1. Background
Diabetes is a widespread chronic disease, with over 90% of cases being type 2, posing significant challenges in managing comorbidities (1). A myriad of genetic and environmental factors have been implicated in the pathogenesis of type 2 diabetes mellitus (T2DM) (2). Lifestyle modification (i.e., diet and exercise) is a key component of the American Diabetes Association Diabetes Self-management Education and Support (National Standards) (3). As the global prevalence of T2DM continues to rise, optimizing the intake of phytochemicals has emerged as a promising approach to management. However, there is insufficient emphasis on prevention, with a greater focus on treatment rather than addressing risk factors. This gap has sparked increased interest in complementary approaches, particularly the use of herbal supplements. These alternatives explore the potential of phytochemicals to improve the management of T2DM (4). Herbal supplements are becoming popular as complementary strategies (5). Numerous contemporary medications and substances are rooted in traditional practices and plant-based extracts, highlighting their importance in today's medical research (6). The World Health Organization states that conventional medicine serves as a comprehensive system vital for health preservation and disease treatment across various cultures (7). There is increasing interest in using natural products to prevent and treat diabetes mellitus and its complications, motivated by their natural sources and typically lower incidence of side effects compared to traditional medications (8).
Herbal supplements like amla (Indian gooseberry) and curcumin (derived from turmeric) are gaining popularity as complementary interventions for T2DM. Turmeric (Curcuma longa), mainly grown in India and part of the ginger family, is celebrated for its therapeutic benefits due to curcuminoids, its active compounds. However, curcumin's hydrophobic nature leads to low bioavailability, necessitating administration with lipid vehicles or other herbs (9). It inhibits hepatic lipogenesis by suppressing SREBP1 gene activity and activating enzymes like CPT1 and ACAT while promoting beige adipocyte formation as a target for obesity treatment (10). In vivo studies indicate that curcumin's oral bioavailability increases significantly in nanoparticle form (11). Moreover, curcumin has anti-inflammatory and cardioprotective effects in individuals with T2DM (12). Some research shows improvements in insulin sensitivity, glucose levels, and lipid profiles (13), but results are mixed, with one meta-analysis finding no significant lipid profile changes (14). However, another concluded beneficial effects of curcumin on glucose metabolism, as well as a significant reduction of low-density lipoprotein (LDL) levels in patients treated with curcumin (15). Notably, combining curcuminoids with turmeric's essential oils (turmerones) enhances their anti-inflammatory potential, suggesting that these phytochemicals can be more effective at lower doses for disease prevention and management without side effects (16, 17).
Amla (Phyllanthus emblica), also known as Indian gooseberry, is rich in antioxidants and may help with T2DM, cardiovascular, and gastrointestinal disorders. When combined with curcumin, it shows potential for improving insulin resistance (18, 19). Amla is known for its high phenolic compound content that functions as a natural antioxidant (20), offering potential benefits for T2DM, gastrointestinal, and cardiovascular disorders (21). It protects against oxidative damage by reducing lipid peroxidation and inhibits mitochondrial COX-2 while regulating Bcl-2 expression, though it has minimal impact on cholesterol metabolism (22). Amla also activates carnitine palmitoyltransferase, aiding lipid oxidation and reducing liver enzymes related to lipogenesis (23). Clinical studies show that Amla can significantly improve lipid profiles (21), Hemoglobin A1c (HbA1c) levels, endothelial function, and systemic inflammation markers in T2DM patients (24). Additionally, Amla works synergistically with Curcuma longa (turmeric) in the Ayurvedic formula Nishamalaki extract "EmbliQur," enhancing insulin resistance alleviation in diabetic models (19). Both herbs improve immune function and insulin sensitivity with bioactive components like curcuminoids (9) and gallic acid (20). Animal studies suggest that their combination (19) can effectively lower fasting blood glucose and triglycerides, indicating a possible additive effect in managing T2DM.
