1. Background
Cervical cancer is the fourth most common cancer among women, with the most significant cause being infection with the HPV virus. Treatment options include surgery, chemotherapy, and radiotherapy, which are used alone or in combination (1).
Quercetin (3,5,7,3',4'-pentahydroxyflavone) is a polyphenolic compound derived from flavonoid-containing plants. Quercetin exhibits anti-inflammatory, antihypertensive, and especially anticancer properties. Some studies have highlighted the role of this compound in regulating proteins such as Akt, EGFR, IL-8, mTOR, NF-kB, PI3K, and TNF-α, as well as various microRNAs, including miR-21 and miR-34a-5p (2, 3).
MicroRNAs are essential for normal animal development and play a role in various biological processes. Abnormal expression of microRNAs is associated with many human diseases (4). miR-21 is one of the first identified microRNAs, with several target proteins, most of which are tumor suppressors such as PTEN. The expression of miR-21 is elevated in certain cancers. miR-21 plays a critical role in cervical tumor metastasis by activating pathways related to invasion and migration (5). Quercetin has been shown to reduce miR-21 expression in some cell types (6). On the other hand, the expression of miR-34a-5p is decreased in cervical tumors, and research has demonstrated that miR-34a-5p plays a significant role in inhibiting cell proliferation and migration (7).
The effect of quercetin on miR-21 and miR-34a-5p in cervical cancer cell lines such as HeLa and Ca Ski has not yet been explored. The results of this study may provide valuable insights into the role of quercetin in cancer cells.
2. Objectives
To investigate the effect of quercetin on the expression levels of miR-21 and miR-34a-5p in HeLa and Ca Ski cervical cancer cell lines.
3. Methods
3.1. Reagents and Cell Culture
Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (FBS), and penicillin-streptomycin were purchased from Gibco (USA). MTT and quercetin (CAS 117-39-5) were obtained from Sigma Aldrich (USA), while dimethyl sulfoxide (DMSO) was sourced from Merck (KGaA, Darmstadt, Germany). Cell-culture-grade chemicals and solutions were acquired from Biosera Europe (Neville, France) or other vendors and were used without further purification. Consumables were procured from SPL Life Sciences (Gyeonggi-do, Korea).
The human HeLa and Ca Ski cervical cancer cell lines were purchased from the National Center of Genetic and Biological Resources of Iran. The cell lines were cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin at 37°C in a 5% CO2 atmosphere.
3.2. Bioinformatic Approaches
We identified the key microRNAs involved in cervical cancer using the HMDD v4.0 database (http://www.cuilab.cn/hmdd). This database classifies and displays the expression of the most relevant microRNAs related to specific cancers, based on laboratory-proven data and the number of published articles. Subsequently, we obtained the top twenty targets of miR-21 and miR-34a-5p, which are most significant in carcinogenesis, from the miRTarBase database. The findings were then visualized using the NetworkAnalyst website.
The PubChem database was used to identify all microRNAs targeted by quercetin that are involved in cancer, as well as all proven quercetin targets in the process of apoptosis. The data were finally illustrated using Cytoscape network drawing software (8).
3.3. Cytotoxicity Screening
The cytotoxicity of quercetin was evaluated using the MTT method as previously described (9). Cells were seeded at a density of 10,000 cells per well in a 96-well plate. A quercetin solution (100 mM) was prepared in DMSO, and different concentrations of the solution were diluted using the culture medium. Cells were treated with varying concentrations of quercetin (10 to 2000 μM) for 48 hours at 37°C. A stock solution of MTT (5 mg/mL) was added, and the plates were incubated for three hours in the dark at 37°C. The resulting formazan was solubilized using DMSO, and optical density was measured at 570 nm using a microplate reader (BioTek ELx800, USA) after two hours of incubation. IC25 and IC50 values were then calculated using GraphPad Prism 8.0.2 software.
