1. Background
Currently, cancer is a major contributor to global human mortality, with its incidence often influenced by a combination of genetic predispositions and environmental factors (1). In developed countries, cancer ranks as the leading cause of death, while in developing nations, it is the second leading cause. According to the most recent report from the World Health Organization's Cancer Research Agency, GLOBOCAN 2020, there were approximately 19.3 million new cancer cases in 2020, with an estimated 10 million deaths resulting from the disease (1, 2). In Iran, a developing country in the Middle East, rapid lifestyle changes, industrialization, and environmental shifts have collectively contributed to alterations in the epidemiological patterns of various cancer types (3, 4). In Iran, cancer is the second most significant chronic non-communicable disease and the third leading cause of mortality, following heart disease and accidents/natural disasters (5). Globally, the most common cancers among men are lung, prostate, colorectal, stomach, and liver cancers, while women are most frequently affected by breast, lung, cervical, uterine, and stomach cancers (3, 5). In Iran, the most prevalent cancers among men are stomach, prostate, bladder, colorectal, and esophageal cancers, while breast, colorectal, esophageal, stomach, and thyroid cancers are most common among women (3-5).
Breast cancer is the most prevalent form of cancer in women, having surpassed lung cancer in 2020 in terms of the number of cases in many countries worldwide. It is estimated that there were 2.3 million new cases of female breast cancer globally in 2020, accounting for 11.7% of all cancer diagnoses (2). Breast cancer is the fifth leading cause of cancer-related deaths among women globally, with a total of 685,000 deaths reported. In Iran, breast cancer is the most common cancer affecting women (6). According to the latest statistics from 2024, the age-standardized incidence rate of breast cancer in Iran is 21.33 per 100,000 individuals, with the average age of diagnosis being 48.49 years (7). Additionally, the age-standardized mortality rate for breast cancer is 14.2 per 100,000 cases, making it the fifth leading cause of death in the country.
Breast cancer is categorized into three main subtypes based on the presence or absence of molecular markers linked to the receptors for progesterone, estrogen, and the human epidermal growth factor receptor 2 (ERBB2, formerly known as HER2) (8). Among breast cancer patients, 70% are hormone receptor-positive and ERBB2-negative, 15 - 20% are ERBB2-positive, and 15% are classified as triple-negative, characterized by the absence of all three standard molecular markers (9).
The treatment approaches for breast cancer are primarily determined by the aforementioned subtypes, as well as the presence or absence of metastasis (10-12). In cases without metastasis, surgical intervention is typically performed, followed by radiation therapy. For patients with metastases, treatment is established systematically, considering the cancer subtype and the anatomical stage of the tumor (11-13). For hormone receptor-positive, ERBB2-negative breast cancer, a combination of endocrine therapy and chemotherapy is employed. In ERBB2-positive cases, targeted antibodies or small molecule inhibitors are used in conjunction with chemotherapy. For triple-negative breast cancer, chemotherapy is the main treatment option (10-12). However, each of these breast cancer treatment methods carries its own set of challenges, which can negatively affect the quality of life for patients (10-13).
Surgical procedures, as invasive treatments, come with inherent risks and complications (12-14). Chemotherapy and radiation therapy, which target rapidly dividing cells, often result in the unintentional destruction of healthy proliferating cells along with cancer cells (15, 16). Additionally, hormonal therapies can lead to drug resistance, while cytotoxic agents may cause adverse effects and toxicities specific to these treatments (12-16).
Both the adaptive and innate immune systems have the ability to recognize transformed cancer cells as "non-self" and subsequently initiate a targeted immune response aimed at inhibiting tumor growth and spread (17, 18). Recently, cancer immunotherapy has gained significant interest as a treatment modality. Immunotherapy focuses on modulating the immune system to target cancer cells, rather than directly targeting tumor cells. Its goal is to enhance or restore the immune system’s capacity to recognize and eliminate cancer cells (18). Unlike traditional treatments, immunotherapy does not destroy healthy cells in the body and can elicit a systemic immune response, often establishing long-lasting memory that may prevent future tumor recurrence.
Immunotherapy encompasses a range of approaches, including the passive transfer of antibodies or T-cells, immune checkpoint inhibition, and vaccination (17-19). One major advantage of the vaccination approach is its ability to stimulate an immune response, offering prolonged protection against cancer and its recurrence (17, 19). Several types of cancer vaccines have been developed, including whole-cell vaccines, peptide vaccines, and dendritic cell vaccines. Dendritic cells, as specialized antigen-presenting cells capable of activating both CD8+ and CD4+ T-cells, play a pivotal role in immunotherapy. Two primary strategies involving dendritic cells can be outlined: (1) vaccines using dendritic cells infused with tumor antigens ex vivo; and (2) vaccines that directly engage toll-like receptors (TLR) and other surface receptors on dendritic cells within the body (in vivo) (17-19).
Tumor-associated macrophages and myeloid-derived suppressor cells (MDSCs) exhibit immunosuppressive properties, and an imbalanced ratio of these suppressive cells relative to dendritic cells, as well as CD4+ and CD8+ T-cells, is associated with decreased survival rates among cancer patients (14). Elevated levels of MDSCs are commonly found in both the bloodstream and the tumor microenvironment in cancer. Targeting MDSCs for inhibition presents a promising therapeutic approach for cancer treatment (15). Phosphoinositide-3-kinases (PI3Ks) are a class of signal-transducing enzymes that play a crucial role in immune responses and cancer progression. The PI3K-γ signaling pathway is particularly important for the activity of myeloid cells, operating downstream of G-protein coupled receptors (GPCRs), such as chemokine receptors, and RAS. For instance, murine syngeneic tumors exhibit slower growth when transplanted into immune-competent mice with genetic inactivation of PI3K-γ (14). This reduction in tumor growth is attributed to the depletion of tumor-associated myeloid cells, which foster an immunosuppressive tumor microenvironment (TME) that promotes tumor growth (14, 15).
