1. Background
Mangifera indica L., commonly known as mango, is an economically significant tropical herb from the Anacardiaceae family, native to South and Southeast Asia (1). Mango leaves, the most critical part of the plant, have traditionally been used as a remedy for a wide range of diseases, including asthma, diarrhea, diabetes, bronchitis, scabies, respiratory problems, urinary disorders, and syphilis (2). Documented studies have confirmed various pharmacological activities of mango leaves, such as analgesic, anti-inflammatory, and antimicrobial effects (3, 4), as well as antibacterial (4, 5), antioxidant (6-8), antipyretic (8), anti-inflammatory (8), anti-diabetic (9, 10), anti-cancer (11-13), hepatoprotective (14, 15), ameliorative (15), and anti-obesity effects (16-20). Although these effects are related to mango extract, the precise effectiveness of each isolated compound is not clearly explained. However, these effects are attributed to mangiferin due to its high concentration in mango.
Phytochemical analysis of M. indica L. leaves has revealed that minerals, vitamins, and phenolic compounds, such as phenolic acids, flavonoids, tannins, terpenoids, saponins, and alkaloids, are the most important classes of compounds (21). Mangiferin, neomangiferin, quercetin, epicatechin gallate, rutin, kaempferol-3-O-rutinoside, cinnamic acid and its derivatives, gallic acid, and protocatechuic acid are reported as the most significant compounds extracted from M. indica leaves (21). Pharmacological studies on mangiferin have demonstrated various medicinal effects, including anti-asthmatic, cardio-protective, anti-apoptotic, anti-inflammatory, immunomodulatory, antidepressant, anti-cancer, antibacterial, anti-diabetic, analgesic, gastro-protective, antioxidant, neuro-protective, anti-Alzheimer, and anti-hyperlipidemic effects (22).
2. Objectives
Given the broad range of pharmacological effects, this study aims to determine the optimal conditions for mangiferin extraction from M. indica L. leaves. Optimizing mangiferin extraction is crucial for maximizing its therapeutic effects and ensuring consistent quality in pharmaceutical formulations. Additionally, this optimization can enhance the cost-effectiveness of mangiferin extract in health supplements.
3. Methods
3.1. Plant Materials
The leaves of M. indica L. were purchased from the Tehran market in September 2023. The plant was identified and authenticated in the herbarium of the Faculty of Pharmacy, Tehran University of Medical Sciences, with herbarium code PMP-438. The leaves were washed with distilled water to remove contaminants. After 48 hours, the dried leaves were ground and powdered.
3.2. Extraction Using Dynamic Maceration Method
The powdered leaves of mango were sieved through a mesh 20. Seven solvents, including ethanol 96%, ethanol 70%, ethanol 50%, methanol 96%, methanol 70%, methanol 50% (all purchased from Pars Chemie), and water, were used to determine the most efficient and effective solvent for the extraction process. Ten mL of each solvent was separately added to different beakers, and 1 g of powdered mango leaves was added to each beaker. A magnet was placed into each beaker containing one gram of leaf powder and solvent. The beakers were covered with aluminum foil to reduce solvent evaporation and then placed for 1 hour on a hotplate stirrer (Heidolph MR Hei-Standard) at 500 rpm. Each extraction and solvent mixture was filtered through filter paper and dried for 48 hours. Each step of extraction was repeated three times for each solvent. The resulting extracts for each solvent were stored for further analysis using the high-performance liquid chromatography (HPLC) method to investigate the amount of mangiferin. This extraction process was conducted for 29 designed experiments of the Box-Behnken method. Ethanol 70% showed the most compelling results in the first step and was chosen as the best solvent for the first step of the extraction procedure.
3.3. High-Performance Liquid Chromatography Analysis Procedure
The HPLC-grade methanol was purchased from Merck (Darmstadt, Germany). Mangiferin European Pharmacopoeia (EP) reference standard with code Y0001751 and CAS Number 4773-96 was provided by Sigma Aldrich (USA), and purified water by the Milli-Q system was used for this study. A KNAUER Smartline (Berlin, Germany) HPLC instrument coupled to a DAD was applied for this study. The wavelength used for all analyses was 254 nm. An RP-18 reversed-phase column (SunFire C18 Column, 5 µm, 4.6 mm × 300 mm, 1/pk, San Francisco, United States) at room temperature was applied for this process. The eluents were 20:50% acetonitrile: Water. The isocratic method was used, and after 15 minutes, analysis was started. The injection volume was 20 μL, and the flow rate was 1 mL/min. After each injection, the column was washed for 30 minutes with water, acetonitrile, and then using 20:80 acetonitrile: Water solvent for ten minutes (23).
3.4. Determination of Mangiferin by High-Performance Liquid Chromatography
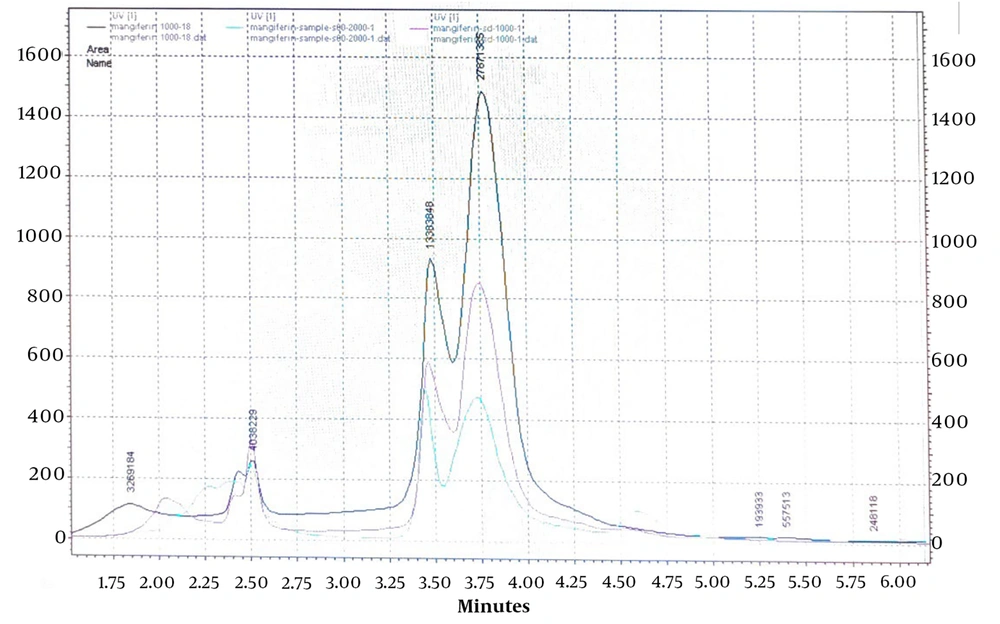
Solvent type, the ratio of solvents in the mobile phase, injection time, column temperature, detector wavelength, and elution method (isocratic or gradient) were considered the most influential factors in the HPLC analysis of this study. A calibration curve was obtained based on the standard concentration of mangiferin. One mg of dried extract of each sample was separately placed into a vial with 1 mL of methanol as solvent and then sonicated for 10 minutes. The solution was filtered through a 0.22 μm filter and then prepared for injection. A sample with a 1 mg/mL concentration was injected into the HPLC device under the mentioned conditions three times. The results showed that although the curves differed, the area under all curves was equal (Figure 1).
