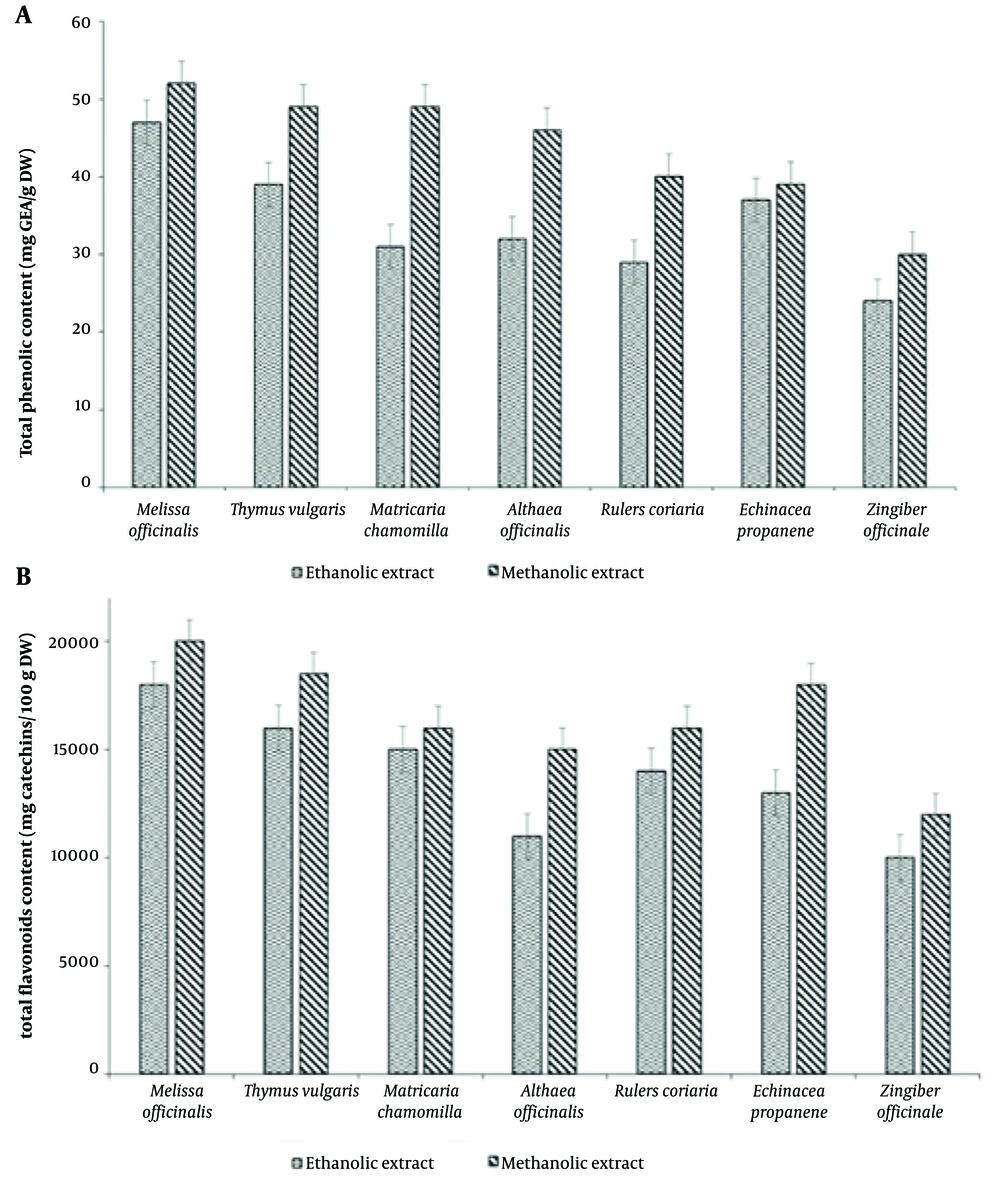
4.4. Total Phenolic and Flavonoid Content
The total phenolic and flavonoid contents of extracts from different plants are shown in Figure 3A and B. The lowest phenolic content was recorded for Zingiber officinale (Figure 3A). The results also indicated that methanolic extracts contained more flavonoid compounds than ethanolic extracts (Figure 3B). Additionally, the highest total phenolic content was found in the methanol extract of Melissa officinalis (50.50 mg GAE/g DW), followed by Rhus coriaria (42.30 mg GAE/100 g DW), Thymus vulgaris (35.21 mg GAE/100 g DW), and Matricaria chamomilla (28.29 mg GAE/100 g DW) (Figure 3B).