3.4. Apoptosis Assay
The HeLa and Ca Ski cells were cultured in 12-well plates at a density of 2×10⁵ cells/well to investigate apoptosis using the Annexin V/PI technique. Cells were treated with 50 μM quercetin for HeLa cells and 200 μM quercetin for Ca Ski cells, with the negative control consisting of culture medium containing DMSO. After 48 hours, apoptosis was evaluated using the Annexin V-FITC/PI apoptosis kit from Abcam (USA). The Partec CyFlow Space flow cytometer (Germany) was used to assess the percentage of apoptosis, and FlowJo software was utilized to analyze the data (10).
3.5. RNA Extraction
Total RNA was extracted 48 hours post-quercetin treatment using TRIzol reagent (Thermo Fisher Scientific, MA). In brief, after removing the medium, TRIzol reagent was directly added to the cell culture flasks, and the lysate was homogenized by pipetting and swirling several times. The lysate was then transferred into an RNase-free tube and mixed with 0.2 mL chloroform. After a 3-minute incubation, the mixture was centrifuged at 12,000 g for 15 minutes. The colorless upper aqueous phase, containing the RNA, was transferred to a new tube. RNA was precipitated using 0.5 mL isopropanol, followed by centrifugation at 12,000 g for 10 minutes at 4°C. The RNA pellet was washed with 75% ethanol, resuspended in 0.03 mL of RNase-free water, and either immediately processed for the next step or stored at -70°C. The quality of the RNA was assessed based on the 260/280 and 260/230 ratios using a NanoDrop spectrophotometer (Thermo Scientific NanoDrop 2000, Finland).
3.6. cDNA Synthesis
Selected miRNA-specific cDNAs were generated using the Parsgenom RT reagent kit (Parsgenome, Iran), following the manufacturer's instructions. Poly A polymerization was performed to synthesize the cDNA. The reaction mixture included 5X reaction buffer, dNTP mix (10 mM), RT enzyme, cDNA synthesis primer (15 pM), and RNA with a Poly A tail (up to 2 μg), prepared according to the kit's instructions to generate specific cDNAs for each microRNA.
3.7. Real-Time qPCR Assay
The expression levels of miR-21 and miR-34a-5p were assessed through qRT-PCR using a StepOnePlus qPCR instrument (Applied Biosystems, Life Technologies Corporation, USA). Real-time PCR was conducted to analyze the cDNAs using the Parsgenom RT reagent kit (Parsgenome, Iran). The final PCR reaction volume (20 μL) consisted of 10 μL Sybr Green master mix, 1 μL primer mix (10 pM), 8 μL distilled water, and 1 μL cDNA. The qRT-PCR protocol included an initial denaturation at 95˚C for 15 minutes, followed by 40 cycles of 95˚C for 30 seconds, 63˚C for 30 seconds, and 72˚C for 30 seconds. A melting curve analysis was performed to confirm the specificity of the products. The qRT-PCR amplification efficiency and cycle threshold (Ct) values were analyzed using the LinRegPCR program. Expression values were normalized using U6 rRNA as a reference gene and presented as relative expression using the 2–ΔΔCt method (11). Relative gene expression values lower than 1 were converted into fold change using the following formula:
3.8. Statistical Analyses
The data is presented as mean ± SD. GraphPad Prism version 8.0.2, was used for statistical analysis. Both methodological and biological replicates were included in the study. A statistically significant difference was defined as P < 0.05. The Kolmogorov-Smirnov test was used to assess the normal distribution of continuous numerical variables. ANOVA, followed by Bonferroni's test, was used to statistically evaluate flow cytometry analyses.