2. Objectives
Overall, evidence supported the efficacy of both herbs in managing T2DM. This randomized controlled trial aimed to evaluate the combined effects of curcumin and Amla compared to a placebo on various health markers in patients with T2DM.
3. Methods and Results
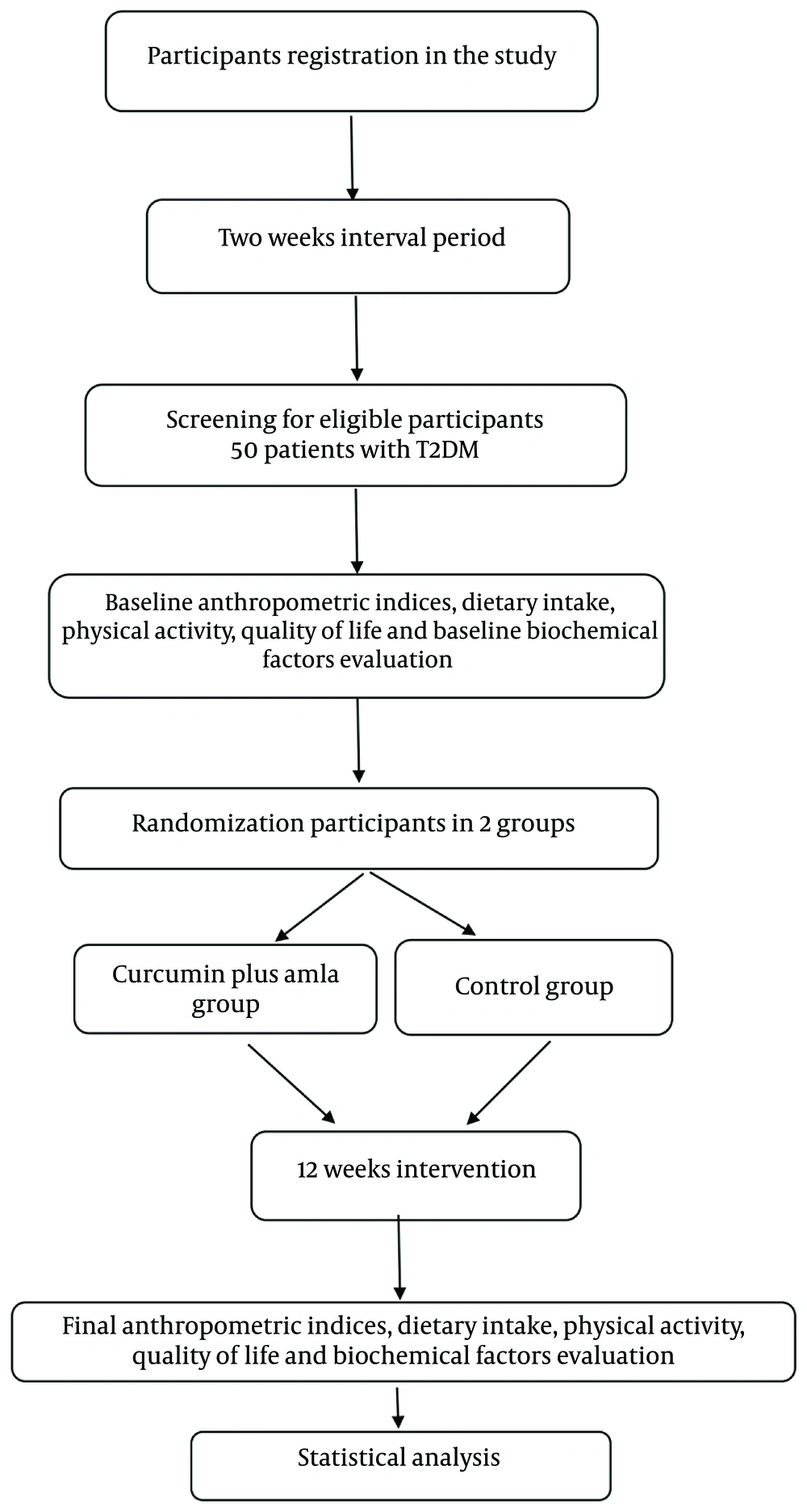
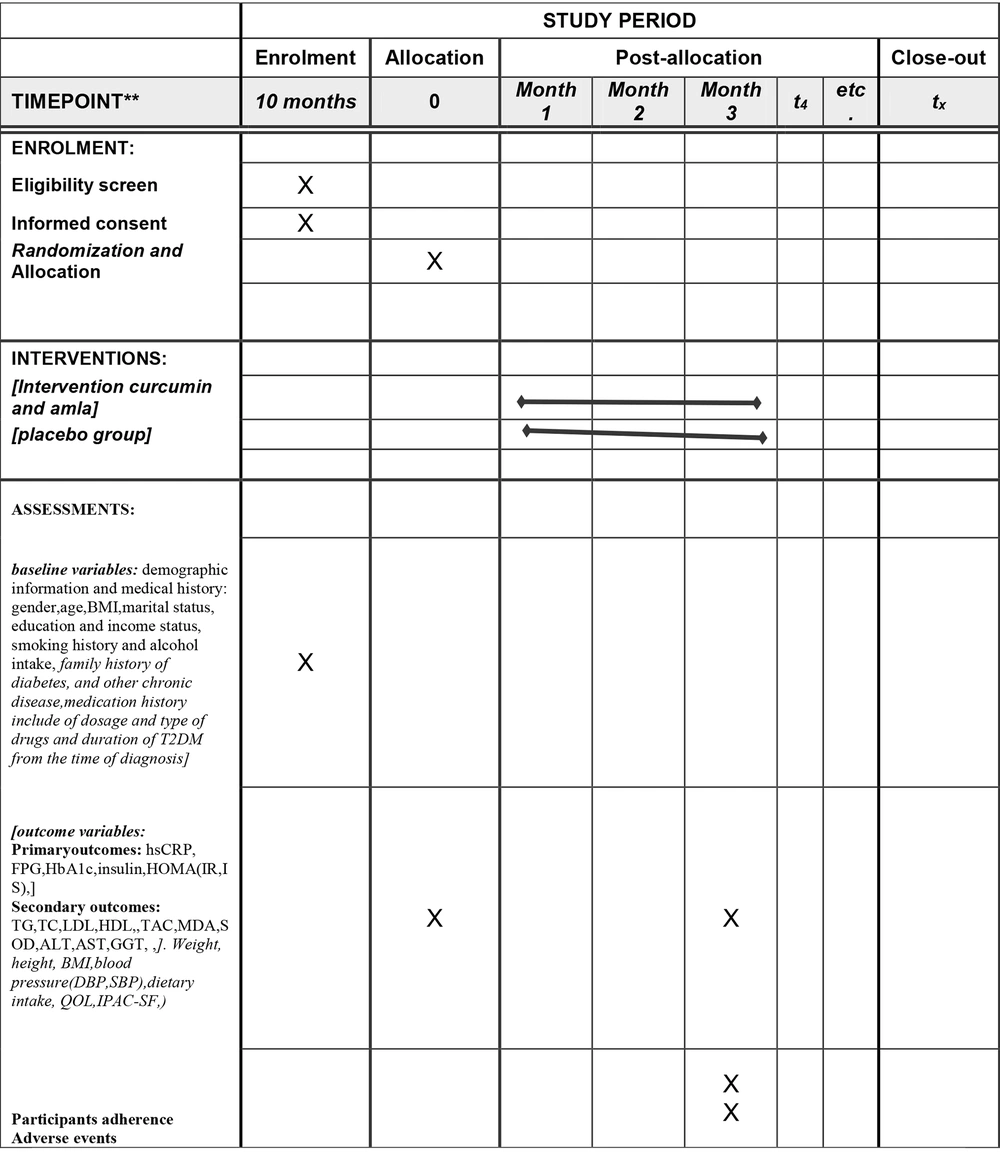
This was a single-center, randomized, double-blind, placebo-controlled, parallel-group study lasting 12 weeks, conducted at the diabetes clinic of Ahvaz Jundishapur University of Medical Sciences, Iran. It enrolled T2DM patients who met specific inclusion criteria through convenience sampling, assigning them to the study groups. Funded by Ahvaz Jundishapur University, the study design was illustrated in Figures 1, and 2, which showed the flow diagram and enrollment schedule.
