1. Background
Oral cancer is a growing public health concern worldwide. According to the latest global cancer statistics, oral cancer ranks as the 16th most common cancer globally (1). In Taiwan, the incidence and mortality rates of oral cancer are particularly high, with 2021 data indicating that it is the fourth leading cause of death among men (2). Although surgery remains the primary treatment modality, inadequate adjuvant therapy often results in poor prognosis and high recurrence rates (3). Therefore, the development of new and effective therapeutic strategies is essential for improving the survival rate and quality of life of patients with oral cancer.
Recently, natural compounds have garnered significant attention for cancer treatment. Shikonin, a natural naphthoquinone derivative extracted from Lithospermum erythrorhizon, exhibits remarkable antitumor activity against various cancer types (4, 5). Studies have demonstrated that shikonin modulates the cell cycle, induces apoptosis and necrosis, and possesses antiangiogenic properties (6, 7).
Cancer progression involves several key processes, including angiogenesis, tumor metastasis, autophagy, and apoptosis (8). Angiogenesis provides tumors with the essential nutrients and oxygen needed to support their growth and metastasis (9). Cancer metastasis, a primary cause of patient mortality, involves complex cellular signaling pathways and the activation of matrix metalloproteinases (10). Autophagy, a cellular self-protection mechanism, plays a dual role in tumor development and progression (11). The dysregulation of apoptosis is a crucial factor in tumor formation and drug resistance (12).
Recent studies have elucidated the autophagy-modulating effects of shikonin in various cancers and non-cancerous diseases. Hu et al. discovered that in colorectal cancer, shikonin induces cellular autophagy by modulating the microRNA-545-3p/GNB1 axis, thereby disrupting cellular carcinogenesis (13). Liu et al. reported that in human bladder cancer, shikonin-induced ROS-dependent cell death involves necroptosis, which inhibits autophagy by regulating RIP3/p62/Keap1 complex formation (14).
Shikonin has also demonstrated significant autophagy-modulating effects in other diseases. Chen et al. investigated the effects of shikonin on autophagy and apoptosis in human promyelocytic leukemia cells (15). Studies by Wang et al. have indicated that shikonin, through the activation of autophagy, has emerged as a potential therapeutic drug for various types of osteoarthritis (16, 17).
These findings not only confirm the autophagy-modulating effects of shikonin in various cancers and diseases but also reveal its potential therapeutic mechanisms. The ability of shikonin to induce autophagy in cancer cells and promote apoptosis positions it as a promising anticancer drug candidate. Despite the demonstrated efficacy of shikonin in multiple cancers, its specific effects and mechanisms of action in oral cancer cells remain unclear.
Given the high incidence of oral cancer and the limitations of existing treatments, conducting in-depth research on the potential therapeutic value of shikonin for oral cancer is crucial. This study aimed to establish an in vitro experimental platform to systematically investigate the effects of shikonin on oral cancer cells, including its regulatory effects on cell survival, metastasis, angiogenesis, autophagy, and apoptosis.
2. Objectives
The study provides new pharmacological evidence for shikonin as a potential therapeutic agent for oral cancer and lays the groundwork for future clinical applications.
3. Methods
3.1. Drug Procurement and Cell Culture
Shikonin (517-89-5) and the other drugs used in this study were purchased from Santa Cruz Biotechnology, Inc. The SAS and SCC-4 cell lines are pivotal in oral squamous cell carcinoma (SCC) research, each offering unique insights into their distinct origins. The SAS cell line, derived from metastatic oral SCC in the lymph nodes, is utilized to study cancer invasion and metastasis, making it essential for evaluating treatments targeting advanced disease stages. In contrast, the SCC-4 cell line originates from a primary oral SCC tumor and represents initial tumor growth and differentiation. Furthermore, the SCC-4 cell line is critical for understanding the early stages of cancer and testing therapies for primary tumors.
The combination of these two cell lines provides diverse perspectives on oral SCC, encompassing tumor formation and metastatic progression, and supports the development of targeted therapies. The human oral cancer cell lines (SCC-4 and SAS) were obtained from the American Type Culture Collection. Cells were cultured in DMEM/α-MEM and F12 media containing 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 incubator. The culture medium was changed every two to three days, and when the cells reached 80 - 90% confluence, they were passaged or subcultured for experimental use.
3.2. Cell Viability Assay
Cell samples were divided into two groups and cultured for 24 hours with or without shikonin treatment. Subsequently, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to the cells at a concentration of 0.5 mg/mL and incubated for an additional 30 minutes. The MTT reaction product was then solubilized in dimethyl sulfoxide (DMSO). Absorbance was measured at 550 nm using a Bio-Tek microplate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA).
3.3. Western Blotting
After quantifying the protein samples, loading dye was added and mixed thoroughly. The samples were denatured at 99°C in an incubator. The SDS-PAGE gel consisted of a 4% stacking gel and a 10% or 12% separating gel. The prepared gel was mounted onto an electrophoresis device, and electrophoresis was conducted for approximately 1.5 hours. Following electrophoresis, protein transfer was performed. Once the transfer was complete, the nitrocellulose membrane was removed, and blocking buffer was added, with shaking at room temperature for 1 hour.
Primary antibodies were then applied and allowed to react at room temperature for 2 hours, followed by three washes with 1× TBS buffer. Next, secondary antibodies were added, and the reaction was carried out at 4°C for 2 hours, followed by a 10-minute wash with TBS. Finally, an enhanced chemiluminescence (ECL) substrate was applied, and the results were visualized using X-ray film.