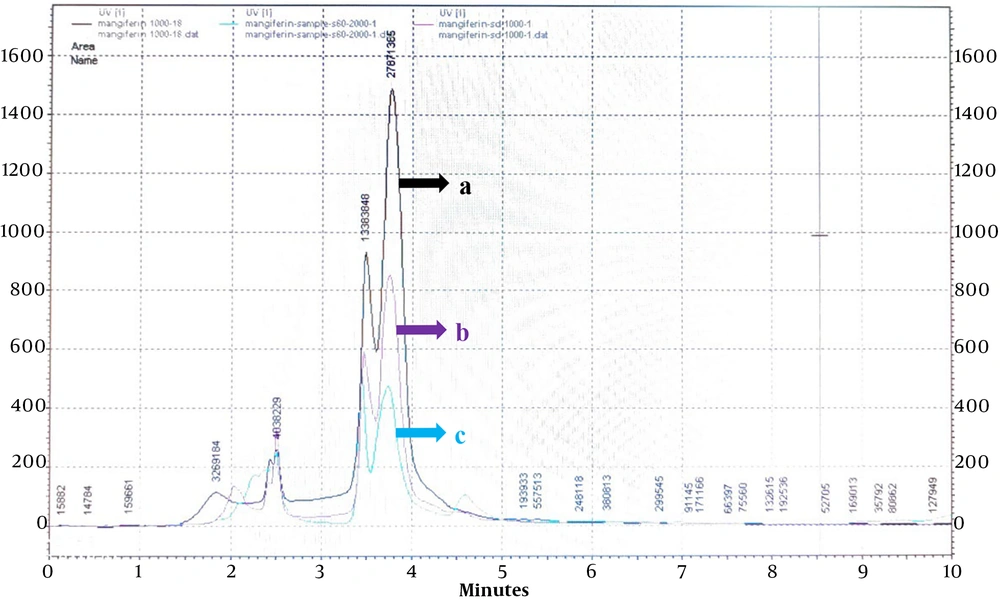
To validate the HPLC method and investigate the correspondence between the chromatograms of reference standard mangiferin and extracted mangiferin from mango leaves, three samples were prepared and analyzed. These samples included standard mangiferin (1 mg/mL), a mixture of extracted mangiferin (2 mg/mL) and standard mangiferin (1 mg/mL), and extracted mangiferin (2 mg/mL). Each sample was separately injected into the HPLC using the same method as described previously. The results of all three injections are presented in Figure 2.
To construct a calibration diagram, samples with concentrations of 1.5, 1, 0.5, 0.25, and 0.625 mg/mL of 70% methanol solvent (HPLC-grade methanol and deionized water) were prepared from the standard reference mangiferin. Each sample was injected into the HPLC device at least three times using the chosen method. The average area under the curve of mangiferin for each concentration was calculated. Based on these data points, a standard graph of mangiferin was plotted, with one axis representing the area under the curve and the other axis representing the concentration of the mangiferin sample (Table 1). The relationship between the concentration of mangiferin and the area under the peak was determined by plotting the graph and obtaining the equation of the line.
| Concentration (mg/mL) | Absorbance (in 254 nm) |
|---|---|
| 1.50 | 642 |
| 1.00 | 415 |
| 0.50 | 144 |
| 0.25 | 84 |
| 0.125 | 41 |
| 0.0625 | 20 |
The Absorbance of Different Concentrations of Standard Reference of Mangiferin in High-Performance Liquid Chromatography
3.5. Analyzing Mangiferin Content with High-Performance Liquid Chromatography and Optimizing the Extraction Method
The method was developed to properly separate HPLC and UV absorption for mangiferin. As previously mentioned, all extracts obtained from the first step of the extraction procedure were dried for 48 hours, and 5 mg of each extract was dissolved in 2.5 mL of HPLC-grade 70% methanol for 10 minutes. The filtered extracts from the experimental design were injected based on the HPLC method described in section 3.3, and the mangiferin concentration of each sample was calculated using the following equation:
Where y is the area under the mangiferin curve divided by 100,000, and x is the concentration of mangiferin in the analyzed sample in mg/mL.
3.6. Optimizing Parameters Based on a Screening Design
The Box-Behnken method was used to design the experiment. This method applies three levels — maximum, middle, and minimum — for each of the variables, determining which level must be met in the experiment. The dynamic maceration extraction method was chosen for its simplicity, cost and time efficiency, reduced solvent consumption compared to the traditional maceration method, and appropriate reproducibility. This method can also be generalized on a larger scale. Based on conducted searches, the optimization of mangiferin extraction from mango plant leaves using the dynamic maceration extraction method has not been previously reported.
Four variables were precisely investigated, expected to have the most significant impact on the extraction process: Solvent mixture, time, temperature, and the percentage of solvent to powder. It has been demonstrated that increasing the temperature from 15°C to 60°C increases the amount of mangiferin extracted. However, as the temperature increased from 60°C to 75°C, a significant decrease in mangiferin was observed (24). Increasing the temperature beyond 60°C decreased the amount of mangiferin in the final extract (25), likely due to solvent evaporation and degradation of mangiferin (24, 26).
Time is another critical factor in optimizing the extraction process of active pharmaceutical compounds in both industrial and laboratory settings. To optimize time, three different levels were utilized. Various solvents, including methanol, water, diethyl ether, acetone, pure hexane, and pure ethanol, were used to compare the dissolution of mangiferin, with pure ethanol demonstrating the highest amount of dissolved mangiferin (27).
The optimization process using ultrasonic waves showed that when the solvent was 44% ethanol, with a liquid-to-solid ratio of 38:1, at 19.2 minutes and 60°C under ultrasound irradiation of 200 W, the optimal extraction condition was achieved (25). Another critical factor affecting the extraction process is the ratio of powdered plant parts to solvent. Different ratios were applied to obtain the most effective ratio for extracting mangiferin from mango leaves (Table 2).
| Levels | Temperature (°C) | Time (min) | Solvent Concentration (v/v %) | Powder-to-Solvent Ratio (mL/g) |
|---|---|---|---|---|
| Maximum level | 60 | 90 | 80 | 7:1 |
| Medium level | 50 | 60 | 60 | 14:1 |
| Minimum level | 40 | 30 | 40 | 21:1 |
Different Parameter Levels are Used in the Extraction Process of Mangiferin
After selecting the variables and considering the appropriate levels for each, the experiment was designed using the Box-Behnken method to optimize the four critical factors in the extraction process: Temperature, time, solvent, and the ratio of powdered leaves to solvent, to obtain the maximum amount of mangiferin (28). Following the determination of time duration in the Box-Behnken design, the resulting liquid was passed through filter paper, and the filter paper was washed with 20 mL of the solvent used for the test. The obtained solution was then poured into a pre-weighed petri dish and placed under a hood to dry for 24 hours. These steps were performed for all 29 trials specified in the Design Expert software.