Furthermore, MDSCs are linked to tumor recurrence following chemotherapy or radiation therapy and play a role in facilitating metastatic spread (14). Findings from preclinical studies underscore the significant role of PI3K-γ in myeloid cell biology and suggest that inhibiting PI3K-γ in MDSCs could be an effective strategy for suppressing tumor growth across various cancer types (14-16). IPI-549 has been shown to reduce the T-cell-suppressive functions of MDSCs derived from both murine and human sources in vitro. These results indicate that IPI-549 enhances antitumor immunity by modifying the tumor-immune microenvironment through the inhibition of tumor-associated myeloid cells (16). Additionally, the upregulation of costimulatory and coinhibitory genes following IPI-549 treatment provides a mechanistic explanation for the observed synergistic effects when combined with immune checkpoint inhibitors. IPI-549 is currently undergoing phase I clinical trials, both as a standalone treatment and in combination with anti-PD-1 antibodies, targeting solid tumors (14, 16).
New therapeutic approaches must undergo thorough examination in laboratory and animal models before being introduced for clinical use in humans. Numerous researchers have demonstrated the efficacy of dendritic cell-based immunotherapy in stimulating the immune system in laboratory studies (17-19). Therefore, the current investigation focuses on an in vivo approach, specifically generating dendritic cells loaded with tumor antigens in a breast cancer mouse model. In this study, CD34+ hematopoietic cells were isolated from the femurs and tibias of Balb/c mice, followed by the cultivation of progenitor cells from the bone marrow in a culture medium supplemented with a cytokine cocktail. The antigens associated with lipopolysaccharides from gram-negative bacteria were then incorporated into the dendritic cells, leading to the maturation of these previously immature cells.
2. Objectives
Once the tumor reaches a volume of 50 - 100 mm², mice that have undergone experimental mammary tumor development will receive immunotherapy with adult dendritic cells that have been generated on at least two occasions. The effects of combining dendritic cells with IPI-549 (Eganelisib) will be evaluated in mice with tumors, investigating the therapeutic potential of this combination.
3. Methods
3.1. Preparation of Tumor Cell Lysate
The 4T1 breast cancer cell line, derived from BALB/c mice and serving as a model for Stage IV human breast cancer, was obtained from the National Cell Bank of Iran (NCBI code: C604, Pasteur Institute, Iran). The cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin, and maintained in a 5% CO2 atmosphere at 37°C until they reached 80% confluency. Cell separation was performed using a 0.25% trypsin/EDTA solution, followed by two washes with RPMI 1640 for 5 minutes at 200 g. The supernatant was discarded, and the cell pellet was reconstituted in 1 ml of PBS, followed by four freeze-thaw cycles alternating between a 37°C water bath and liquid nitrogen. This process was followed by sonication for 20 seconds using an ultrasonic processor on ice. The cell lysate was subjected to two rounds of centrifugation, first at 300 g for 6 minutes, and then at 13,000 g for 35 minutes, both at 4°C. The supernatant was collected, and protein concentration was determined using a previously described protocol. The cell lysate was filtered through a 0.22 μm membrane and stored at -80°C for future use (20).
3.2. The Preparation of Dendritic Cells from the Bone Marrow of BALB/c Mice
To generate dendritic cells from bone marrow, female BALB/c mice aged 8 to 12 weeks were utilized. The mice were euthanized via cervical dislocation, and their bodies were sterilized with 70% alcohol. Both legs were removed, and the skin and muscle tissues were carefully dissected to expose the femur and tibia. These bones were then placed in 70% alcohol for one minute for sterilization, followed by a rise in calcium- and magnesium-free PBS under a sterile hood.
The femur and tibia were transferred to a Petri dish containing RPMI 1640 medium, ensuring complete immersion. Using sterile scissors, both ends of the bones were cut off. A 1 mL syringe equipped with a 25 G needle was used to flush out the bone marrow by drawing the medium into one end of the bone and allowing the bone marrow to exit through the opposite end into a Petri dish. This procedure was repeated until the bones became white and translucent, indicating that no bone marrow remained.
The extracted bone marrow fragments were gently crushed with the syringe piston, without causing cell death. The bone marrow cell suspension was passed through a nylon mesh with 74 µg diameter pores and collected into a 50 mL tube. This suspension was centrifuged at 250 g for five minutes, and the supernatant was carefully removed.
The remaining pellet was treated with 1 mL of ammonium chloride solution to lyse red blood cells and incubated at 37°C for two minutes. The resulting suspension of 107 live cells was placed in a 24-well plate with a final volume of 1 mL per well. The cells were cultured in a medium containing 2 ng/mL GM-CSF and 10 ng/mL IL-4. On the third and fifth days, two-thirds of the culture medium was replaced with fresh medium containing the same cytokines. On the sixth day, tumor antigens (50 µg/mL) were added to the cells, followed by the addition of an extra 0.1 mg/mL of antigen on the seventh day.
On the ninth day, dendritic cells were harvested, and their characterization and viability were assessed using trypan blue staining and counting (21).
3.3. In vivo Design
Female BALB/c mice, aged between 6 and 8 weeks, and bred through inbreeding, were obtained from the Pasteur Institute animal breeding division in Tehran, Iran. The mice were randomly assigned to five groups (n = 10). Eight mice were used for in vivo examinations, while six were utilized for survival analysis. The five experimental groups were organized as follows:
- Group I (control group): This group consisted of ten mice that did not undergo any specific treatment and served as the control.
- Group II (bone marrow harvesting group): Ten mice in this group had their bone marrow harvested for the development and maturation of dendritic cells.
- Group III (tumor induction group): Ten mice in this group were induced with mammary tumors. This control group received only sterile saline.