3.4. Quantitative Polymerase Chain Reaction
Total ribonucleic acid (RNA) was extracted using the TRIzol reagent under RNase-free conditions. Subsequently, 1 μg of total RNA was subjected to reverse transcription at 50°C for 2 minutes, followed by 60°C for 30 minutes. The template, equivalent to 20 ng of total RNA, was denatured at 95°C for 5 minutes. Amplification was performed through 40 cycles, with each cycle consisting of denaturation at 94°C for 20 seconds and annealing at 50°C for 1 minute. mRNA expression was detected using the TaqMan fluorescence labeling system and the StepOnePlus PCR Detection system.
3.5. Migration Assay
A transwell plate (Corning) with a polyethylene terephthalate transparent membrane containing evenly dispersed 8 μm-diameter pores was placed in a 24-well cell culture plate. Subsequently, 1 × 10⁵ cells were suspended in 200 μL of culture medium without FCS and seeded into the transwell plate. After 12 hours, the cells were fixed with 95% ethanol for 10 minutes, washed with PBS, and stained with 25% hematoxylin for 30 minutes. Following three washes with PBS, the cells on the upper side of the membrane were removed with a cotton swab. The cells on the lower side of the membrane were observed under a microscope in five fields of view and counted to calculate the average cell number.
3.6. Colony Formation Assay
A total of 5 × 10⁵ cells were seeded into six-well plates and cultured with different drug concentrations for 21 days. The cells were fixed with 10% methanol and 10% acetic acid in PBS for 5 minutes, washed with PBS, and stained with 0.001% crystal violet for 30 minutes. After washing, the colonies were observed in the culture dish, and the number and area of the colonies were calculated.
3.7. Detection of Changes in Calcium ion (Ca2+) Release
The cell suspension was aspirated into centrifuge tubes and treated with the drugs for varying durations. During the last 10 minutes of treatment, Fluo-3/AM calcium ion dye (1 μL Fluo-3/AM working solution in 500 μL PBS) was added to each tube in a final volume of 500 μL. A blank tube containing only 500 μL of PBS without drugs or dye was also prepared. The samples were placed in a 37°C water bath in the dark. Subsequently, the samples were analyzed using a flow cytometer, and 10,000 cells were collected from each sample to assess the extent of calcium ion release.
3.8. Detection of Changes in Mitochondrial Membrane Potential
Cells were seeded in a six-well plate at a density of approximately 2 × 10⁵ cells/mL per well and allowed to adhere for 24 hours. After cell adhesion, different concentrations of drugs were added, and the cells were continuously cultured for 48 hours. Next, the supernatant from the culture dish was collected into a 1 mL centrifuge tube, and the culture dish was washed with PBS. The wash liquid was also collected in the centrifuge tube.
Subsequently, 0.1% trypsin was added to the culture dishes to cover their surfaces. After incubation for 3 minutes, the dish was gently tapped to suspend the adhered cells, which were then aspirated into the centrifuge tube from the previous step. Centrifugation was performed at 1500 rpm for 5 minutes to remove the supernatant. JC-1 dye was added to the cell suspension to achieve a uniform suspension, and the cells were stained for 30 minutes. The mitochondrial membrane potential of the cells was determined using a flow cytometer, and 10,000 cells were analyzed to assess changes in the mitochondrial membrane potential.
3.9. Detection of Cell Apoptosis
An In Situ Cell Apoptosis Detection Kit (Roche Diagnostics, East Sussex, UK) was used to detect apoptosis in tumor samples based on the principle of terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate-biotin nick end-labeling (TUNEL). Tissue sections were permeabilized with 0.1% Triton-X100 for 20 minutes, followed by a 60-minute reaction with fluorescein-labeled deoxyuridine triphosphate (dUTP) and TdT. Subsequently, a 30-minute reaction with an alkaline phosphatase-conjugated anti-fluorescein antibody was performed, followed by staining with a fast red solution.
Tissues without TdT labeling were used as negative controls. Two sections from each sample were analyzed, and 10 random images were selected for observation at 400× magnification to calculate the staining results. Finally, the samples were re-stained with 4',6-diamidino-2-phenylindole dihydrochloride (DAPI; 2 μg/mL, Sigma-Aldrich Inc.) at 37°C for 30 minutes. Cells showing TUNEL-positive and DAPI-positive reactions along with condensed or fragmented chromatin in the nucleus were considered apoptotic.
After three replicates of the experiment, nine random images were selected for observation at 200 × magnification using an Axiovert 200 inverted fluorescence microscope equipped with an Axiocam HRM-cooled charge-coupled device camera and Axio Vision 4 image analysis software (Carl Zeiss, Göttingen, Germany). The number of apoptotic and total cells was counted, and the apoptotic ratio was calculated.
3.10. Animal Experiment
Male BALB/c nude mice were purchased from BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan) and acclimatized for one week. SCC-4 cells were seeded in a 10 cm dish and allowed to adhere for 24 hours. After cell adhesion, the supernatant from the culture dish was collected in a 1 mL centrifuge tube, and the culture dish was washed with PBS. Subsequently, 0.1% trypsin was added to the culture dish to cover the surface. After 3 minutes of incubation, the dish was gently tapped to suspend the adhered cells.