The Box-Behnken experiment design was based on four variable factors and five central points, resulting in 29 defined experiments. The design of the experiment was conducted using Design Expert software. In addition to determining the levels of each variable for each experiment, the order of experiments was also established. The designed experiments using the Box-Behnken method are presented in Table 3.
| Std. | Run No. | Factor 1 (Temp.) | Factor 2 (Time) | Factor 3 (Solvent Conc.) | Factor 4 (S/P) |
|---|---|---|---|---|---|
| 1 | 22 | 40 | 30 | 60 | 14 |
| 2 | 12 | 60 | 30 | 60 | 14 |
| 3 | 15 | 40 | 90 | 60 | 14 |
| 4 | 14 | 60 | 90 | 60 | 14 |
| 5 | 10 | 50 | 60 | 40 | 7 |
| 6 | 28 | 50 | 60 | 80 | 7 |
| 7 | 9 | 50 | 60 | 40 | 21 |
| 8 | 17 | 50 | 60 | 80 | 21 |
| 9 | 1 | 40 | 60 | 60 | 7 |
| 10 | 26 | 60 | 60 | 60 | 7 |
| 11 | 4 | 40 | 60 | 60 | 21 |
| 12 | 16 | 60 | 60 | 60 | 21 |
| 13 | 8 | 50 | 30 | 40 | 14 |
| 14 | 3 | 50 | 90 | 40 | 14 |
| 15 | 6 | 50 | 30 | 80 | 14 |
| 16 | 2 | 50 | 90 | 80 | 14 |
| 17 | 29 | 40 | 60 | 40 | 14 |
| 18 | 23 | 60 | 60 | 40 | 14 |
| 19 | 18 | 40 | 60 | 80 | 14 |
| 20 | 21 | 60 | 60 | 80 | 14 |
| 21 | 5 | 50 | 30 | 60 | 7 |
| 22 | 19 | 50 | 90 | 60 | 7 |
| 23 | 13 | 50 | 30 | 60 | 21 |
| 24 | 25 | 50 | 90 | 60 | 21 |
| 25 | 11 | 50 | 60 | 60 | 14 |
| 26 | 20 | 50 | 60 | 60 | 14 |
| 27 | 27 | 50 | 60 | 60 | 14 |
| 28 | 7 | 50 | 60 | 60 | 14 |
| 29 | 24 | 50 | 60 | 60 | 14 |
Experiment Design for the Optimization Process
To obtain the maximum amount of mangiferin, the practical factors in the extraction process must be optimized. The response surface method was employed for this purpose. This methodology is widely used to enhance process efficiency or increase product yield by modeling and identifying the optimum conditions of variables. A quadratic polynomial relationship was utilized in the response surface method. In this approach, a model is defined for each independent variable to examine the main and interaction effects of the factors on each dependent variable. The multivariate model is represented by the following equation:
In this equation, Y is the predicted response, b0 is a constant coefficient, b1, b2, b3 are linear coefficients, b11, b22, b33 are quadratic effects, and b12, b13, b23 are interaction effects. X1, X2, X3 are independent variables (29).
3.6.1. Statistical Analysis
The mathematical relationship between the response and the independent variables is modeled using a quadratic polynomial function. This equation evaluates linear or quadratic relationships and any interactions between independent variables and the response. The regression method and response surface diagrams were used for specialized statistical data analysis. The significance of independent variables on the response was evaluated using the ANOVA test with a P-value less than 0.05, applying Fisher’s test in GraphPad Prism. For better interpretation of the factors and the logical relationship between their interactions, three-dimensional diagrams were utilized.
4. Results and Conclusions
4.1. Solvent Selection
To determine the most effective solvent for the extraction process of mango leaves, various concentrations of ethanol and water were tested, including 96%, 70%, and 50% ethanol, as well as 96%, 70%, and 50% water. The results of the extraction process are presented in Table 4. As indicated, 70% ethanol yielded the highest amount of extract and was selected as the solvent for the extraction procedure. Consequently, 40%, 60%, and 80% ethanol were chosen as the minimum, middle, and maximum levels for further experimentation.
| Solvent | Extraction Weight (mg) | Extract Weight to Powdered Leaves (%) |
|---|---|---|
| 96% ethanol | 146.40 ± 2.49 | 14.6 |
| 70% ethanol | 224.50 ± 6.91 | 22.4 |
| 50% ethanol | 221.10 ± 4.86 | 22.1 |
| 96% ethanol | 93.70 ± 5.71 | 9.3 |
| 70% ethanol | 180.90 ± 4.10 | 18 |
| 50% ethanol | 196.20 ± 3.70 | 19.6 |
| Water | 177.50 ± 5.36 | 17.7 |
Result of Different Solvents Used in the Extraction Process of Mangiferin
4.2. Response Surface Method Experiment Design
Response surface methodology (RSM) employs a collection of mathematical and statistical techniques to analyze the effect of each variable on the response and investigate interactions between variables. Ultimately, it provides a non-linear model with multiple variables to determine extremum and optimization points. This method efficiently optimizes variables and reduces the number of trials, thereby minimizing time, cost, and experimental errors. The variables in this study included temperature (40, 50, and 60°C), time (30, 60, and 90 minutes), solvent concentration (40%, 60%, and 80%), and the ratio of solvent to powdered leaves (1:7, 1:14, and 1:21) (30, 31). The overall results of each trial, including extract weight, the area under the mangiferin peak, mangiferin concentration in each solution, and the yield of the extraction process, are presented in Table 5.