4. Results
4.1. Bioinformatic Studies
4.1.1. MicroRNAs Involved in Cervical Cancer
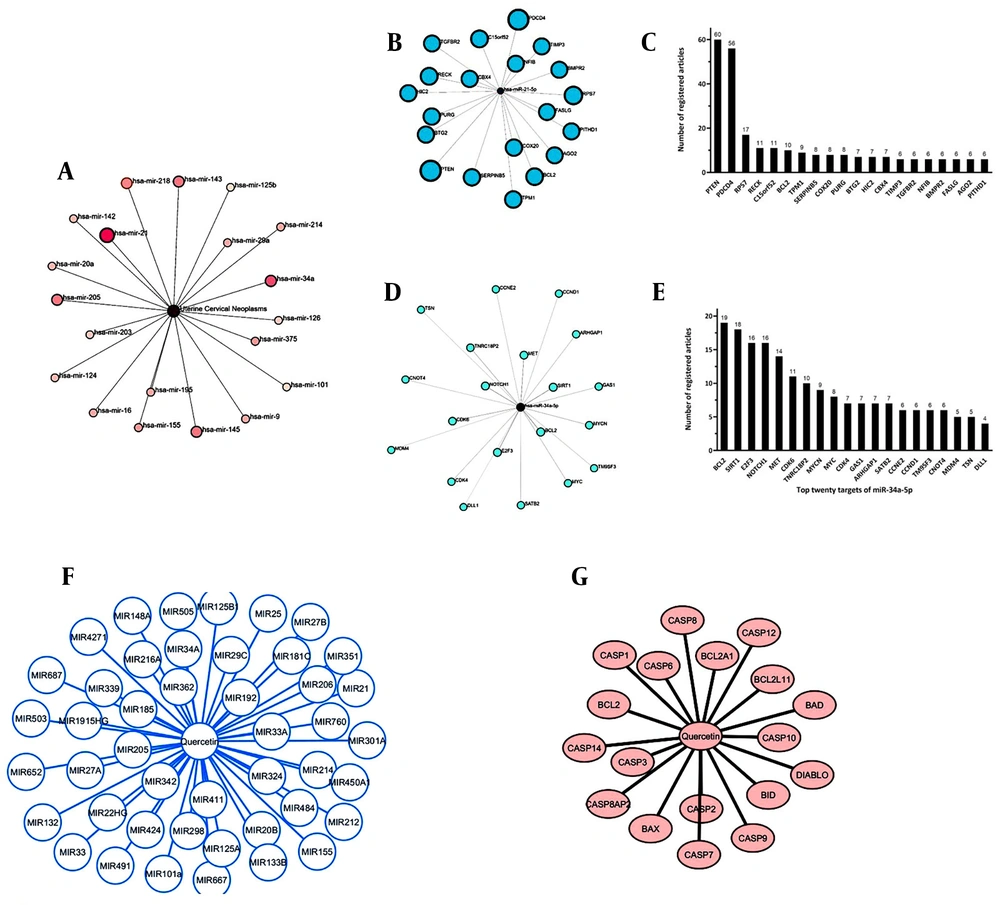
According to the bioinformatics findings, based on the number of articles registered until July 20, 2024, miR-21 (48 articles) and miR-34a-5p (28 articles) have the highest number of registered articles related to cervical cancer (Figure 1A).
Bioinformatic Studies. A, microRNAs involved in cervical cancer; B, prediction of miR-21; C, top twenty targets of miR-21 using the miRTarBase database based on the ranking of the number of articles registered until July 20, 2024; D, miR- 34a-5p targets; E, top twenty targets of miR-34a-5p using the miRTarBase database based on the ranking of the number of articles registered until July 20, 2024; F, some target microRNAs of quercetin involved in the cancer process; G, prediction the effect of quercetin on apoptosis
4.1.2. Prediction of miR-21 and miR-34a-5p Targets by Bioinformatics
Targets of miR-21 (Figure 1B) and miR-34a-5p (Figure 1D) were mapped using bioinformatics, and the top twenty targets were selected based on the highest scores as of July 20, 2024. These targets were also identified as factors involved in carcinogenesis (Figure 1C and E).
4.1.3 Important Target microRNAs of Quercetin Involved in the Cancer Process
All target microRNAs of quercetin involved in the regulation of cancer-related genes were identified through analysis, revealing that miR-21 and miR-34a-5p are among the targets of quercetin (Figure 1F).