The cell suspension was aspirated into a centrifuge tube and centrifuged at 1500 rpm for 5 minutes. The supernatant was removed, and serum-free culture medium was added to evenly disperse the cells. The cell density was approximately 1 × 10⁶ cells/L. A 200 μL cell suspension was then subcutaneously injected into the lower abdomen of each mouse. Drug administration was initiated when the tumor size reached 100 mm³, and the tumor size and body weight of each mouse were monitored until day 28.
All experimental methods were approved by Tunghai University of Medicine (Taichung, Taiwan) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Temperature, humidity, and light-dark cycle were maintained under controlled conditions.
3.11. Histological Analysis
Specimens were fixed in 4% formaldehyde, embedded in paraffin, and sectioned into 4 μm-thick slices. Hematoxylin-eosin (HE) and Alcian blue staining (Sigma-Aldrich Inc., St. Louis, MO) were performed to examine and analyze the morphology of the tumor tissue.
3.12. Statistical Analysis
For all quantified results, the mean ± SD of at least three experiments was calculated using GraphPad Prism 5.0 software. Student’s t-test was employed for statistical comparison between two groups, while two-factor analysis of variance (ANOVA) with the Bonferroni post hoc test or the Mann-Whitney U test was used for statistical comparisons involving more than two groups, as appropriate. In all cases, statistical significance was defined as a P-value of less than 0.05.
4. Results
4.1. Shikonin Exhibits Specific Inhibitory Effects on Oral Cancer Cells
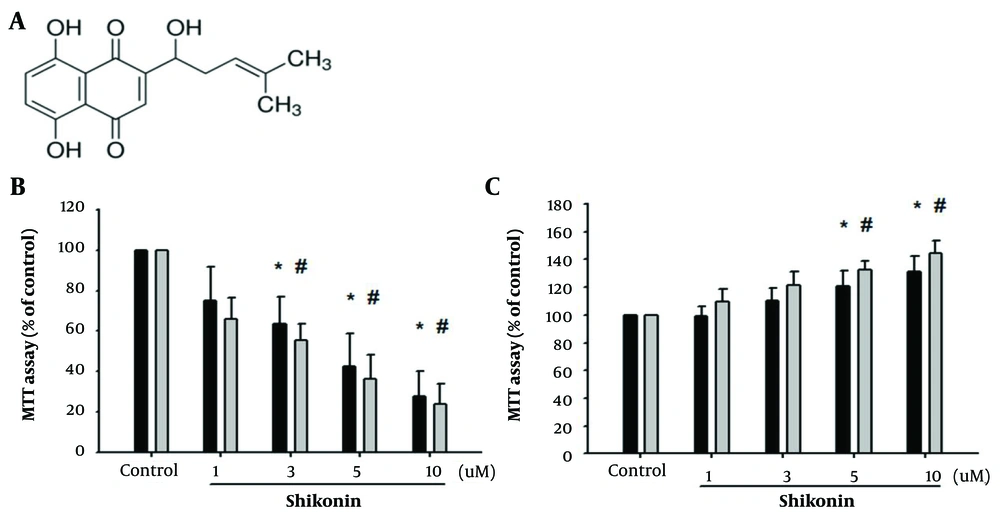
Shikonin is a naphthoquinone derivative, and its structure is shown in Figure 1A. Previous studies have demonstrated the significant benefits of shikonin for cancer treatment (1, 2). However, its effects on oral cancer have not been fully elucidated. Oral cancer encompasses various pathological types, including squamous cell carcinoma (SCC), verrucous carcinoma, adenoid cystic carcinoma, and mucoepidermoid carcinoma. SCC accounts for more than 90% of all oral cancers and is associated with a poor prognosis.
A, structure of shikonin known as a naphthoquinone derivate; B, shikonin effectively inhibited oral cancer cells in a concentration-dependent manner, as observed through (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) experiments. (* represented SAS cell line was significantly inhibited by shikonin and # represnted SCC-4 cell line was significantly inhibited by shikonin); C, accidental discovery that shikonin promoted the proliferation of muscle cells (* represented significant proliferation of SAS cell line the and # represnted the SCC-4 cell line).
Some systemic cancers, including those of the digestive tract, breast, lung, liver, prostate, multiple myeloma, and malignant lymphoma, can also metastasize to the oral cavity. To clarify the impact of shikonin on oral cancer cells, we conducted a cell viability analysis on two SCC cell lines, SCC4 and SAS, which have been less commonly used in previous studies on shikonin and oral cancer. The purpose of this study was to demonstrate the specificity of shikonin for oral cancer cells and confirm its inhibitory effect through MTT experiments.
The results revealed that shikonin effectively inhibited the growth of oral cancer cells in a concentration-dependent manner (Figure 1B). Furthermore, we investigated the effects of shikonin on normal tissues by establishing C2C12 cells as mature myoblasts and G7 cells as skeletal muscle cells for comparison with normal tissues and oral cancer cells. Preliminary findings indicated that shikonin specifically inhibited cancer cells without affecting normal tissues. Interestingly, this study unexpectedly showed that shikonin promoted the proliferation of muscle cells (Figure 1C).