| Std. | Run No. | Factor 1 (Temp.) | Factor 2 (Time) | Factor 3 (S. Conc.) | Factor 4 (S/P) | Extract (mg) | AUC (× 100000) | Mangiferin Conc. (mg/mL) | Yield (Mg-g) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 22 | 40 | 30 | 60 | 14 | 247.50 | 131.81 | 0.361 | 44.75 |
| 2 | 12 | 60 | 30 | 60 | 14 | 250.60 | 128.58 | 0.354 | 44.38 |
| 3 | 15 | 40 | 90 | 60 | 14 | 253.10 | 131.93 | 0.361 | 45.80 |
| 4 | 14 | 60 | 90 | 60 | 14 | 260.25 | 131.14 | 0.360 | 46.85 |
| 5 | 10 | 50 | 60 | 40 | 7 | 180.52 | 120.12 | 0.334 | 30.23 |
| 6 | 28 | 50 | 60 | 80 | 7 | 199.45 | 133.65 | 0.365 | 36.48 |
| 7 | 9 | 50 | 60 | 40 | 21 | 258.27 | 131.64 | 0.361 | 46.65 |
| 8 | 17 | 50 | 60 | 80 | 21 | 264.95 | 140.05 | 0.380 | 50.40 |
| 9 | 1 | 40 | 60 | 60 | 7 | 188.90 | 132.97 | 0.364 | 34.40 |
| 10 | 26 | 60 | 60 | 60 | 7 | 193.80 | 129.31 | 0.355 | 34.49 |
| 11 | 4 | 40 | 60 | 60 | 21 | 264.02 | 139.99 | 0.380 | 50.20 |
| 12 | 16 | 60 | 60 | 60 | 21 | 258.50 | 132.28 | 0.362 | 46.88 |
| 13 | 8 | 50 | 30 | 40 | 14 | 237.20 | 126.90 | 0.350 | 41.56 |
| 14 | 3 | 50 | 90 | 40 | 14 | 249.92 | 134.86 | 0.368 | 46.06 |
| 15 | 6 | 50 | 30 | 80 | 14 | 250.47 | 140.00 | 0.380 | 47.63 |
| 16 | 2 | 50 | 90 | 80 | 14 | 246.52 | 140.88 | 0.382 | 47.13 |
| 17 | 29 | 40 | 60 | 40 | 14 | 229.10 | 134.34 | 0.367 | 42.08 |
| 18 | 23 | 60 | 60 | 40 | 14 | 235.35 | 122.65 | 0.340 | 40.09 |
| 19 | 18 | 40 | 60 | 80 | 14 | 250.75 | 132.12 | 0.362 | 45.42 |
| 20 | 21 | 60 | 60 | 80 | 14 | 244.20 | 138.34 | 0.376 | 45.97 |
| 21 | 5 | 50 | 30 | 60 | 7 | 185.00 | 129.46 | 0.356 | 32.95 |
| 22 | 19 | 50 | 90 | 60 | 7 | 188.25 | 132.24 | 0.362 | 34.13 |
| 23 | 13 | 50 | 30 | 60 | 21 | 269.12 | 133.01 | 0.364 | 49.03 |
| 24 | 25 | 50 | 90 | 60 | 21 | 269.90 | 136.70 | 0.372 | 50.31 |
| 25 | 11 | 50 | 60 | 60 | 14 | 253.45 | 134.17 | 0.367 | 46.51 |
| 26 | 20 | 50 | 60 | 60 | 14 | 252.82 | 136.65 | 0.372 | 47.11 |
| 27 | 27 | 50 | 60 | 60 | 14 | 256.32 | 136.10 | 0.371 | 47.60 |
| 28 | 7 | 50 | 60 | 60 | 14 | 256.25 | 135.85 | 0.370 | 47.51 |
| 29 | 24 | 50 | 60 | 60 | 14 | 258.47 | 137.77 | 0.375 | 48.49 |
Overall Results of 29 Trials Under Different Conditions
A quadratic polynomial equation is employed as a model in RSM. At the response level, the defined model for each independent variable investigates the effect of main and interaction factors on each dependent variable. The multivariate model is represented by the following equation:
In this equation, Y is the predicted response, b0 is a constant coefficient, b1, b2, b3 are linear coefficients, b11, b22, b33 are quadratic effects, and b12, b13, b23 are interaction effects. X1, X2, X3 are independent variables (29).
4.3. Data Analysis Using Design Expert Software
After designing the experiment, conducting tests under specified levels of each variable, and determining the response of each to predict the system's behavior even at untested points between the maximum and minimum levels of the variables, selecting a suitable model for data analysis is essential. Among linear, quadratic, and cubic models, the one most compatible with the data and variance analysis should be applied. Table 6 presents the models used to determine the extraction yield based on mangiferin and the variables.
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | P-Value |
|---|---|---|---|---|---|
| Mean vs. total | 55712.75 | 1 | 55712.75 | ||
| Linear vs. mean | 754.43 | 4 | 188.61 | 24.55 | < 0.0001 |
| 2FI vs. linear | 12.84 | 6 | 2.14 | 0.22 | 0.9635 |
| Quadratic vs. 2FI | 159.5 | 4 | 39.88 | 46.39 | < 0.0001 |
| Cubic vs. quadratic | 5.39 | 8 | 0.67 | 0.61 | 0.748 |
| Residual | 6.64 | 6 | 1.11 | N/A | N/A |
| Total | 56651.56 | 29 | 1953.50 | N/A | N/A |
Evaluated Models Showing the Relationship Between Mangiferin Yield and Variables in Response Surface Method
F-value and P-value were utilized to determine the correlation and compatibility of data with the suggested models. In ANOVA, F-value and P-value are calculated for quadratic and cubic equations. A higher F-value and a lower P-value indicate better data compatibility with the proposed model. As shown in Table 6, the quadratic equation, with an F-value of 46.39 and a P-value of < 0.0001, respectively, had the most compatibility with data, and this equation was chosen as the proper model.
In addition, a lack of fit (LOF) test was applied to determine the validation of the proposed model. In this test, P-values show that the LOF with data is insignificant. Based on the results exhibited in Table 7, the quadratic equation had an insignificant LOF with data. This showed that the selected model (quadratic model) is the most desired for data analysis in ANOVA.
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | P-Value |
|---|---|---|---|---|---|
| Linear | 182.27 | 20 | 9.11 | 17.30 | 0.0067 |
| 2FI | 169.43 | 14 | 12.1 | 22.98 | 0.004 |
| Quadratic | 9.93 | 10 | 0.99 | 1.88 | 0.2835 |
| Cubic | 4.54 | 2 | 2.27 | 4.31 | 0.1006 |
| Pure error | 2.11 | 4 | 0.53 | - | - |
Evaluated Models in Terms of Lack of Fit in ANOVA Analysis
The quadratic equation also showed the main effects of each variable and the interactions of the effects on each other. This exhibited more fitness and compatibility with the suggested model. Table 8 shows the properly designed model.