- Group IV (dendritic cell extract group): This group included ten mice that were administered dendritic cell extract.
- Group V (dendritic cells with IPI-549 group): Ten mice in this group were administered dendritic cells loaded with IPI-549 (Eganelisib; Merck Company, Germany).
3.4. Mice Tumor Inoculation
BALB/c mice received a subcutaneous inoculation of 50 μL of phosphate-buffered saline (PBS) containing (105) viable tumor cells, administered into the right flank mammary fat pad (22). Tumor growth was assessed three times a week using calipers for morphometric measurements. The tumor volume was determined using a specific formula, and all results were presented as mean ± SE (standard error) (23).
3.5. Treatment
Immunotherapy commenced on day 14 following tumor cell inoculation, at which point the tumor volume had reached a size of 50 - 100 mm3. All formulations were initially dissolved in 0.1 mL of normal saline and administered via subcutaneous injection on the left flank, opposite the site of tumor growth. The same dosage of the treatment was administered again 14 days later. Throughout the experiment, both tumor growth and body weight were monitored. Seven days after the final immunization, the animals were euthanized, and the tumors were excised and weighed. The tumor inhibition rate (TIR) was calculated using the formula provided in reference (24).
Where Wt represents the tumor weight in the test group, and Ws represents the tumor weight in the control group.
3.6. Survival Rate
After the study period, six mice from each group were kept under standard conditions. The experiments were concluded when the tumor volume exceeded 2 × 103 mm3 or when the mice died. Daily mortality was recorded, and the survival rate was analyzed using the Kaplan–Meier test, as outlined in reference (25).
3.7. Delayed-Type Hypersensitivity Assay
A week following the final immunization, the animals were challenged with 0.02 mL of 1 mg/mL tumor cell lysate (TCL) administered to the left footpad (test group), while 0.02 mL of normal saline solution was administered to the right footpad (control group). Footpad swelling was measured at 24, 48, and 72 hours post-treatment using a dial caliper. The footpad swelling index was calculated following the method described in reference (26).
3.8. MTT Assay
The lymphocyte proliferation rate was assessed using the MTT assay. One week after the final immunization, spleens were excised, and a single-cell suspension of splenocytes (1 × 105 cells/100 μL/well) was prepared and cultured in triplicate in flat-bottom 96-well plates using RPMI 1640 medium. The cells were then stimulated with 20 μg/mL of the tumor antigen, 0.05 mL of PHA solution (1 mg/mL) as a positive control, or medium alone as a negative control. The cultures were incubated at 37°C for 72 hours in a humidified atmosphere with 5% CO₂. Afterward, 0.02 mL of sterile MTT solution (5 mg/mL) was added to each well, and the plates were incubated again. Next, 100 μL of DMSO was added to dissolve the formazan product, and the plates were shaken thoroughly. Absorbance was measured at 570 nm using an ELISA reader (25).
3.9. Cytokines Assay
Splenocytes at a concentration of 2 × 10⁶ cells/mL were cultured and stimulated with 0.02 mg/mL of the tumor antigen, along with 0.05 mL of PHA solution as a positive control. After 72 hours of incubation, the culture supernatants were collected, and IL-4 levels were measured using ELISA kits, following the manufacturer’s instructions (Peprotech, USA) (25).
3.10. CD107 Expression Analysis
The functional activity of cytotoxic cells was assessed using the CD107a degranulation marker test, following a previously described method with some modifications (27). Splenic cells from both treated and control mice were stimulated with 0.1 mg/mL of 4T1 tumor cell lysate for 6 hours at 37°C in the presence of FITC-conjugated anti-mouse CD107a monoclonal antibodies or an isotype control. The cells were then washed with FACS buffer, analyzed using a flow cytometer (Partec, Görlitz, Germany), and the data were processed using FlowMax software.
3.11. Real-time Quantitative PCR Assay
Total RNA was extracted from the tumor tissue using the Easy Red total RNA extraction kit (Intronbio, Korea). RNA concentration and purity were assessed using the NanoDrop™ 1000 spectrophotometer (Thermo Fisher Scientific, USA). To eliminate any potential genomic DNA contamination, 1 µg of RNA was treated with an RNase-free DNase kit (Thermo Fisher Scientific, USA) before proceeding with cDNA synthesis, which was performed using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA). The breast cancer gene 1 (BRCA1) was selected for examination, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serving as an internal control. Specific primers for BRCA1 were designed, with the forward primer sequence F: TGA AGA CTG CTC GCA GAG TGA TA and the reverse primer sequence R: AGC TTC CAG GTG AGC CAT TTC, sourced from willowfort.co.uk. RT-qPCR was conducted on the Rotor-gene Q Real-time PCR cycler (Qiagen) in a total reaction volume of 0.02 mL. The reaction mixture consisted of 0.01 mL of Master Mix (2×) (Thermo Fisher Scientific, USA), 0.5 μL each of forward and reverse primers (10 pmol/μL), 1 μL of cDNA, and nuclease-free water to reach a final volume of 0.02 mL. Gene expression results were normalized to GAPDH and analyzed using the 2 − ΔΔCt method (28).
3.12. Statistical Analysis
The data were analyzed using SPSS-22 software, with each experiment performed in triplicate. ANOVA was applied, followed by the LSD test, to identify significant differences (P < 0.01) between the groups.