4.1.4 Prediction the Effect of Quercetin on Apoptosis
Some important targets of quercetin involved in the process of apoptosis were identified using the PubChem database. According to these findings, quercetin has been shown to increase the expression of apoptosis-activating proteins and decrease the expression of apoptosis-inhibiting proteins (Figure 1G).
4.2. Cytotoxicity Assay
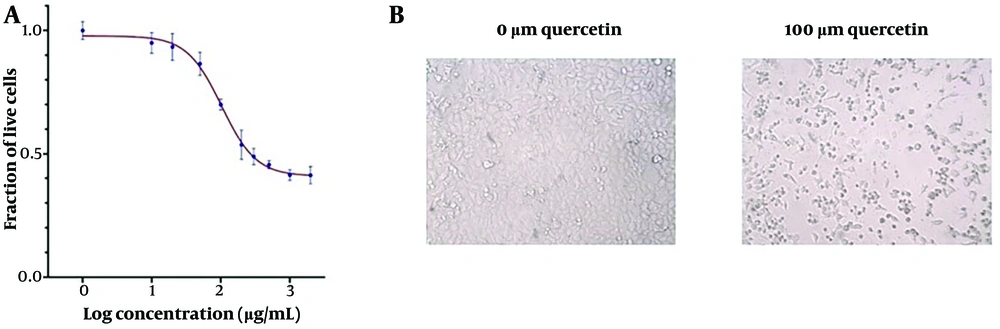
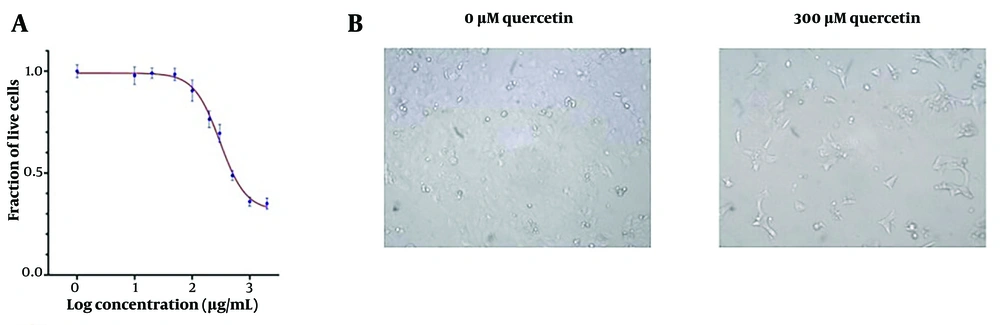
Quercetin suppressed the viability of HeLa cells with IC25 and IC50 values of 54.6 μM and 102.9 μM, respectively, after 48 hours of treatment (Figure 2A and B). The IC25 and IC50 for Ca Ski cells after 48 hours of quercetin treatment were 172.2 μM and 304.1 μM, respectively (Figure 3A and B).