| Sources | Sum of Squares | Degree of Freedom | Mean Square | F-Value | P-Value Prob > F |
|---|---|---|---|---|---|
| Model | 920.29 | 8 | 115.04 | 124.22 | < 0.0001 |
| X2 (min) | 8.3 | 1 | 8.30 | 8.96 | 0.0072 |
| X3 [solvent concentration (v/v%)] | 57.9 | 1 | 57.90 | 62.53 | < 0.0001 |
| X4 [solvent-to-powder ratio (mL/g)] | 686.9 | 1 | 686.90 | 741.73 | < 0.0001 |
| X1X4 | 2.91 | 1 | 2.91 | 3.14 | 0.0917 |
| X2X3 | 6.25 | 1 | 6.25 | 6.75 | 0.0172 |
| 16.08 | 1 | 16.08 | 17.36 | 0.0005 | |
| 20.53 | 1 | 20.53 | 22.17 | 0.0001 | |
| 148.03 | 1 | 148.03 | 159.84 | < 0.0001 | |
| Residual | 18.52 | 20 | 0.93 | ||
| LOF | 16.41 | 16 | 1.03 | 1.95 | 0.2731 |
| Pure error | 2.11 | 4 | 0.53 | N/A | N/A |
| Correlation total | 938.81 | 28 | N/A | N/A | N/A |
| R-squared | 0.9803 | - | - | - | - |
| Adj R-squared | 0.9724 | - | - | - | - |
| Pred R-squared | 0.9506 | - | - | - | - |
The ANOVA Analysis for the Response Surface Quadratic Model
To achieve better fitness and compatibility, improper terms were removed. Accordingly, all terms with insignificant P-values (P-value > 0.1) were excluded from the final equation. The terms X1, X1X2, X1X3, X2X4, X3X4 and
The correlation coefficient R2 and adjusted correlation (Adjusted R2) were 0.98 and 0.972, respectively, indicating that the proposed model accounts for more than 97% of the response variables. The predicted correlation (Predicted R2) of the model was 0.95, showing a strong correlation between predicted and actual values.
The final equation is as follows:
Where Y is the amount of extracted mangiferin from 1 gram of powdered mango leaves, X2 is time, X3 is the ethanol concentration as a solvent, and X4 is the solvent (mL) ratio to powdered mango leaves (g).
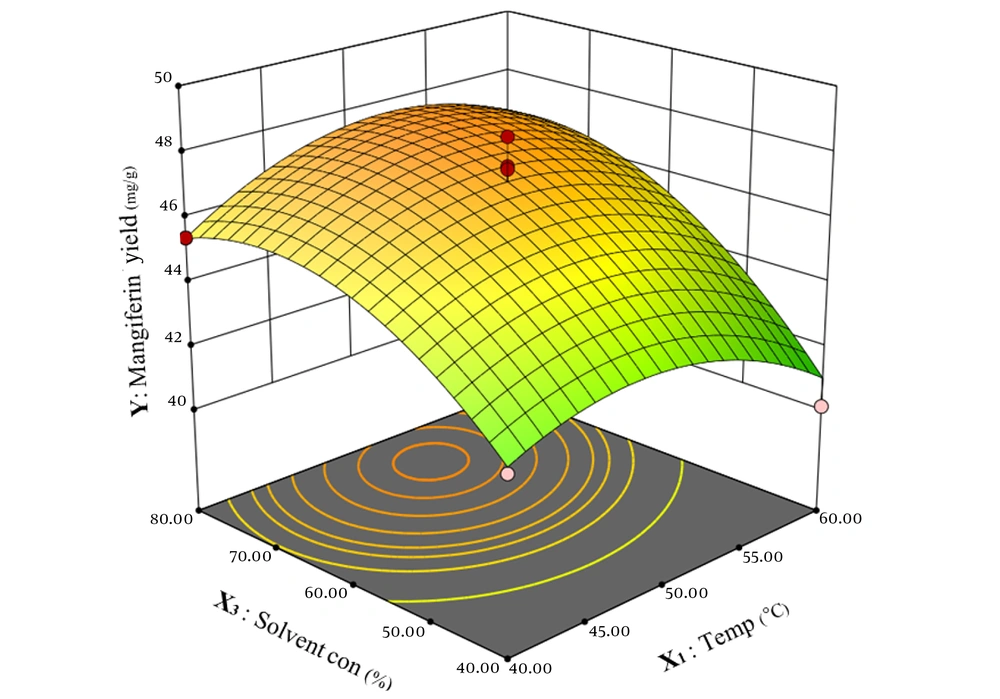
Using Design Expert software, a three-dimensional response surface graph was drawn. This plot examines the effects of two variables simultaneously and provides insight into the correlation between variables and response. The simultaneous relationship between two variables and the amount of mangiferin or extraction yield based on the amount of mangiferin is depicted, while the other two variables are at their median level.
Figure 3 illustrates the simultaneous correlation of temperature and solvent concentration on mangiferin yield in the extraction process. As depicted in the figure, the effects of the two variables — temperature and solvent concentration — on the response are linear. Increasing the temperature from 40°C to approximately 50°C enhances the amount of mangiferin extracted. However, temperatures above 60°C lead to a reduction in the total extract and mangiferin content.
Similarly, solvent concentration exerts a comparable effect on mangiferin yield. As the ethanol concentration increases from 40% to about 70%, the amount of mangiferin improves non-linearly. Conversely, increasing ethanol concentration from 70% to 80% results in a reduction in mangiferin extraction.
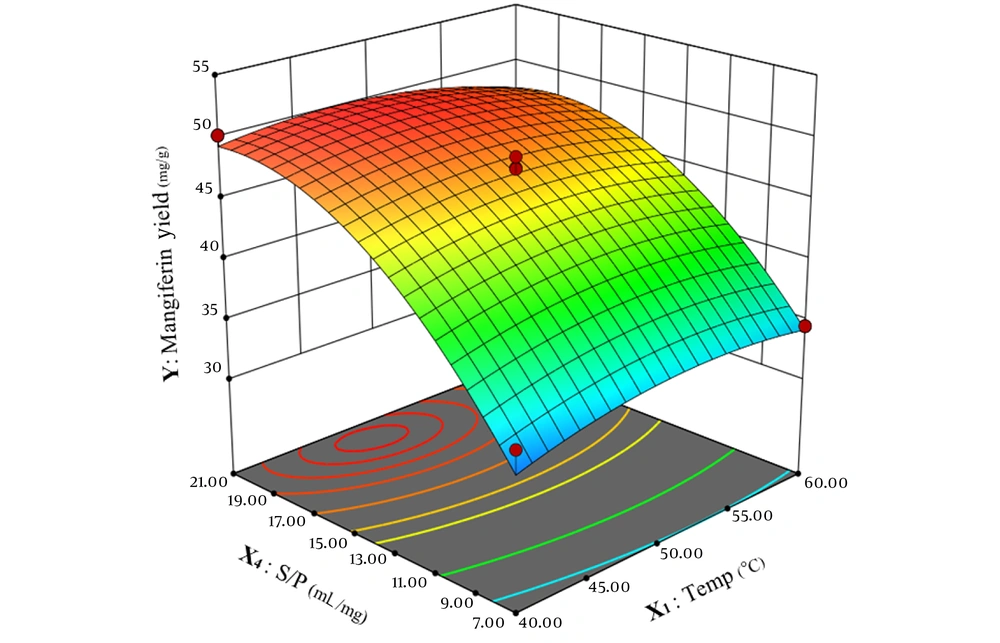
Figure 4 illustrates the simultaneous effects of temperature and the solvent-to-powdered leaves ratio on mangiferin yield. The plot indicates that increasing the ratio of solvent to powdered leaves from 7 to 19 results in an increase in both the total extract and the amount of mangiferin. However, further increasing this ratio beyond 19 shows no additional effect on the extraction process, with the total extract and mangiferin remaining constant. The temperature exhibits the same effect on the extraction process, and raising the temperature from 40°C to 45°C enhances the extraction process, and the amount of mangiferin increases in the extract. However, the temperature between 50°C to 60°C reduces the extraction yield, and as a result, the extracted mangiferin declines.