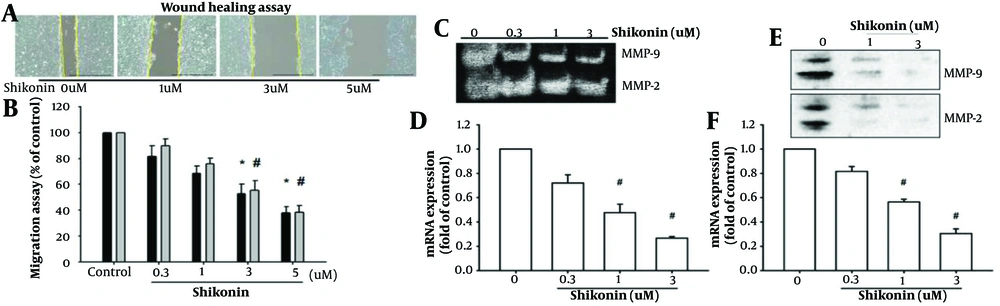
4.2. Shikonin Inhibits Cancer Cell Migration and Wound Healing Ability by Suppressing Matrix Metalloproteinases
Metastasis is a critical factor in cancer progression (3, 4). We investigated the ability of shikonin to inhibit cancer cell migration. To ensure that the shikonin-mediated inhibition of cancer cell migration was not solely due to the induction of apoptosis in oral cancer cells, as observed in previous experiments where concentrations exceeding 5 μM significantly caused cancer cell death, we tested the effect of different concentrations (0, 0.3, 1, 3, and 5 μM) of shikonin on cell migration.
The preliminary experimental results indicated that as the concentration of shikonin increased, the migration ability of oral cancer cells decreased. This finding suggests that shikonin effectively inhibits the migration of oral cancer cells, as depicted in Figure 2A and B.
A and B, Shikonin effectively inhibited the migration of oral cancer cells as the concentration increased (* represented significantly inhbitied migration of SAS cell line and # represented significantly inhbitied migration of SCC-4 cell line); C, enzyme activity tests; D and F, mRNA expression, which represented the expression of matrix metalloproteinases-2 (MMP-2) and MMP-9 (# represented significantly suppressed); E, were suppressed by shikonin stimulation; at the lethal dose of 5 μM, which induced cell death, shikonin further inhibited the expression of MMP-2 and MMP-9.
Next, we aimed to identify the specific protein that shikonin regulates to inhibit cancer cell migration. At the lethal dose of 5 μM, which induced cell death, shikonin was found to inhibit the expression of matrix metalloproteinases-2 (MMP-2) and matrix metalloproteinases-9 (MMP-9) proteins (Figure 2E). Additionally, mRNA expression and enzymatic activity tests revealed that the expression of MMP-2 and MMP-9 was suppressed by shikonin stimulation (Figure 2C, D, and F).
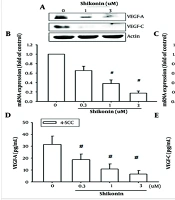
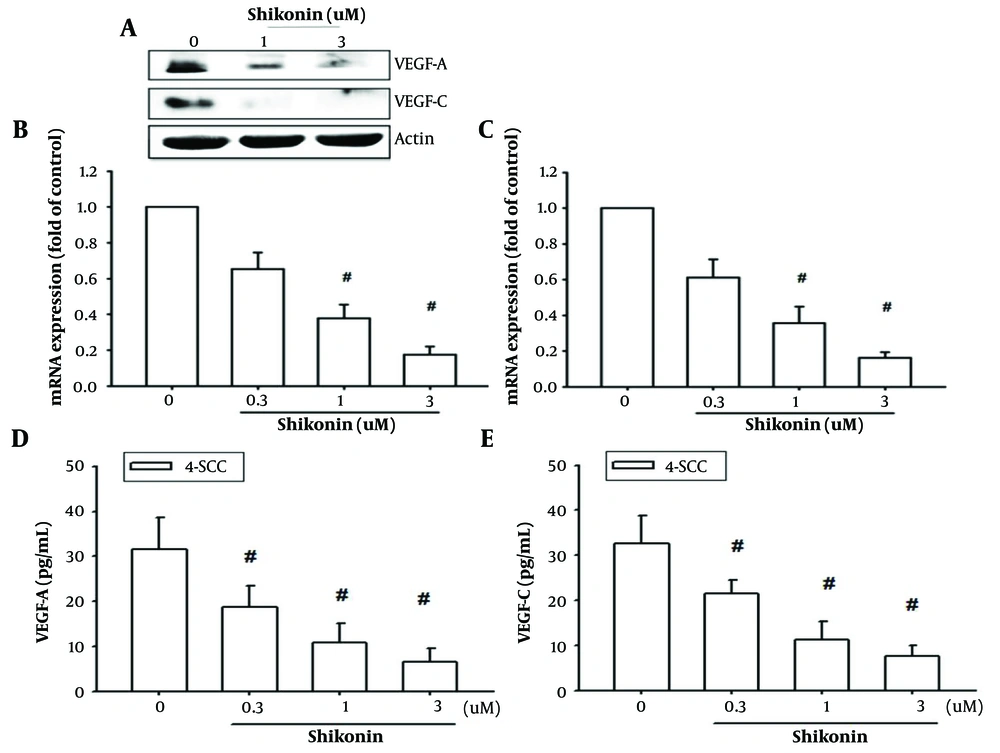
4.3. Shikonin Inhibits the Production of Vascular Endothelial Growth Factor-A and Vascular Endothelial Growth Factor-C in Oral Cancer Cells
Both vascular endothelial growth factor-A (VEGF-A) and vascular endothelial growth factor-C (VEGF-C) have been identified as crucial factors in inducing angiogenesis and lymphangiogenesis (5, 6). These proteins play significant roles in directly generating blood or lymphatic vessels to supply nutrients to cancer cells, facilitate cancer cell metastasis, and cooperate with other oncogenes to promote tumor cell proliferation. This underscores the importance of VEGF-A and VEGF-C as key proteins in cancer cell malignancies.