Schedule of enrolment, intervention, and assessments. Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; hs-CRP, high-sensitivity C-reactive protein; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HC, hip circumference; HDL-C, high-density lipoprotein cholesterol; HOMA, homeostasis model of assessment; QOL, quality of life; IPAQ-SF, short form of international physical activity Questionnaire; IR, insulin resistance; IS, insulin sensitivity; MDA, malondialdehyde TAC, Total blood antioxidant capacity;; MET, metabolic equivalent of task; SBP, systolic blood pressure; DBP, diastolic blood pressures TC, total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; T2DM, type 2 diabetes mellitus; VLDL, very low density lipoprotein; BMI, Body Mass Index.
3.1. Primary Endpoints
The primary endpoints included evaluating serum levels of fasting plasma glucose (FPG), HbA1c, serum insulin, insulin resistance (IR), insulin sensitivity (IS), and high-sensitivity C-reactive protein (hs-CRP) differences between the intervention and control groups. Secondary endpoints were to assess the average differences in blood pressure, dietary intake, anthropometrics, and other serum biochemical and non-biochemical levels in both groups:
(1) Triglycerides (TG), total cholesterol (TC), LDL, and high-density lipoprotein (HDL).
(2) Total blood antioxidant capacity (TAC), malondialdehyde (MDA), and superoxide dismutase (SOD).
(3) Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyltransferase (GGT).
(4) Changes in systolic and diastolic blood pressure.
(5) Changes in anthropometric parameters (weight, height, and body mass index).
(6) Changes in health-related quality of life as assessed by the Persian version of the SF-12 questionnaire.
3.2. Study Population and Settings
Participants were contacted via phone, text, or social media platforms such as WhatsApp, Telegram, or local applications about the study and invited to an initial appointment for informed consent. Eligible patients were evaluated by an endocrinologist according to ADA guidelines and randomized into an intervention group (curcumin and Amla co-supplementation) or a control group. The trial (NRC-0102) received approval from the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences on August 13, 2022 ethical code: IR.AJUMS.REC.1401.196 and trial code: IRCT20220902055853N1. The study followed CONSORT reporting standards, Good Clinical Practice (GCP), and the Declaration of Helsinki. Participants were informed about the research objectives and provided written consent before participating.
3.3. Randomization and Blinding
Patients were assigned to study groups using block randomization with a block size of four to ensure equal proportions. They were stratified by age and gender. The random number sequence was generated by Sealed Envelope Ltd, available online. This study followed a double-blind design, with identical-looking intervention and placebo capsules. An independent individual coded the capsules (A and B), and both study personnel and participants remained unaware of group assignments until the trial concluded to maintain blinding and ensure unbiased outcomes.