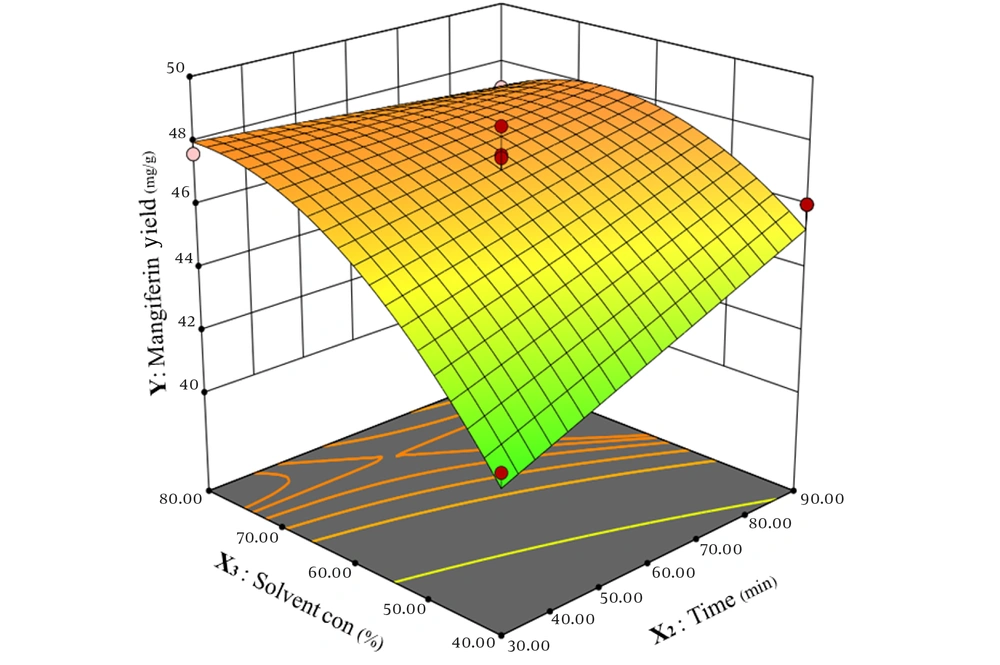
As shown in Figure 5, time exhibits a linear effect on mangiferin extraction. Increasing the extraction time from 30 minutes to 90 minutes results in a rise in the amount of extracted mangiferin. Solvent concentration, as another variable, demonstrates a non-linear effect on mangiferin extraction. Increasing the ethanol concentration from 40% to 80% enhances the amount of extracted mangiferin. However, at temperatures above 70°C, the extraction of mangiferin reaches its peak when using ethanol with a lower concentration.
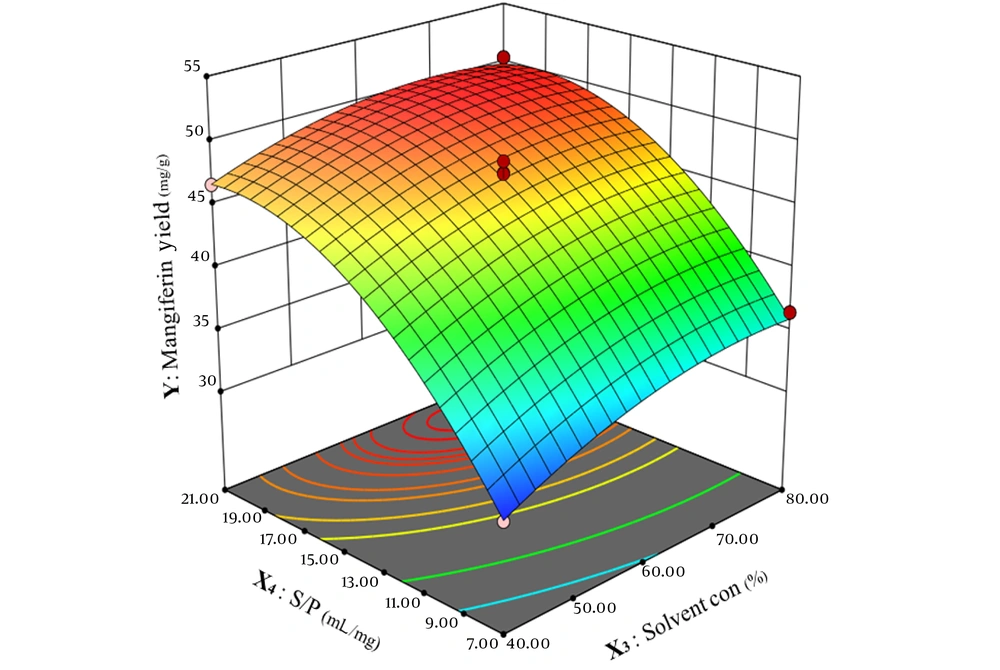
According to Figure 6, the effect of solvent concentration and solvent-to-powdered mango leaves is non-linear. The amount of extracted mangiferin reaches its peak when methanol concentration is 65% to 75%, and the ratio of solvent-to-powder is 18 to 21.