Therefore, we aimed to evaluate the effects of shikonin on VEGF-A and VEGF-C expression and clarify its potential to inhibit angiogenesis. The results demonstrated that shikonin significantly suppressed the mRNA and protein expression of both VEGF-A and VEGF-C (Figure 3A - C). Furthermore, ELISA analysis of protein expression in the culture supernatant of oral cancer cells revealed that shikonin significantly inhibited the secretion of VEGF-A and VEGF-C (Figure 3D and E). These findings suggest that shikonin effectively inhibits angiogenesis in cancer cells.
Shikonin may potentially inhibit angiogenesis in cancer cells. Panel A, B, and C, showed significantly suppressed the mRNA and cellular proteins expressions of both vascular endothelial growth factor A (VEGF-A) and VEGF-C; panel D and E, exhibited the secretion of VEGF-A and VEGF-C were significantly inhibited by shikonin based on the enzyme-linked immunosorbent assay (ELISA) analysis. # represented significanted suppressed when compared to the control group.
4.4. Effects of Shikonin on Autophagy in Oral Cancer Cells
Stimulation with high concentrations of shikonin inhibits cancer cell proliferation and autophagy (7, 8). However, the detailed mechanisms underlying protein regulation during shikonin-induced autophagy in oral cancer cells remain unclear. Therefore, this study further validated the effects of shikonin on autophagy and aimed to determine its influence on autophagy-related proteins.
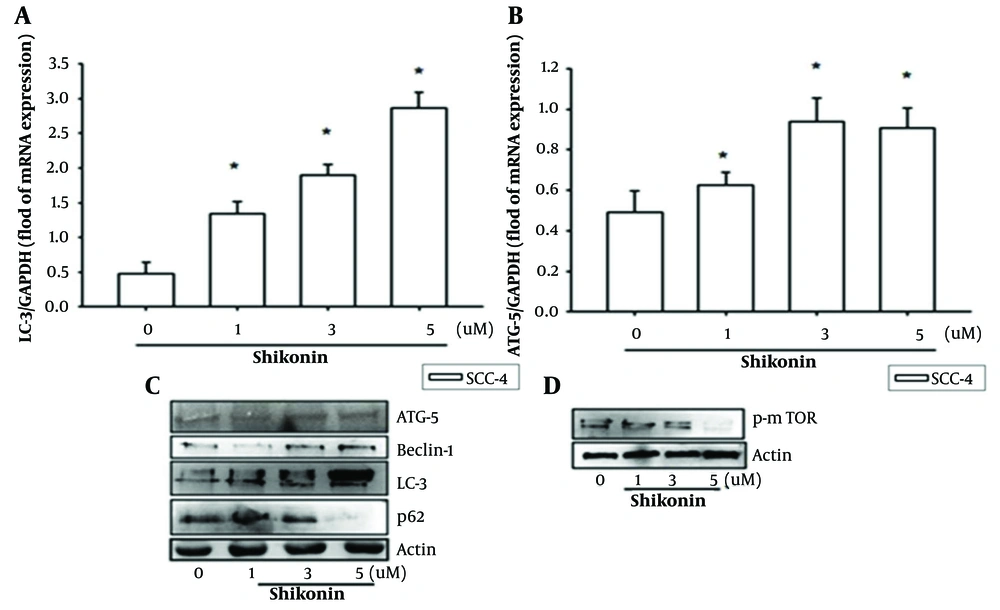
We found that the levels of autophagy-related proteins, such as Beclin-1, ATG5, and light chain-3 (LC-3), increased with the concentration of shikonin, reaching a peak at cytotoxic doses of 3 μM and 5 μM, which were above the IC50 (Figure 4A - C). Additionally, mTOR and other related proteins were inhibited, thereby initiating autophagy (Figure 4D).
Shikonin can significantly affect cancer cell growth, migration, and autophagy, as shown by the following results. A, B, and C, levels of autophagy-related proteins such as Belin-1, autophagy related 5 (ATG-5), and 1A/1B-light chain 3 (LC-3) increased with the concentration of shikonin, reaching a peak at cytotoxic doses of 3 μM and 5 μM; D, Mammalian target of rapamycin(m-TOR) and other related proteins were suppressed, initiating autophagy. * represented significantly increased when compared to the control group.
These results indicate that shikonin significantly affects cancer cell growth, migration, and autophagy. Future studies should investigate the potential induction of apoptotic responses at cytotoxic doses.