4.3. Effect of Quercetin on Apoptosis
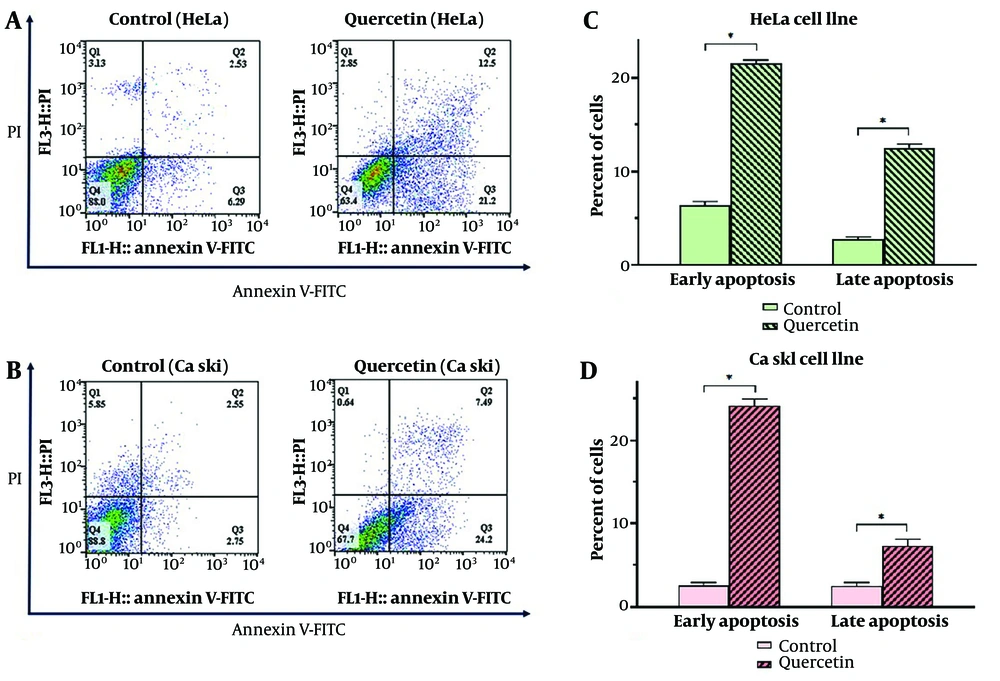
The effect of quercetin on apoptosis in HeLa (Figure 4A) and Ca Ski (Figure 4B) cervical cancer cell lines was investigated at the IC25 concentration of quercetin. Treatment with quercetin significantly induced both primary and secondary apoptosis in HeLa (Figure 4C) and Ca Ski (Figure 4D) cell lines at 50 μM and 200 μM of quercetin, respectively, compared to the control (P < 0.05).
Flow cytometry diagram of apoptosis induction in HeLa (A) and Ca Ski (B) cell lines by quercetin. Evaluation of primary and secondary apoptosis in HeLa (C) and Ca Ski (D) cell lines by quercetin. Any indicative value mean ± SD (n = 3). Data were analyzed using two-way analysis of variance (ANOVA) with Bonferroni test. * P < 0.05
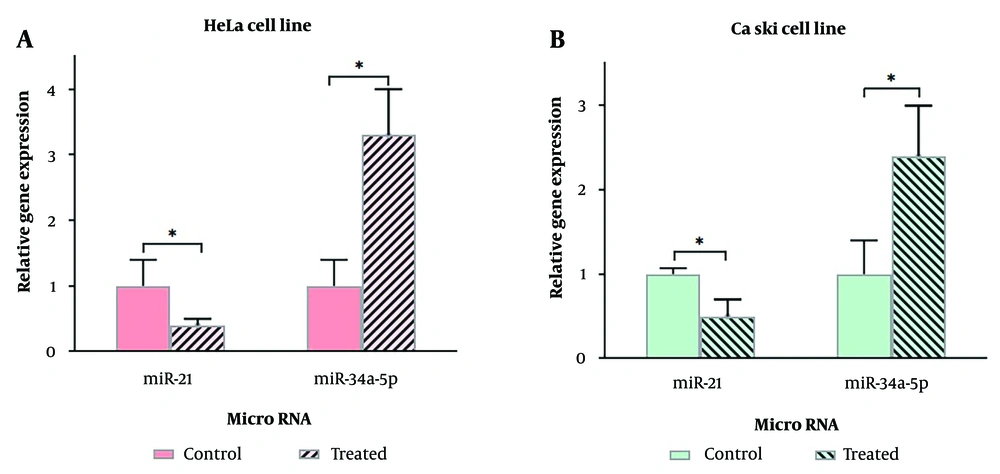
4.4. Expression of miR-21 and miR-34a-5p
The expression levels of miR-21 and miR-34a-5p in HeLa (Figure 5A) and Ca Ski (Figure 5B) cervical cancer cell lines were evaluated after treatment with 100 μM and 300 μM quercetin for 48 hours, respectively.
In HeLa cells treated with quercetin, the expression levels of miR-21 and miR-34a-5p compared to control cells showed a significant difference of 0.4 ± 0.1 (fold change = -2.5 ± 0.1, P = 0.045) and 3.3 ± 0.7 (fold change = 3.3 ± 0.7, P = 0.010), respectively (P < 0.05).