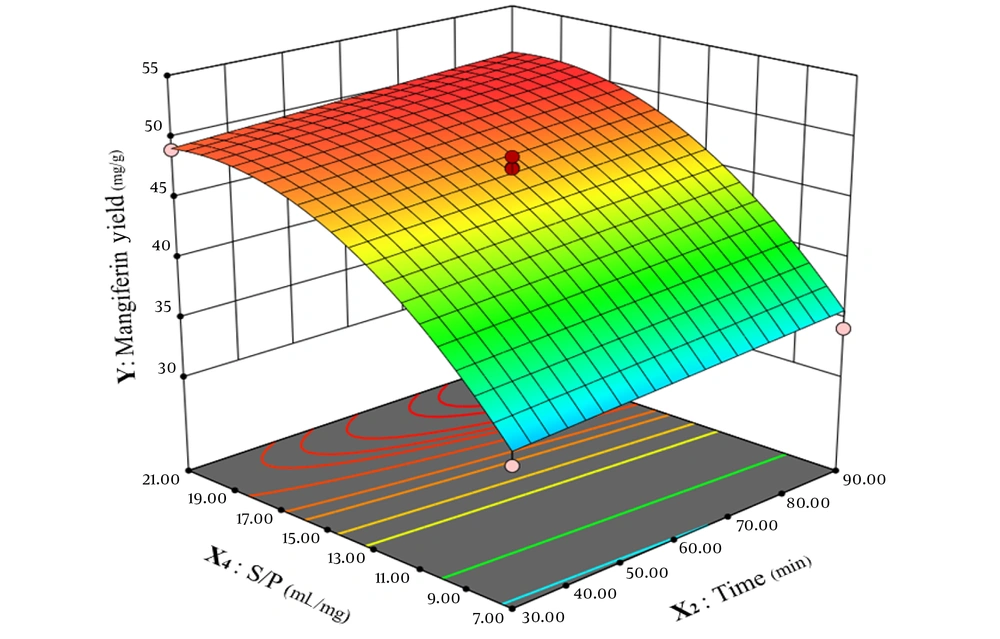
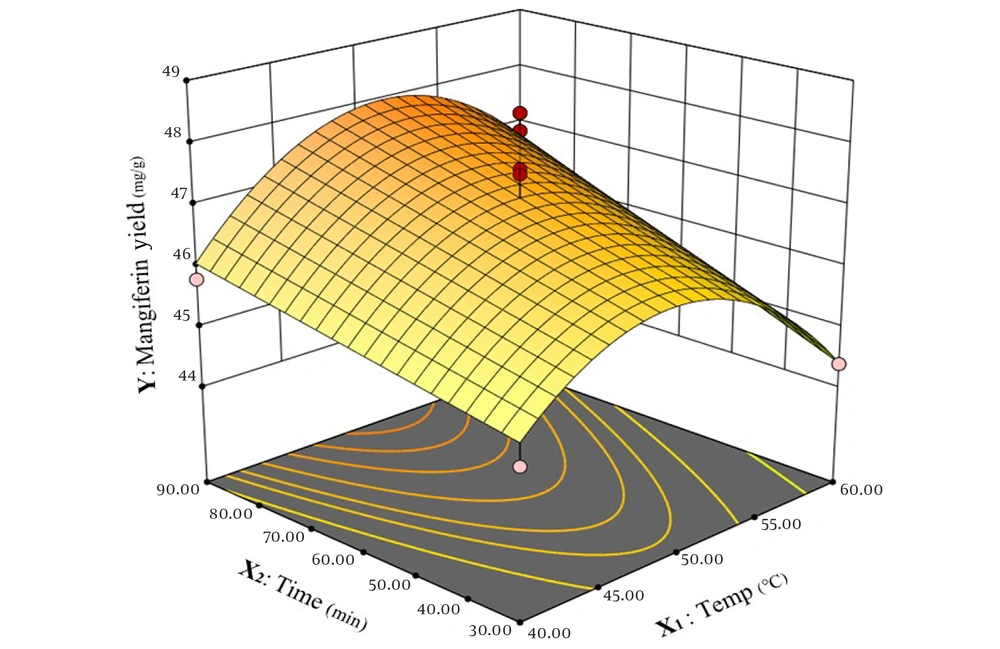
As depicted in Figure 7, time shows the linear correlation to mangiferin extraction and enhancing time from 30 minutes to 90 minutes, improving the extraction process of mangiferin and the amount of mangiferin raised on a constant base. On the other hand, the correlation between solvent-to-powdered leaves of mango and mangiferin extract is non-linear, and elevating this ratio from 7 to 20 increases mangiferin extraction yield while raising the ratio of solvent-to-powdered leaves from 20 to 21 has no significant effect on mangiferin amount in the extract.
As illustrated in Figure 8, temperature has a non-linear effect on the extraction of mangiferin. The relationship between temperature and the extraction process of mangiferin does not follow a constant rate, with the highest yield of mangiferin obtained at 50°C. Additionally, there is a linear correlation between extraction time and the amount of mangiferin extracted, reaching a peak after 90 minutes. To determine the optimal points from the resulting equation for each variable, the derivative of each equation was computed. This process resulted in four equations with four unknown parameters, and the optimal points were obtained by solving these equations. Based on these points, the relationship and fit between the predicted and experimental amounts of mangiferin were investigated using the point with the highest yield to evaluate the model experimentally. According to the proposed model, the maximum amount of mangiferin extracted from 1 gram of powdered mango leaves was achieved under the following conditions: Temperature of 54°C, extraction time of 88 minutes, a solvent-to-powdered leaves ratio of 19:1, and a solvent concentration of 66% ethanol. It is predicted that under these conditions, 50.4 mg of mangiferin can be extracted from 1 gram of powdered leaves. The extraction of mangiferin was conducted based on the conditions described in section 3.3 to validate the experimental model. The obtained extract was then analyzed by HPLC, and the results are presented in Table 9.
| Std | Factor 1 (Temp) | Factor 2 (Time) | Factor 3 (S Con) | Factor 4 (S/P) | Extract (mg) | AUC (× 100000) | Mangiferin Con (mg/mL) | Yield (Mg-g) | Predicted Yield (Mg-g) | SD |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | 88 | 66 | 19 | 267 | 139.71 | 0.379 | 50.68 | 50.40 | 0.65 |
Optimal Conditions for Mangiferin Extraction in Dynamic Maceration
As shown in Table 9, the experimental yield (50.68 mg) and predicted yield (50.4 mg) are remarkably close, validating the suggested model through experimental responses. This study successfully optimized the extraction of mangiferin from M. indica (mango) leaves using the dynamic maceration method, providing significant insights into the variables affecting extraction efficiency. Utilizing RSM, the research identified optimal conditions for maximizing mangiferin yield: A temperature of 54°C, extraction time of 88 minutes, 66% ethanol concentration, and a solvent-to-leaf powder ratio of 1:19. Under these conditions, an impressive yield of 50.68 mg of mangiferin per gram of powdered leaves was achieved. The findings underscore that temperature, time, solvent concentration, and the ratio of solvent to powdered leaves influence extraction efficiency. Previous studies confirmed these results, suggesting that increased temperature enhances diffusion and solubility, thereby improving extraction rates. However, it is essential to note that excessively high temperatures may lead to solvent evaporation and potential degradation of mangiferin, which can diminish yield.
This study employed the dynamic maceration method to obtain an extract with the highest amount of mangiferin from M. indica (mango) leaves. The response surface statistical method was used to identify the variables involved in the extraction process that result in the highest amount of mangiferin extracted from the plant leaves. The response surface method efficiently investigates the simultaneous effects of several variables on the response and detects the interaction between the variables. Furthermore, it provides a model of the relationship between the different factors and their response, expressed as a function of the variables. The design of the experiment using the response surface method allows for the prediction of variables' effects on the response across the range of variable values, determining the levels of the variables that cause the maximum or minimum response by predicting the response value.