4.5. Effects of Shikonin on the Colony-formation Ability and Apoptosis of Oral Cancer Cells
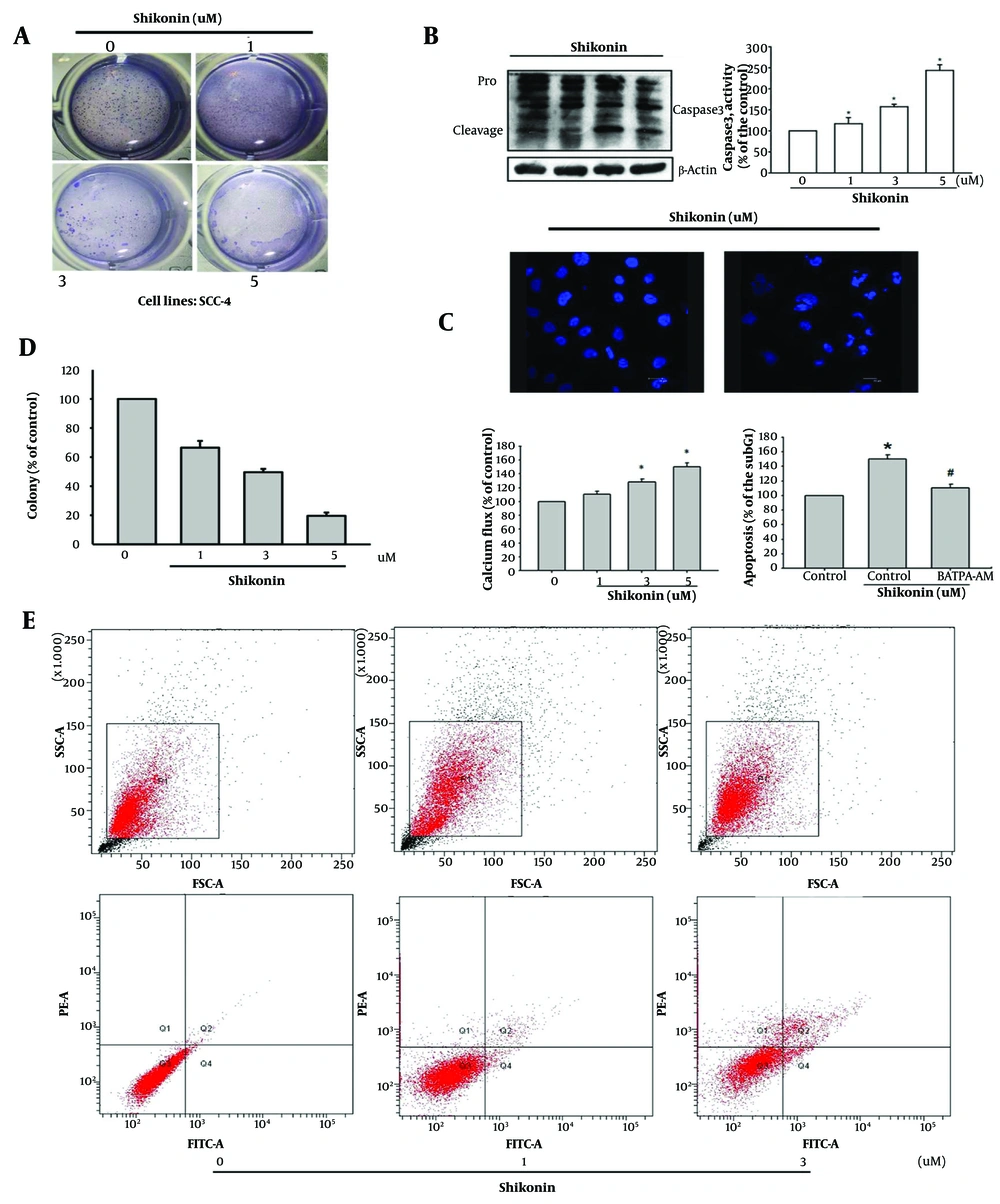
The ability of cancer cells to detach from their environment and independently form colonies is a critical indicator of cell survival (9). This study found that shikonin inhibited the colony-forming ability of oral cancer cells, leading to apoptosis (Figure 5A). In experiments investigating the effects of shikonin on apoptosis in oral cancer cells, it was observed that shikonin increased the activity of the apoptotic protein Caspase 3 (Figure 5B). The TUNEL experiment, which involved nuclear staining, demonstrated that high concentrations of shikonin induced nuclear fragmentation and apoptosis in oral cancer cells (Figure 5C).
A, shikonin inhibited the colony formation ability of oral cancer cells (SCC-4), leading to apoptosis; B, elevated activity of the apoptotic protein Caspase 3; C, in the terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) experiment, in which the nucleus is stained, high concentrations of shikonin induced nuclear fragmentation and apoptosis in oral cancer cells; D, shikonin caused the release of calcium ions in cancer cells, resulting in changes in the mitochondrial membrane potential and consequent cell death. Conversely, pretreatment of a calcium ion chelator (BAPTA-AM) can stop shikonin-induced cell apoptosis; E, higher proportion of Annexin V + labeling, a hallmark of early phase of apoptosis, was detected in cells treated with increasing doses of shikonin. * represened significantly increased and # represented significantly suppresed.
Moreover, shikonin stimulated the release of calcium ions in cancer cells, leading to changes in the mitochondrial membrane potential and subsequent cell death. Conversely, pretreatment with the calcium ion chelator (BAPTA-AM) inhibited shikonin-induced apoptosis (Figure 5D). Annexin V/PI double labeling was used to detect phosphatidylserine externalization, a hallmark of the early phase of apoptosis. A higher proportion of Annexin V-positive labeling was observed in cells treated with increasing doses of shikonin (Figure 5E).