3.4. Inclusion Criteria
- Diagnosed with T2DM for at least one year
- Aged 25 to 65
- BMI 25-34.9 kg/m²
- Using oral medications to manage T2DM
- Willing to actively participate
3.5. Exclusion Criteria
- Have chronic conditions (e.g., liver, kidney, heart, thyroid, rheumatic, pulmonary, metabolic, digestive issues, malignancies, and allergies)
- Pregnancy or lactation
- Use of insulin or recent food/vitamin supplements or fiber supplements
- Use of other medicinal plants within the past 3 months
- Weight loss > 10% in the last 6 months
- Smoking and/or alcohol intake
- Recent use of illicit or prescribed medications
- Use of steroid anti-inflammatory drugs
3.6. Intervention
Participants were randomized into two groups: (1) the curcumin and amla co-supplementation group and (2) the placebo group. They received two capsules daily for 12 weeks, with each 500 mg capsule containing either 250 mg of curcumin and 250 mg of Amla extract or 500 mg of roasted rice powder as a placebo. The curcumin extract (BCM-95® / Curcugreen®) included a blend of curcuminoids and turmeric essential oil for enhanced bioavailability, standardized to contain at least 95% total curcuminoids, 86% curcuminoids, and 65% curcumin. The Amla extract was standardized to contain a minimum of 35% total polyphenols, 7% triterpenoids, and omega-3 fatty acids. The intervention and placebo capsules, which were visually identical, were provided by Arjuna Natural Pvt. Ltd., India.
3.7. Discontinuation of Treatment
Upon a patient's withdrawal from the project, researchers documented the reason and date and conducted the same tests scheduled for the 12-week mark, if the patient consented. If non-cooperation resulted from side effects related to the study intervention, necessary follow-up continued until the patient's condition returned to normal. Withdrawal could have occurred for the following reasons:
- The patient expressed unwillingness to continue.
- Significant side effects hindered participation.
- The patient migrated.
- Adherence to capsule intake was less than 85% at any visit.
3.8. Outcome Measures
At baseline and after 12 weeks of intervention, demographic data such as sex, date of birth, education, income, medication history, T2DM diagnosis, smoking, alcohol consumption, family disease history, and dietary habits were collected.
3.8.1. Dietary Assessment
A three-day dietary recall questionnaire (one weekend day and two weekdays) was utilized, conducted by an expert nutritionist and analyzed with modified Nutritionist IV software.
3.8.2. Physical Activity Assessment
Physical activity was measured using the short form of the International Physical Activity Questionnaire (IPAQ), reported in minutes per week for each activity category, through direct interviews conducted by blinded staff.
3.8.3. Anthropometric Measurements
Weight and height were recorded using a Seca scale and a wall stadiometer, with participants wearing minimal clothing and no shoes. Body Mass Index (BMI) was calculated.
3.8.4. Blood Pressure Measurement
Blood pressure was measured using a calibrated sphygmomanometer, with the patient seated after resting for 3 to 5 minutes. Participants were instructed to avoid caffeine, exercise, and smoking for at least 30 minutes before the measurement.
3.9. Assessment of Quality of Life
The Persian version of the SF-12 questionnaire will be employed to evaluate health-related quality of life (QOL) (25). This questionnaire comprised 12 questions across eight scales:
(1) Physical functioning (PF)–limitations in moderate activity and climbing stairs
(2) Role limitations due to physical problems (RP)–impact on work and activities
(3) Bodily pain (BP)–interference with normal work due to pain
(4) General health (GH)–overall health perception
(5) Vitality (VT)–energy levels
(6) Social functioning (SF)–impairment due to physical or emotional issues
(7) Role limitations due to emotional problems (RE)–impact on activities and diligence
(8) Mental health (MH)–feelings of calmness and depression
Response scales ranged from 2 to 6 points, with raw scores for items between 1 and 6. Selected raw scores from BP, GH, VT, and one item from MH were transformed into eight scale scores, each ranging from 0 (worst) to 100 (best), where higher scores indicated better QOL.
3.10. Assessment of Biochemistry Variables
At baseline and after 12 weeks, 10 cc of fasting blood were collected from patients. The samples were centrifuged at 1500 to 2000 rpm for 10 minutes to separate serum, which was stored at -80°C until analysis. Biochemical analyses included:
- The hs-CRP concentration: Measured using the ELISA method with standard kits.
- The HbA1c: Assessed via spectrophotometry.