4. Results
This research aims to explore the efficacy of dendritic cells loaded with IPI-549 (Eganelisib) under ex vivo conditions, specifically examining its role as a therapeutic supplement in modulating the immune system's response in mice with breast cancer. IPI-549 is a highly effective, first-in-class inhibitor of PI3K-γ, exhibiting an IC50 of 1.2 nM and showing significant selectivity, being at least 150 times more selective than class I PI3K isoforms and other kinases (29-33). Preclinical studies on pharmacokinetics (PK) and the processes of distribution, absorption, excretion, and metabolism have demonstrated that Eganelisib has an oral bioavailability of at least 31% in various preclinical species (33). Moreover, the pharmacokinetic profile indicates it facilitates complete and sustained inhibition of PI3K-γ with daily administration (33). In vitro studies revealed that Eganelisib did not directly affect cancer cell proliferation or T-cell activation (34). Similar to studies involving PI3K-γ knockout models, in vivo investigations with Eganelisib across various syngeneic models have demonstrated immune activation within the myeloid compartment. This activation facilitated T-cell activation and infiltration, ultimately contributing to delayed tumor growth (16, 34). Additionally, combining Eganelisib with immune checkpoint inhibitors (ICIs) targeting PD-L1, PD-1, or CTLA-4 successfully overcame resistance to ICIs in tumor models with high myeloid content and checkpoint refractoriness (35-37). Collectively, the data suggest that PI3K-γ inhibition by Eganelisib can reprogram immunosuppressive tumor-associated myeloid cells, thereby altering the tumor microenvironment and enhancing anti-tumor efficacy. This non-redundant approach complements ICIs, supporting the use of Eganelisib in conjunction with ICIs for the treatment of solid tumors (29, 35-37).
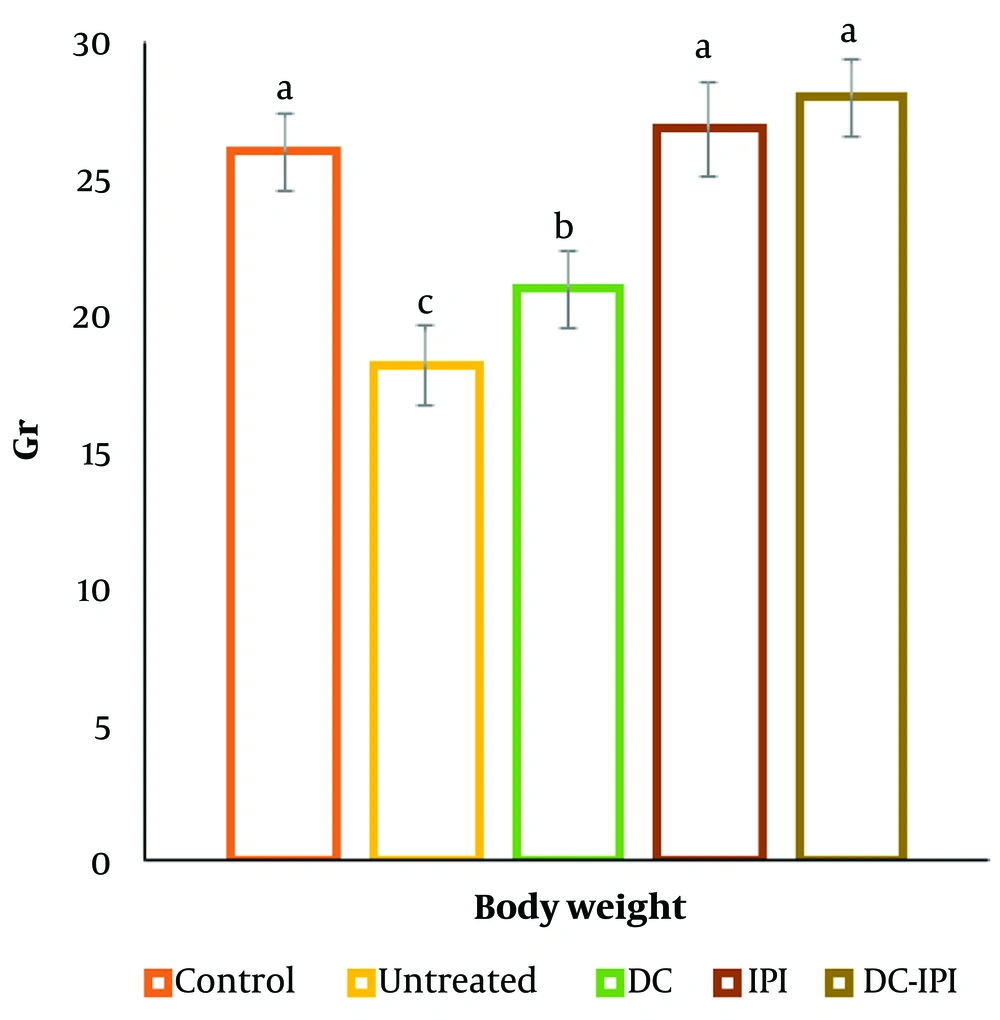
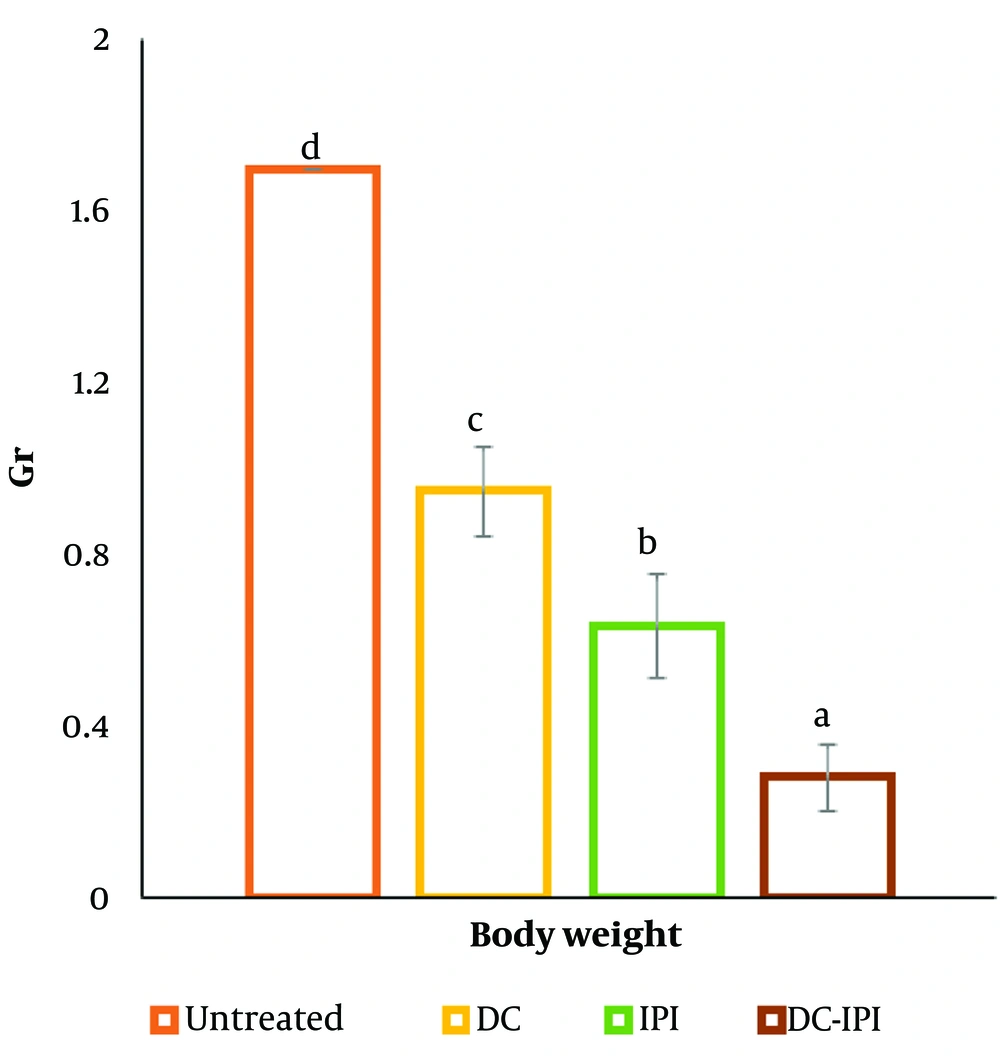
4.1. Body Weight, Tumor Volume, Tumor Growth Inhibition, and Tumor Weight