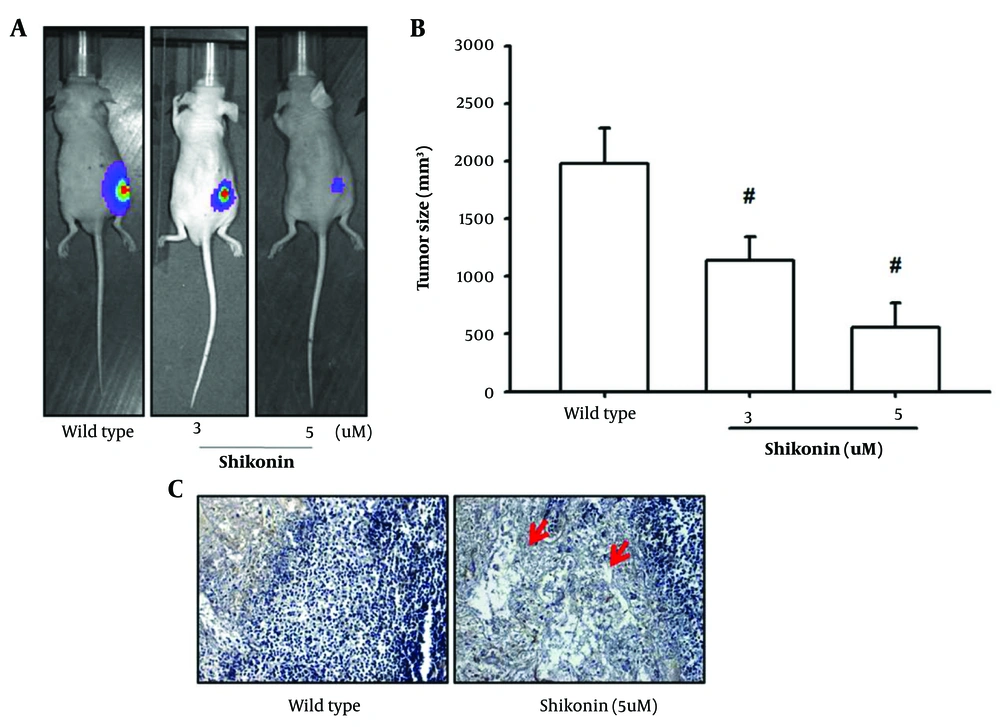
Furthermore, a mouse model was established by subcutaneously transplanting SCC-4 oral cancer cells into the backs of mice. Once the tumor masses reached a size of 1 cm × 1 cm, shikonin was administered regularly to observe its impact on tumor formation. After 20 days of treatment, tumor formation was inhibited, and tumor growth was significantly limited. Histological examination of the extracted tumors, stained with HE, revealed signs of apoptosis, including necrosis and cavities in tumors treated with shikonin. These in vivo experiments demonstrated that shikonin effectively induced apoptosis in oral cancer cells (Figure 6A - C).
A, mouse model was established by subcutaneously transplanting the SCC-4 oral cancer cell line into the back of the mice; B, tumor formation was inhibited, and tumor growth was significantly limited; C, histological examination of the extracted tumors stained with hematoxylin-eosin (HE) revealed signs of apoptosis, such as necrosis and cavities in the tumors treated with shikonin. # represented significantly inhibited when compared to the wild type.
5. Discussion
Shikonin, a naphthoquinone derivative extracted from the root of L. erythrorhizon, is widely used in traditional Chinese medicine (10, 11). Over the past few decades, numerous studies have demonstrated various biological effects of shikonin, including anti-HIV (12), anti-inflammatory (18, 19), antibacterial (20), and anticancer (21, 22) properties. Its anticancer function is particularly notable for its ability to induce cell apoptosis and necroptosis.
Previous studies have shown similar effects of shikonin on oral cancer cell lines, such as Tca-8113, Ca9-22, and SCC-25. It induces apoptosis through the caspase pathway and effectively inhibits cancer cell growth in cell cycle experiments (23, 24). However, the reproducibility and relationship between autophagy and apoptosis in different cell lines, as well as the effects of varying autophagy concentrations, remain unclear. Additionally, its potential to inhibit angiogenesis has not been thoroughly studied.
Therefore, this study aimed to address these uncertainties by confirming whether shikonin exhibits similar effects on the SCC-4 and SAS oral cancer cell lines, exploring the effects of different concentrations of shikonin on oral cancer, investigating the relationship between autophagy and apoptosis, and elucidating the potential benefits of shikonin in the inhibition of angiogenesis.
The results of this study demonstrated that shikonin exerts a dose-dependent inhibitory effect on SCC-4 and SAS oral cancer cell lines, significantly suppressing cell growth. Moreover, the effective concentration required for inhibiting oral cancer growth is lower than the previously reported 20 μM, with an IC50 value achieved at concentrations as low as 3 μM. Shikonin also influences autophagy by activating downstream proteins, initiating caspase pathways, and releasing calcium ions, ultimately leading to apoptosis.
Recent studies (from 2021 to 2024) have provided additional evidence supporting the autophagy-inducing properties of shikonin in various cancer types and other diseases. Hu et al. demonstrated that shikonin induces autophagy in colorectal cancer cells by modulating the microRNA-545-3p/GNB1 axis (13). Liu et al. reported that shikonin-induced ROS-dependent cell death involves complex interactions between necroptosis and autophagy (14). Furthermore, Chen et al. and Wang et al. observed autophagy-activating effects of shikonin in leukemia cells and osteoarthritis models, respectively (15, 16). These studies collectively reinforce our findings on the ability of shikonin to modulate autophagy in oral cancer cells and suggest a consistent mechanism across different cellular contexts.
The convergence of these recent findings with our results underscores the potential of shikonin as a promising therapeutic agent that acts via the activation of autophagy, warranting further investigation in oral cancer treatment. These findings align with previous studies on the effectiveness of shikonin in treating breast and colorectal cancer (25-27).