4.5. Anti-quorum Sensing Properties of the Extracts
In this method, the ability of methanol and ethanol extracts from 7 plant species to suppress the quorum-sensing-dependent phenotype in CV026 reporter bacteria (violet pigment production) was evaluated (Table 4). The results showed that methanol and ethanol extracts from four plant species—Thymus vulgaris, Matricaria chamomilla, Rhus coriaria, and Dracocephalum—suppressed the quorum-sensing phenotype in the CV026 reporter bacterium (production of violet pigments). In this experiment, DMSO solvent was used as the negative control, and standard tetracycline disks were used as the positive control.
| Plants | Inhibition of Violacein |
|---|---|
| Thymus vulgaris | + |
| Matricaria chamomilla | + |
| Melissa Officinalis | - |
| Zingiber officinale | - |
| Echinacea Purpurea | + |
| Rhus coriaria | + |
| Althaea officinalis | - |
The results showed that violacein pigment production was inhibited, indicating that the extracts had anti-QS properties against P. aeruginosa. When different concentrations of methanol and ethanol extracts from the aforementioned plants were added to the wells, a clear halo formed around the well. The population sensitivity of the studied plants was determined by the appearance of a cream-colored halo around the wells against the purple background of CV026. The inner ring of the halo demonstrated antimicrobial properties and was free of any living cells. As the concentration of the extracts increased, violacein production decreased by up to 98%.
4.6. Anti-biofilm Properties of the Extracts
The examination of plant extracts on P. aeruginosa biofilm formation showed that the filtered supernatant was able to inhibit biofilm formation. The biofilm inhibition rates were as follows: Thymus vulgaris (80.03%), Matricaria chamomilla (76.05%), Melissa officinalis (50.08%), Zingiber officinale (33.33%), Echinacea purpurea (33.33%), Rhus coriaria (68.04%), and Althaea officinalis (46.15%).
4.7. Pyocyanin Inhibition
Table 5 shows the results of optical absorption of pigment production in gelatin agar in the presence of different plant ethanol extracts in P. aeruginosa. The presence of plant extracts (23) resulted in decreased pigment production, as indicated by a reduction in light absorption. The amount of light absorption of the pigment significantly decreased compared to the control (P < 0.001).
| The Concentration of 100 mg/mL Ethanolic Extract | Optical Absorption (OD) of Pseudomonas aeruginosa Bacteria |
|---|---|
| Distal water | 0.507 ± 0.002 |
| Thymus vulgaris | 0.300 ± 0.01 b |
| Matricaria chamomilla | 0.287 ± 0.001 c |
| Melissa officinalis | 0.494 ± 0.015 |
| Zingiber officinale | 0.471 ± 0.011 b |
| Echinacea epurpurea | 0.385 ± 0.001 b |
| Rhusc oriaria | 0.327 ± 0.002 b |
| Althaea officinalis | 0.308 ± 0.001 c |
a Values are expressed as mean ± SD.
b P < 0.05.
c P < 0.001.
The highest production of pyocyanin occurred in the presence of the ethanolic extract of Melissa officinalis, while the lowest production was observed with the ethanolic extract of Matricaria chamomilla.
4.8. Swarming
Appendix 12 in Supplementary File shows the results of swarming changes in the presence of different plant extracts. Although Thymus vulgaris and Matricaria chamomilla extracts significantly inhibited biofilm and pyocyanin production in P. aeruginosa, they did not have a significant effect on bacterial motility. The highest swarming diameter was observed with the ethanolic extract of Melissa officinalis, and the lowest swarming diameter was observed with the ethanolic extract of Matricaria chamomilla.