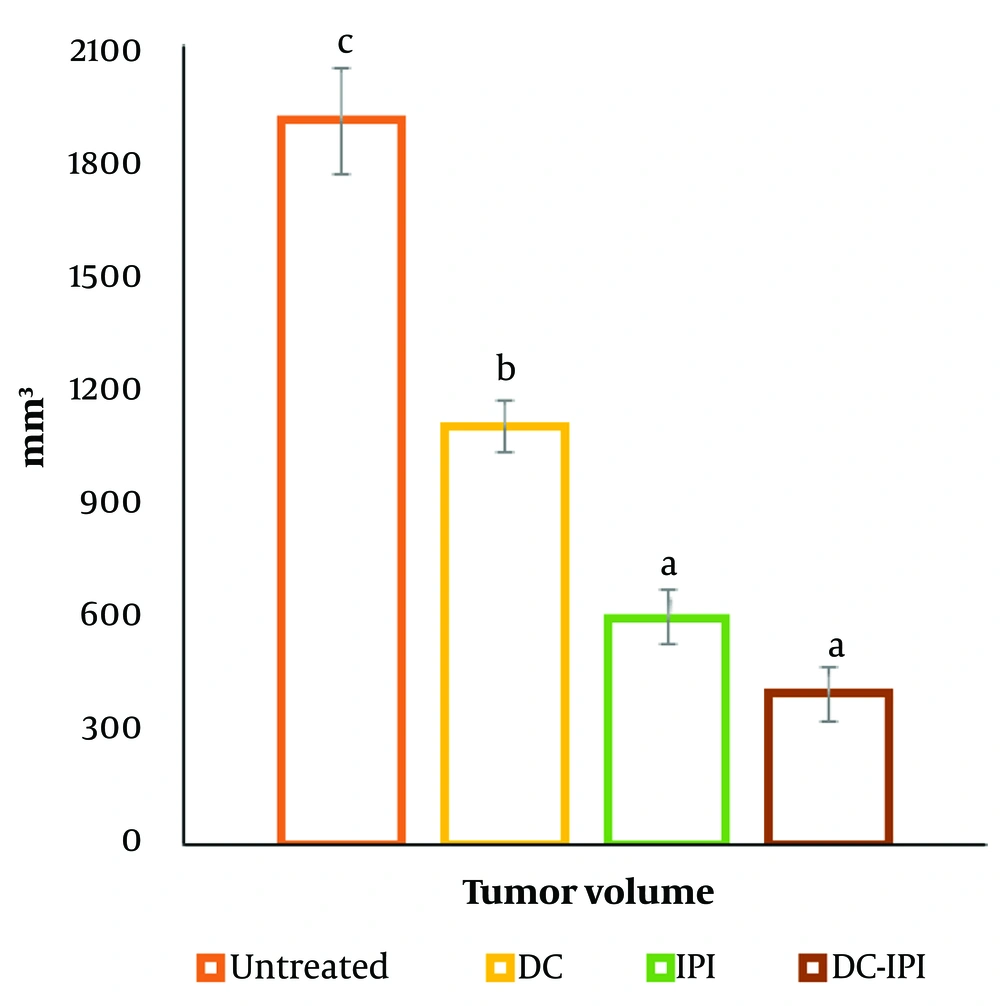
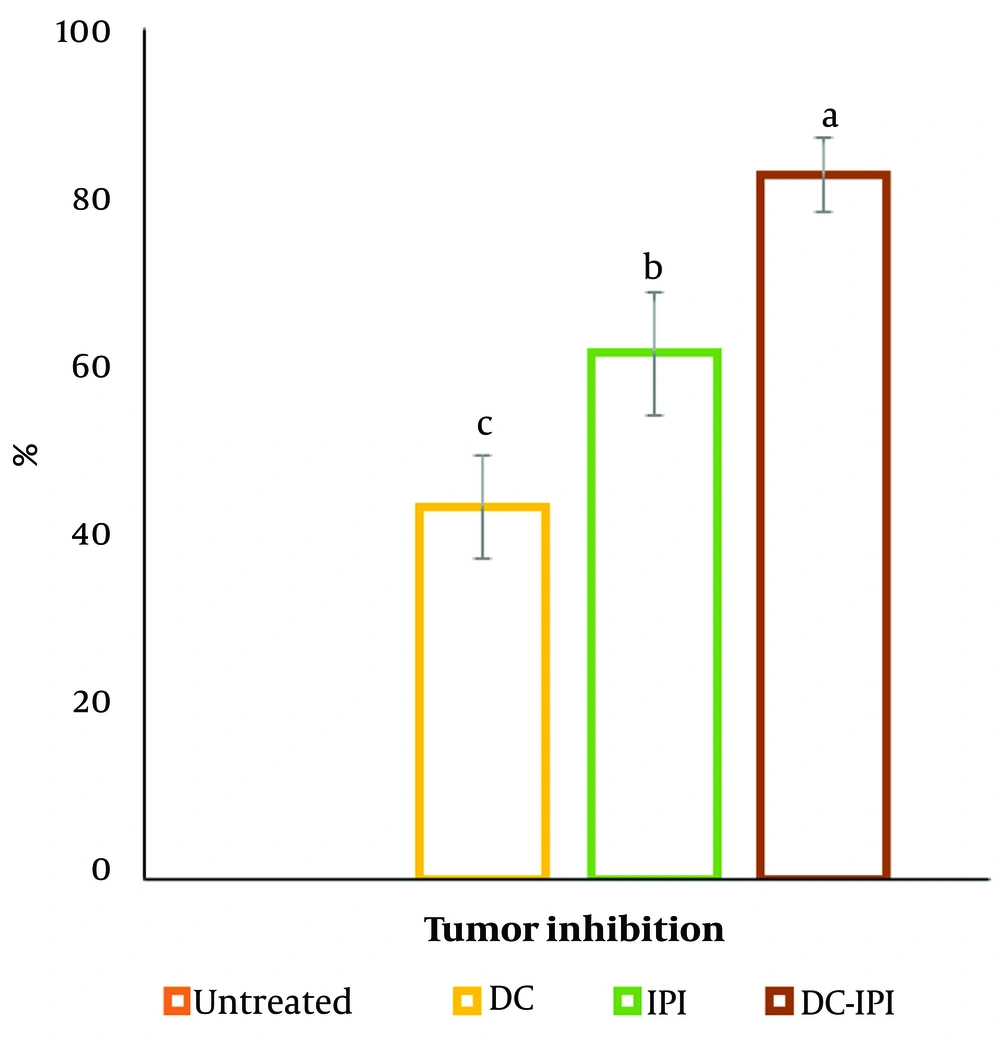
The group administered DC-IPI exhibited a significantly greater resistance to weight loss, with these mice showing a 10% weight increase from the start of the treatment (Figure 1). As shown in Figure 2, the groups immunized with DC-IPI demonstrated a prolonged ability to inhibit tumor growth. Analysis of tumor weight and volume across the different groups revealed that the most substantial reduction occurred in the DC-IPI group (Figures 2 and 3). Furthermore, as illustrated in Figure 4, the evaluation of tumor growth suppression in the DC-IPI group showed that DC-IPI effectively inhibited tumor progression.
4.2. Cytokine Release
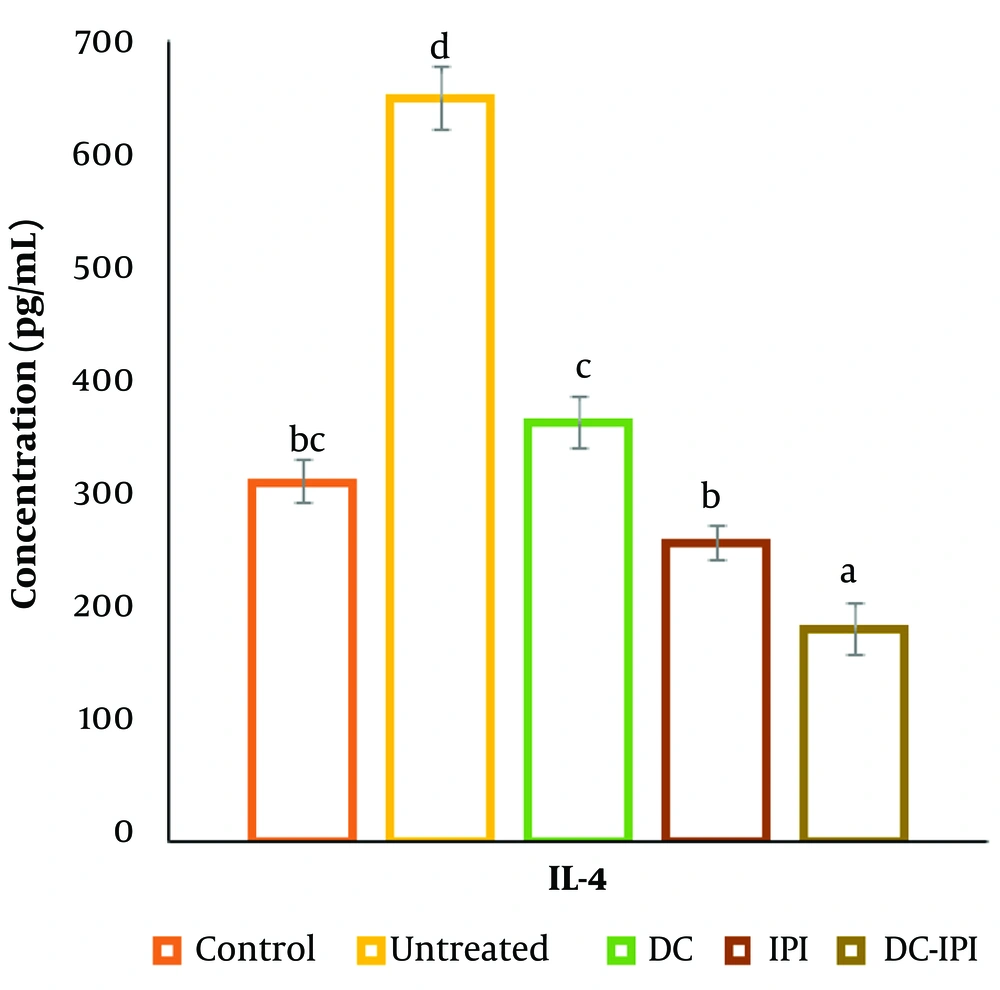
As illustrated in Figure 5, the groups immunized with DC-IPI demonstrated a prolonged ability to reduce IL-4 concentrations. Among all groups, the DC-IPI group exhibited the most significant reduction in IL-4 levels.