Interestingly, this study revealed that shikonin spares skeletal muscle cells and promotes their proliferation. This unexpected discovery indicates the potential of shikonin to facilitate skeletal muscle cell growth. However, this aspect was not explored further as it was beyond the scope of this study. Future research is warranted to investigate the feasibility of using shikonin to promote skeletal muscle cell proliferation.
Our study also revealed that shikonin significantly inhibits the synthesis of VEGF-A and VEGF-C, two key factors driving angiogenesis in oral cancer cells. Although angiogenesis was not directly simulated in vitro or in vivo in this study, these findings align with previous research on naphthoquinone derivatives, demonstrating their role in suppressing angiogenesis. For example, shikonin, along with acetylshikonin and isobutyrylshikonin, has been shown to inhibit VEGF activity in human umbilical vein endothelial cells and reduce tumor formation and angiogenesis in animal models (28). Moreover, beyond its anti-angiogenic effects in cancer, shikonin has demonstrated efficacy in inhibiting angiogenesis in rheumatoid arthritis (18), highlighting its therapeutic potential against excessive angiogenesis in various diseases.
In terms of cancer metastasis, shikonin exhibited inhibitory effects on the migration and adhesion capabilities of SCC-4 and SAS oral cancer cells. The expression levels of metastasis-related proteins, such as MMP-2 and MMP-9, decreased in a concentration-dependent manner, as evidenced by zymography experiments showing suppressed MMP activity. This inhibitory effect has also been observed in other cancer types, including lung cancer, hepatocellular carcinoma, and thyroid tumors (29-31).
One notable finding of this study is that the induction of autophagy by shikonin acts as a precursor to apoptosis. Previous studies have debated whether autophagy plays a protective or pro-apoptotic role in shikonin-treated cells. Kim et al. reported that autophagy activation protects cells from shikonin-induced apoptosis (32), whereas other studies have suggested that autophagy activation contributes to apoptosis (33). Our results demonstrated that shikonin-induced autophagy led to cell death once a lethal dose was reached, confirming that autophagy could not protect cells against the effects of high concentrations of shikonin.
These findings underscore the considerable potential of shikonin as a therapeutic agent against oral cancer.
5.1. Conclusions
This study demonstrated that shikonin significantly inhibits the mRNA and protein expression of VEGF-A and VEGF-C, thereby suppressing angiogenesis. It also impedes cell migration by inhibiting the expression of MMP-2 and MMP-9. As the drug concentration increased, autophagosome formation was induced in both oral cancer cell lines (SCC-4 and SAS) through the promotion of LC3 cleavage. Concurrently, Caspase 3 activation occurred, and calcium ions, indicative of endoplasmic reticulum stress, were released.
In addition, shikonin effectively inhibited oral cancer growth in a mouse model. Thus, shikonin exhibited remarkable inhibitory effects on cell growth, angiogenesis, and migration. However, this study primarily focused on comprehensively investigating the cancer treatment aspects of shikonin through cell and animal experiments.
Future research should explore detailed pathways and pharmacokinetics to achieve a deeper understanding of its mechanisms. These findings are expected to provide indispensable insights into the molecular mechanisms underlying oral cancer treatment and contribute to future advancements in the field.
5.2. Limitations and Future Work
Although our study provides compelling evidence for shikonin's anticancer effects on oral cancer, several limitations must be acknowledged. First, while we conducted preliminary in vivo experiments to examine tumor size reduction and histological changes, these animal studies were limited in scope. Specifically, they did not fully explore the effects of shikonin on metastasis and angiogenesis or its potential systemic side effects in living organisms. Second, our molecular investigations, although informative, primarily focused on a selected set of pathways and markers. While this targeted approach provided valuable insights, it may have overlooked other significant molecular mechanisms influenced by shikonin. Finally, this study did not address the long-term effects of shikonin treatment or its potential interactions with other therapeutic agents commonly used in oral cancer management.
To address these limitations and advance our understanding of shikonin's therapeutic potential, future studies should focus on several key areas. Comprehensive in vivo studies are essential to elucidate the effects of shikonin on oral cancer progression. These studies should employ advanced imaging techniques and a broader array of biomarkers to track cancer development and treatment responses in real time, providing a more holistic view of the efficacy and safety profile of shikonin. At the molecular level, high-throughput technologies such as RNA-seq and proteomics can be employed to uncover additional targets and signaling pathways affected by shikonin, both in vitro and in vivo. This broader mechanistic insight would not only enhance our understanding of shikonin's mode of action but also potentially reveal new therapeutic targets.
Furthermore, investigating the potential synergistic effects of shikonin in combination with current standard-of-care treatments for oral cancer could open new avenues for combination therapies. Such studies may result in more effective treatment regimens with fewer adverse effects. As we move toward potential clinical applications, future research should also prioritize optimizing the pharmacokinetic profile of shikonin and developing innovative drug delivery systems. These advancements could address challenges related to bioavailability and target specificity, which are critical for translating laboratory findings into clinical success. The initiation of preclinical safety studies and early-phase clinical trials will be a crucial step in assessing the viability of shikonin as a novel therapeutic agent for oral cancer.
By systematically addressing these research priorities, we can build on our current findings, overcome the limitations of this study, and potentially pave the way for the integration of shikonin into clinical practice, offering new hope for patients with oral cancer.