5. Discussion
Pseudomonas aeruginosa is the most common pathogen isolated from patients with secondary infections. Resistance to antimicrobial agents plays a significant role in the mortality rate of patients infected with P. aeruginosa. Nowadays, plant extracts have been reported as important natural antimicrobial agents. The antimicrobial activity of various plant extracts has been known for many years, and in recent years, a large number of plant extracts and their compounds have been investigated for their antimicrobial properties against various bacterial and fungal strains. Therefore, in the present study, seven plants, including Thymus vulgaris, Rhus coriaria, Melissa officinalis, Echinacea purpurea, Zingiber officinale, Althaea officinalis, and Matricaria chamomilla, were studied. This study showed that the highest number of strains were isolated from urine samples, accounting for 34%. In similar studies, however, the highest frequency has been reported from lower respiratory tract samples (24). Additionally, in another study, researchers found that the most detected isolates were from wound cultures in outpatients and the respiratory system (2, 25). Therefore, the obtained results suggest that in this geographical area, personal hygiene may be lower than in other regions, or perhaps the number of patients with urinary tract infections (UTIs) is higher.
On the other hand, antibiotic resistance is one of the main limitations of effective treatment against bacteria. Resistance can occur due to gene mutations or the acquisition of new genes. These new genes are typically transferred from one cell to another through mobile genetic elements, such as plasmids, transposons, and bacteriophages, which can facilitate the spread of drug resistance in bacteria. Previous studies indicate that the level of resistance to different antibiotics in P. aeruginosa strains is relatively high, with results varying depending on the time and location of strain isolation. Due to the high genetic diversity of these strains, it is difficult to determine a universally effective antibiotic for treating different patients. For example, a recent study found that 48% of isolates from clinical samples were resistant to antibiotics (26). In the present study, we evaluated the effects of various native plant extracts on these isolates and assessed their impact on biofilm formation, pigment production, and swarming—key factors in bacterial pathogenicity.
Since the pathogenicity, or factors related to the pathogenicity, of bacteria are controlled by QS, discovering natural compounds that can affect QS properties could serve as a novel approach in the control of pathogenic bacteria. The QS system of a pathogenic bacterium can be disrupted in various ways, with the most common being the enzymatic degradation of messenger molecules (27). Pseudomunas aeruginosa has two QS systems, las and rhl, which enable communication between cells. This bacterium has numerous pathogenic factors, including adhesins, secretion systems, and other secretory proteins, which are essential for colonization, infection, and pathogenicity. These two systems can regulate pathogenic gene expression in P. aeruginosa (28). The las system consists of transcriptional activators, LasR and LasI, which lead to the auto-inducible synthesis of N-(3-oxododecanoyl) homoserine lactone (PAI-1). The induction of lasB (which encodes elastase) and other pathogenic genes requires LasR and PAI-1 (29). The rhl system consists of transcriptional activators, RhlR and RhlI, which drive the synthesis of N-butyryl homoserine lactone (PAI-2).
Disrupting AHL production through LuxI and preventing its binding to the LuxR receptor protein are additional methods for inhibiting QS. In this study, extracts from Thymus vulgaris, Matricaria chamomilla, Echinacea purpurea, and Rhus coriaria exhibited anti-QS properties. The rhlI, LasR, LasI, and rhlR genes are critical for QS. The present study measured the relative changes in gene expression (fold change) in the presence of different extracts. The results suggest that these extracts downregulated gene expression in all tested isolates.
The downregulation of LasI, lasR, rhlI, and rhlR genes could be due to the binding affinity of the extracts to quorum-sensing regulatory proteins, competitively reducing the binding of natural auto-inducers (30). Ulloa-Urizar et al. demonstrated that M. macrocarpa, D. loretense, T. impetiginosa, E. camaldulensis, and U. tomentosa plants possessed antibacterial properties against P. aeruginosa (31).