In Ca Ski cells treated with quercetin, the expression levels of miR-21 and miR-34a-5p compared to control cells showed a significant difference of 0.5 ± 0.2 (fold change = -2 ± 0.2, P = 0.016) and 2.4 ± 0.8 (fold change = 2.4 ± 0.8, P = 0.047), respectively (P < 0.05).
In both cell lines, quercetin decreased the expression of miR-21 and increased the expression of miR-34a-5p.
5. Discussion
In the present study, the role of quercetin in preventing the proliferation of cervical cancer cells was investigated. Additionally, the effect of quercetin on the expression of two important microRNAs involved in cervical cancer was evaluated.
Surgery, chemotherapy, and radiation therapy are commonly used in the treatment of cervical cancer. However, the side effects of certain drugs used in chemotherapy protocols have limited their use (12). Recently, the important role of natural plant extracts, including supportive supplements, in cancer treatment has gained attention (2).
Bioinformatics studies have highlighted the role of various microRNAs in cervical cancer. The results of this research showed that miR-21 (48 articles) and miR-34a-5p (28 articles) have the highest number of registered studies related to cervical cancer. It was also found that quercetin can affect the expression levels of these two microRNAs. However, based on a literature review, this study is the first to investigate the effect of quercetin on the expression of miR-21 (an oncogenic microRNA) and miR-34a-5p (a tumor suppressor microRNA) in HeLa and Ca Ski cervical cancer cell lines. The HeLa and Ca Ski cell lines are immortalized cells derived from cervical cancer tissue caused by HPV-18 and HPV-16, respectively.
Quercetin is a polyphenol compound with proven anti-cancer effects and minimal side effects (2). The results of this study demonstrated that quercetin could suppress the proliferation of HeLa and Ca Ski cancer cells. The effect of quercetin on the proliferation of cervical cancer cells in this study is consistent with its effects on breast and ovarian cancer cell lines (13). In a study reported by Xu et al., quercetin enhanced the inhibitory effect of cisplatin on the migration and invasion of cervical cancer cells. In that study, the combination of quercetin and cisplatin increased cisplatin's effect on the expression of ezrin protein in HeLa and SiHa cells, and a decrease in MMP2 expression was also observed. According to Xu's study and the present results, quercetin may have beneficial effects when used alongside standard treatments by promoting apoptosis and inhibiting the proliferation, migration, and invasion of cervical cancer cells (14).
The present study also showed an increase in apoptosis in cells treated with quercetin. Several studies have pointed to quercetin's role in suppressing anti-apoptotic proteins and increasing the expression of pro-apoptotic proteins in cervical cancer (15). In this study, factors involved in the process of apoptosis under the influence of quercetin were investigated using data from the PubChem database. Some of these targets include pro-apoptotic proteins such as BAX, BID, BAD, and BAK, and anti-apoptotic proteins like Bcl-2 and Bcl2A1, according to bioinformatic analyses. Additionally, quercetin was found to increase the expression of caspase family proteins, which promote apoptosis through both the extrinsic and intrinsic pathways.
MicroRNAs are considered important regulators of gene expression and signaling pathways. Studies have shown that changes in the expression of these molecules can disrupt cellular systems, such as the cell proliferation cycle and migration, potentially leading to serious consequences, including cancer (16). In the present study, the effect of quercetin on the expression of miR-21 in HeLa and Ca Ski cervical cancer cell lines was investigated, and it was found that quercetin reduces the expression of miR-21. Several studies have identified miR-21 as an onco-miR, with increased expression being linked to many cancers, including cervical cancer (17). A study conducted on three cervical cancer cell lines SiHa, HeLa, and C33a demonstrated that elevated miR-21 expression is associated with increased metastasis and tumor growth in cervical cancer (18). In 2021, Alshammari et al. explored the relationship between quercetin-induced reduction of miR-21 expression and the improvement of non-alcoholic fatty liver disease in a mouse model (6). Thus, the results of the present study highlight the positive effect of quercetin, a plant extract with minimal side effects, in aiding the treatment of cervical cancer by suppressing miR-21 expression.