- Serum Glucose, TG, TC, HDL: Measured using photometric assays (Parsazmoun).
- The LDL cholesterol: Calculated using the Friedewald formula: LDL-C = TC - HDL-C - (TG/5).
- Fasting Insulin: Measured by enzyme-linked immunosorbent assay.
- Insulin Resistance: Quantified via HOMA-IR: HOMA-IR = [fasting insulin × fasting glucose]/405 (26).
- Insulin Sensitivity: Assessed using QUICKI = 1/[log(I0) + log(G0)] (27).
- MDA, TAC, SOD: Evaluated spectrophotometrically at 534 nm.
- Serum ALT, AST, and GGT: Measured using a conventional auto-analyzer (Autoanalyzer BT3000) with standard diagnostic kits (Pars Azmoon Co, Iran).
3.11. Participant Adherence
Participants received dosing guidelines and were required to return any unused supplements at monthly visits. Reminders were sent via social media or text messages to encourage timely intake. Participants were instructed to contact the study appraiser if they experienced any adverse effects.
3.12. Adverse Events and Trial Monitoring
Participants were reminded daily to track their supplement intake and received weekly counseling from a clinical advisor via phone or social media. Any adverse effects were documented and reported to the Data Monitoring and Ethics Committee. The research advisors oversaw and monitored the study to ensure adherence to the CONSORT protocol.
3.13. Statistical Analysis
The data were analyzed using SPSS, version 22. Normality was assessed using the Kolmogorov-Smirnov (KS) test. Quantitative data were reported as mean and standard deviation, while qualitative data were expressed as percentages. Various statistical tests, such as the paired t-test, Mann-Whitney test, independent t-test, and Wilcoxon test, were used for comparisons. ANCOVA was utilized to control for confounding variables. Intervention effectiveness was evaluated by calculating relative mean differences for specific variables. For handling missing data, all individuals who were randomized and took at least one dose of either curcumin with Amla or placebo were included in the intention-to-treat analysis, with additional per-protocol analysis conducted. A significance level of P < 0.05 was applied for all analyses.
3.14. Sample Size
The sample size was determined with 90% power and a 95% confidence interval, focusing on hs-CRP levels (1.59 ± 0.98 for the Amla group and 1.47 ± 2.94 for the placebo group) (24). Hence, 19 participants were calculated per group. Considering a 30% dropout rate, the sample size for each group was set at 25 participants.
3.15. Patient and Public Involvement
Participants did not take part in the study's development and design. They were informed about the research objectives and outcomes, reminded daily to take their supplements, and received weekly counseling from a clinical advisor. The Data Monitoring Committee oversaw the results and shared completed outcome measurements and trial publications with participants.
4. Discussion
The main focus of this study was to examine the impact of combining curcumin and Amla on the levels of hs-CRP and glycemic status. It also evaluated their effects on serum biochemical levels and non-biochemical markers in individuals with T2DM. The global rise in T2DM has led to growing interest in safe and effective herbal remedies from traditional medicine in East Asia, India, China, and Iran (28). The effects of curcumin in prediabetes and T2DM patients were studied, but inconsistent results raised questions about its role in diabetes management (29-34). Amla consumption, with its anti-inflammatory and antioxidant properties, could have helped regulate blood sugar and lipid levels, reducing the risk of diabetes and other disorders (24, 35-38). Recent research highlighted the health benefits of curcuminoids, the active compounds in turmeric, particularly their notable anti-inflammatory effects (39). However, the results of studies were inconsistent due to variations in the study population, type of curcumin used, duration of intervention, and other factors (40, 41).
This study assessed the impact of co-supplementation of curcumin and Amla on various health markers in T2DM patients. It was a single-center trial with limitations related to self-reported dietary intake and patient inclusion criteria. If the effectiveness of this supplementation was observed, it could be used as a therapeutic supplement for T2DM patients alongside other treatments.