In this study, the response method was used to find the maximum response, applying the four variables of temperature, time, solvent concentration, and solvent-to-powdered leaves ratio, which significantly affected mangiferin extraction. The results showed that the optimal conditions to obtain the maximum amount of mangiferin from ethanol extract of mango leaves are as follows: Temperature, 54°C; time, 88 minutes; 66% ethanol; and solvent-to-powdered leaves ratio, 1:19. Under these conditions, 50.68 mg of mangiferin was extracted from 1 gram of powdered leaves, indicating that mangiferin comprises almost 5% of dried leaves and 19% of the total mango extract. Depending on extraction conditions, the maximum amount of mangiferin ranges from 30 to 60 mg. Factors such as the type of mango plant, climate conditions, soil, cultivation season, and extraction methods and conditions, including solvent and its concentration, extraction time, temperature, and pH, are essential in mangiferin extraction.
In optimizing mangiferin extraction from mango leaves using RSM and the Box-Behnken design method, four variables — ethanol concentration as the solvent, the ratio of solvent to mango leaf powder, temperature, and time of the extraction process utilizing ultrasonic waves — were investigated and optimized to obtain the maximum amount of mangiferin from mango leaves. The study showed that the optimal conditions were 44% ethanol, a solvent-to-powder ratio of 1:38, time of 19.2 minutes, and a temperature of 60°C. Under these conditions, the extraction efficiency of mangiferin was 58.46 ± 1.27 mg per gram of dry mango leaf powder (25). Ultrasound-assisted extraction of mangiferin from M. indica L. leaves by RSM demonstrated that the optimal extraction conditions were 44% ethanol, a liquid-to-solid ratio of 38:1, and extraction for 19.2 minutes at 60°C under ultrasound irradiation of 200 W. Under optimal conditions, the yield of mangiferin was 58.46 ± 1.27 mg/g (32).
Using ultrasonic waves, two variables — temperature and time — were investigated and optimized to enhance the extraction of mangiferin and phenolic compounds from mango fruit peel. The optimal conditions for these variables were determined to be 54°C and 10 minutes, respectively, resulting in an extracted mangiferin amount of 3.2 mg per gram of dry powder (33). In a study aimed at optimizing the variables involved in harvesting and extracting mangiferin from the leaves of Swertia chirata of the Gentianaceae family, a complete factorial experiment design method was employed. The highest amount of mangiferin was obtained using an ultrasound extraction method with 50% ethanol solvent and a duration of 30 minutes, while variables such as temperature (40°C) and device power (200 W) remained constant. Under these conditions, the efficiency of mangiferin was 4.86% ± 0.19 of the plant's total dry leaf weight.
To optimize the extraction of mangiferin from Phaleria macrocarpa fruits using a water system below the critical temperature and response surface method, temperature and extraction duration were investigated and optimized. The results indicated that the optimum temperature and extraction time were 105°C and 6 hours, respectively (34). Under these conditions, the amount of mangiferin extracted was 38.7 mg per gram of dry powder (35). In the dynamic maceration extraction method, the duration of extraction and solvent exposure to heat is much longer than in ultrasonic wave extraction. In this study, the solvent temperature first reached the desired level in the experiment design before adding the mango leaf powder to initiate the extraction process. Therefore, the results obtained in this study confirm previous findings on the effect of temperature on mangiferin extraction. Temperature influences diffusion, solubility, surface tension, and viscosity, providing the necessary energy to break the bonds between raw material components and making them available to the solvent. Thus, increasing the extraction temperature enhances the diffusion of compounds in the solvent and increases the extraction rate. However, this relationship is not necessarily linear, as solvent evaporation and potential oxidation and degradation of mangiferin at temperatures above 55 - 60°C may decrease the amount of mangiferin in the extract (26, 36-39).
Based on the obtained results, the relationship between extraction time and the amount of extracted mangiferin is linear. Increasing the extraction time from 30 to 90 minutes increases mangiferin efficiency with a relatively constant slope, as long-term exposure of the sample to the solvent provides sufficient time for the desired compounds to be extracted (40). Zou et al. compared conventional extraction and microwave extraction methods for mangiferin extraction. In this study, 1 gram of dried mango leaf powder was added to 30 mL of 40% ethanol and mixed for 15 minutes using a stirrer to enhance solvent penetration. The mixture was then left at room temperature for 30, 60, 90, and 120 minutes to investigate mangiferin extraction. The results showed that increasing the extraction time from 30 to 120 minutes enhanced mangiferin extraction, although extending the time from 90 to 120 minutes reduced the amount of extracted mangiferin from mango leaves (32).
The relationship between the solvent-to-powdered leaves ratio of mango leaves and the amount of extracted mangiferin is non-linear. Increasing this ratio from 7 to 20 elevates the yield of mangiferin non-linearly and then remains almost constant. Generally, a small liquid-to-solid ratio leads to lower extraction efficiency, while a larger ratio increases the solvent’s capacity to dissolve plant compounds, enhancing extraction efficiency. However, increasing this ratio also raises the volume of solvent used, leading to solvent wastage and decreased economic efficiency of extraction. Therefore, selecting an appropriate solvent volume is essential (41).
Water, ethanol, and methanol are the most common solvents used in extracting plant compounds. Water is less expensive than other solvents, non-toxic to humans and the environment, and capable of dissolving a wide range of compounds. However, a mixture of water with other solvents provides higher extraction efficiency than using water alone (32). Mangiferin, a molecule with medium polarity, is expected to dissolve in water, methanol, and ethanol (26). Similar studies have demonstrated that these factors are more significant than others. Kaur et al. and Ramirez-Brewer et al. showed that these variable parameters are more effective than others (34, 42). Zou et al. investigated that the optimal conditions for mangiferin yield were a 40% ethanol concentration, a 30:1 mL/g liquid-to-solid ratio, and an extraction time of 20 minutes at 60°C (25). A study in 2019 demonstrated that acetone is more permeable than ethanol for the root of Salacia chinensis, yielding 92 mg of mangiferin per gram of root (43).
Before designing the experiment, different percentages of water, ethanol, and methanol solvents were compared regarding the weight of the extract obtained from mango leaves. The results showed that a combination of water and ethanol as a solvent produced the highest amount of extract from mango leaves. Based on the results, the relationship between ethanol concentration and mangiferin extraction efficiency is non-linear. Mangiferin extraction increases significantly with an increase in ethanol concentration from 40% to approximately 70%, while a concentration increase to 80% ethanol causes a slight decrease in efficiency. The statistical robustness indicates a strong correlation between experimental data and model predictions.
In conclusion, optimizing mangiferin extraction from M. indica leaves through dynamic maceration using RSM enhances our understanding of the extraction process and lays a foundation for future research to improve efficiency and yield in phytochemical extractions. Dynamic maceration offers advantages in terms of simplicity and lower initial equipment costs, and this method is preferable to the ultrasonic method due to a lower rate of degradation. This standardization is crucial for pharmaceutical and nutraceutical applications. However, other factors such as pH, plant maturity, and post-harvesting handling should be explored. Additionally, other eco-friendly extraction methods and green solvents may influence the yield of this process. Further investigations into varying environmental conditions and alternative methods could yield even more efficient extraction protocols for this valuable compound.