Another microRNA examined in this research was miR-34a-5p, which has reduced expression levels in many cancers, including cervical cancer (19). miR-34a-5p, as a tumor suppressor, negatively affects processes related to cancer progression, such as epithelial-mesenchymal transition (EMT), invasion, and metastasis (20). Quercetin was found to increase the expression of miR-34a-5p in cancer cells. A study showed that quercetin-induced miR-34a-5p activates p53, which in turn promotes apoptosis and cell death in the HepG2 cell line, associated with liver carcinoma (21). Additionally, in a 2020 study, Chai et al. investigated the relationship between miR-34a-5p and quercetin in a non-small cell lung carcinoma cell line and its effect on apoptosis. The results demonstrated that quercetin plays a significant role in increasing the expression of miR-34a-5p, and this increase was associated with enhanced apoptosis (22). In the present study, the effect of quercetin on miR-34a-5p expression in Ca Ski and HeLa cervical cancer cell lines was investigated, and the results showed an increase in miR-34a-5p expression when these cells were treated with quercetin.
miR-21 is considered an onco-miR, while miR-34a-5p acts as a tumor suppressor. Therefore, reducing miR-21 and increasing miR-34a-5p levels is desirable in cancer treatment. In the present study, quercetin was observed to decrease miR-21 and increase miR-34a-5p levels in HeLa and Ca Ski cervical cancer cell lines.
According to previous studies, several mechanisms have been proposed regarding quercetin's effect on the expression of miR-21 and miR-34a-5p. p53, STAT3, NF-κB, and IL-6 are associated with quercetin's effect on miR-21, while p53, HIF-1α, and ZEB1 are related to its effect on miR-34a-5p. Quercetin increases p53 levels in cervical cancer cells. It has been suggested that quercetin interacts with the HPV E6 protein in HeLa and SiHa cell lines, potentially preventing p53 degradation by disrupting the formation of the E6AP/E6/p53 complex (23). Some studies highlight p53's role in reducing miR-21 expression (24) and increasing miR-34a-5p expression (25) in various cancers. Therefore, quercetin may decrease miR-21 and increase miR-34a-5p expression through the p53 pathway.
Furthermore, research points to quercetin's role in reducing STAT3 (26) and IL-6 (27) expression, as well as inhibiting NF-κB (28). Several studies indicate that miR-21 is upregulated by STAT3, IL-6 (29), and NF-κB (30). As a result, quercetin may reduce miR-21 levels by influencing these factors.
Recent studies have confirmed that quercetin suppresses the expression of HIF-1α (31) and ZEB1 (32). Additionally, researchers have reported that HIF-1α (33) and ZEB1 (34) play a role in suppressing miR-34a-5p. Based on this, quercetin may induce the expression of miR-34a-5p by inhibiting HIF-1α and ZEB1.
Quercetin shows promise as a natural compound with low side effects that could complement standard approaches in the treatment of cervical cancer. However, animal studies have shown that quercetin can exacerbate adverse effects in already pre-damaged kidneys. Therefore, quercetin may pose a risk for cervical cancer patients with impaired renal function (35). While the occurrence of harmful effects from quercetin in humans remains low, its consumption cannot be considered entirely without adverse effects. Future studies are suggested to further explore the mechanisms through which quercetin affects miR-21 and miR-34a-5p expression in cervical cancer.
5.1. Conclusions
Quercetin has the ability to inhibit the growth of HeLa and Ca Ski cervical cancer cell lines and induce apoptosis. Additionally, quercetin reduces the expression of miR-21 and increases the expression of miR-34a-5p in these cells. Given its anti-cancer properties, quercetin shows promise as a potential therapeutic agent with fewer side effects, making it a valuable complement to standard treatments for cervical cancer.