Furthermore, many studies have shown that natural flavonoid and phenolic compounds are extensively used to suppress biofilm formation and QS-regulated factors of P. aeruginosa. Indeed, different flavonoids, such as catechin, suppress the expression of QS genes and can reduce pathogenicity factors in P. aeruginosa. Other flavonoids, such as baicalein and quercetin, have also demonstrated anti-QS and anti-biofilm properties, inhibiting the expression of LasI genes in P. aeruginosa (32). The results obtained are consistent with previous studies, which showed that naringenin disrupts the production of QS-regulated factors in P. aeruginosa. Flavonoids bind to the LasR regulator, thereby competing with its physiological activator, N-(3-oxo-dodecanoyl)-L-homoserine lactone (3OC12-HSL). This process prevents protein binding to DNA (33). A study conducted on garlic extract found that concentrations lower than the growth inhibitory concentration caused morphological changes, increased hemolysis of the strain, and decreased pyocyanin production. The mechanism of the flavonoid effect on cellular responses includes the inhibition of proteins in the cytoplasm, such as the IκB kinase complex (IKK) and mitogen-activated protein kinases (MAPKs), as well as in the extracellular environment, such as interleukins and other cytokines (34). Thyme contains 0.8% to 2.6% essential oil, and its active ingredients include thymol, carvacrol, tannins, flavonoids, saponins, and bitter substances. The main products of Shirazi thyme are carvacrol, thymol, and eugenol (35).
Scientists have shown that carvacrol exerts an antimicrobial effect by inducing pore formation in the cell membrane of gram-positive bacteria and disrupting the outer membrane of gram-negative bacteria (36). Carvacrol has been found to alter cell membrane permeability, causing nucleic acids and proteins to leak, which results in cell death. This property makes carvacrol a potentially effective antimicrobial agent, as it can disrupt the integrity of bacterial cells and inhibit their growth (37). Furthermore, laboratory analyses and pharmacological tests have shown that the main compounds of chamomile include 27.3% camphor, 24.12% parasmin, 13.03% 1 and 8-cineole, 5.82% terpene, 5.16% carvacrol, and 3.14% borneol (38). Terpenes, flavonoids, phenolic acids, and coumarins can pass through or interact with bacterial cell membranes, potentially causing disruption or leakage. This can lead to oxidative stress, disturbance of protein metabolism, and mitochondrial dysfunction within the cells (39). Research has shown that the root of the marshmallow plant contains flavonoids, phenolic acids, and coumarins, which have been identified through chromatography (40).
In our study, we demonstrated that all plant extracts reduced pyocyanin synthesis, biofilm formation, and swarming. The presence of flagella and bacterial motility plays an important role in biofilm formation. Bacteria use their flagella to reach substrates, and once attached to a surface, they survive through motility, leading to biofilm formation. Elastase and protease are important for the early colonization of organisms on host tissue. Moreover, pyocyanin production impedes multiple cellular functions, including iron uptake, and enhances pathogenicity expression (20, 41). Therefore, it can be considered that the pathogenicity of P. aeruginosa depends on various pathogenic factors, with pyocyanin potentially playing a key role in this regard. Additionally, for measuring QS, rhamnolipids, protease, and elastase are good markers for evaluation (32).
5.1. Conclusions
Pseudomonas aeruginosa is an opportunistic pathogen with numerous pathogenic factors and a high genetic capacity for resistance to many antibiotics. Understanding the genes responsible for resistance and their mechanisms is crucial for developing effective treatments. The resistance genes in this bacterium can be chromosomal, plasmid-based, or transposon-mediated, and alterations in the bacterium's genome can lead to drug resistance. According to the results obtained in this study, genes related to QS are present in high frequencies in drug-resistant strains of this bacterium, and their expression is reduced when the bacteria are treated with natural compounds and plant extracts. Therefore, the use of plant extracts to inhibit gene expression and reduce drug resistance may be recommended for controlling and preventing infections caused by this bacterium. The findings of this study indicate that plant extracts significantly reduce the production of P. aeruginosa pathogenesis, including pyocyanin production. Additionally, these medicinal plants can inhibit biofilm formation, exopolysaccharide production, and bacterial swarming. Other medicinal plants could also be used as anti-quorum-sensing and, therefore, antibacterial agents for the treatment of P. aeruginosa infections.