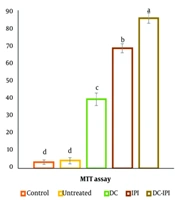
4.3. Splenic Lymphocyte Proliferation
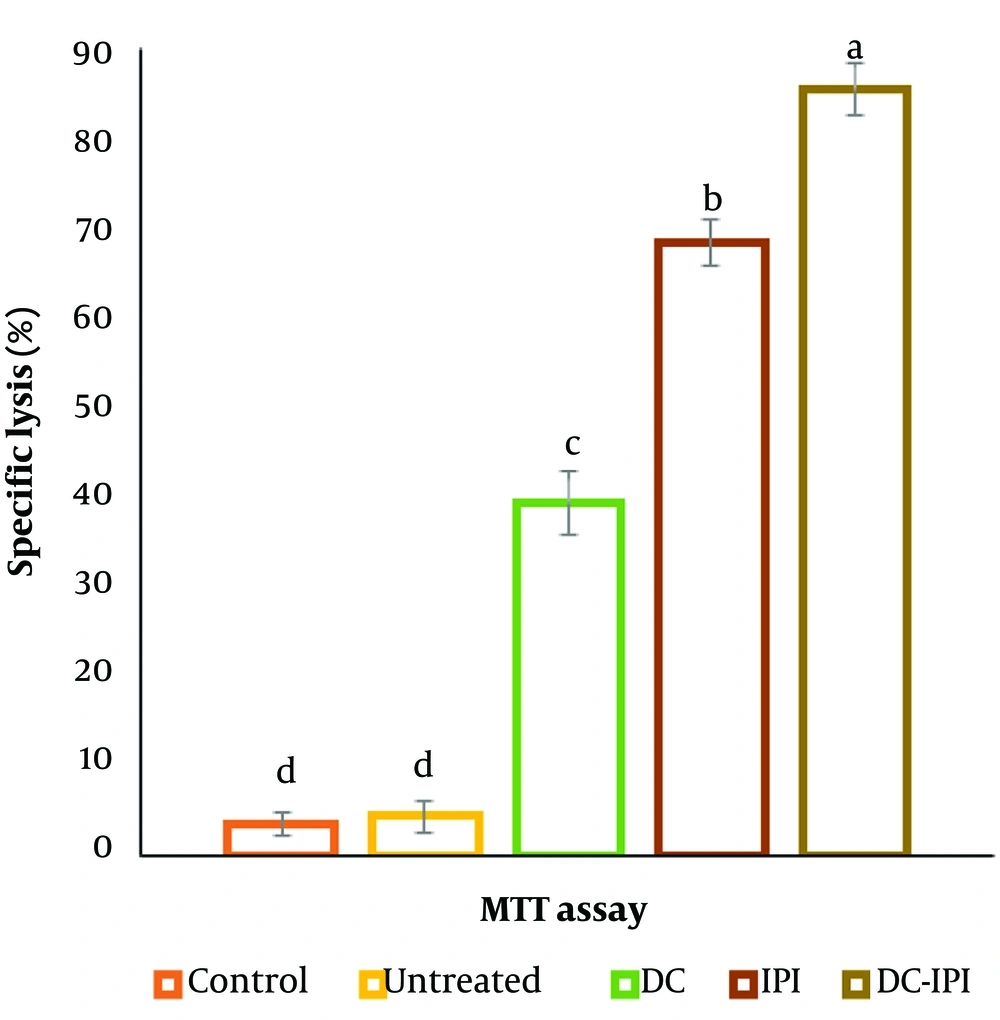
The results of the MTT test demonstrated a significant enhancement in the splenic lymphocytes' proliferative response within the treatment group that received DC-IPI, as shown in Figure 6.
4.4. Delayed-Type Hypersensitivity Responses
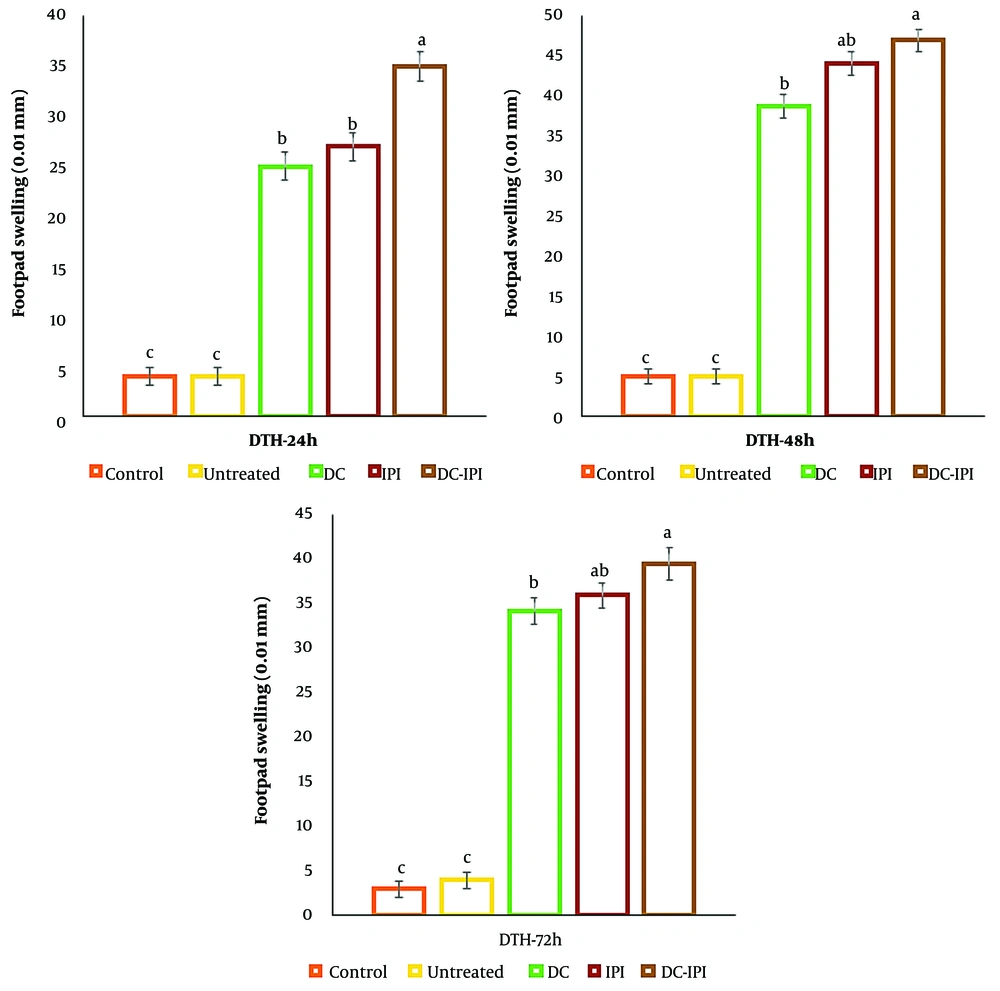
The favorable outcome of the skin test suggests the presence of an activated population of Th1 cells in response to tumor antigens. To confirm this, the levels of inflammation resulting from the injection of tumor antigens into the footpads of mice from both the control and treatment groups were evaluated at 24, 48, and 72 hours post-injection. The results revealed that the delayed-type hypersensitivity (DTH) reaction was significantly more pronounced in the group treated with DC-IPI (Figure 7).
4.5. CD107a Assay
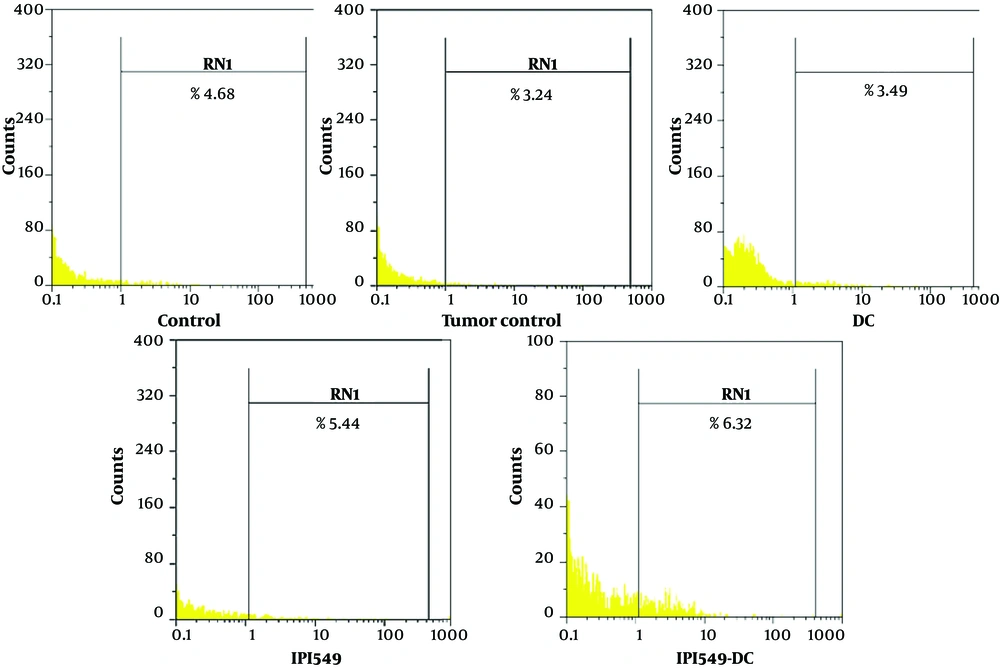
The lysosome-associated membrane protein (LAMP-1)/CD107a assay is utilized to assess the functionality of cytotoxic cells, such as natural killer (NK) cells and cytotoxic T-lymphocytes (CTLs), as it is expressed during the degranulation process of these cells. Flow cytometric analysis revealed that CD107a expression levels were significantly elevated in animals treated with DC-IPI, indicating enhanced cytotoxic cell activity (Figure 8).
The analysis of flow cytometric related to the CD107a expression. Splenic cells obtained from both treated and control mice were stimulated using tumor cell lysate along with FITC-conjugated anti-mouse CD107a monoclonal antibodies or isotype controls. Subsequently, these cells were analyzed using a flow cytometer (Partec, G¨orlitz, Germany), and the resulting data were processed with FlowMax software.
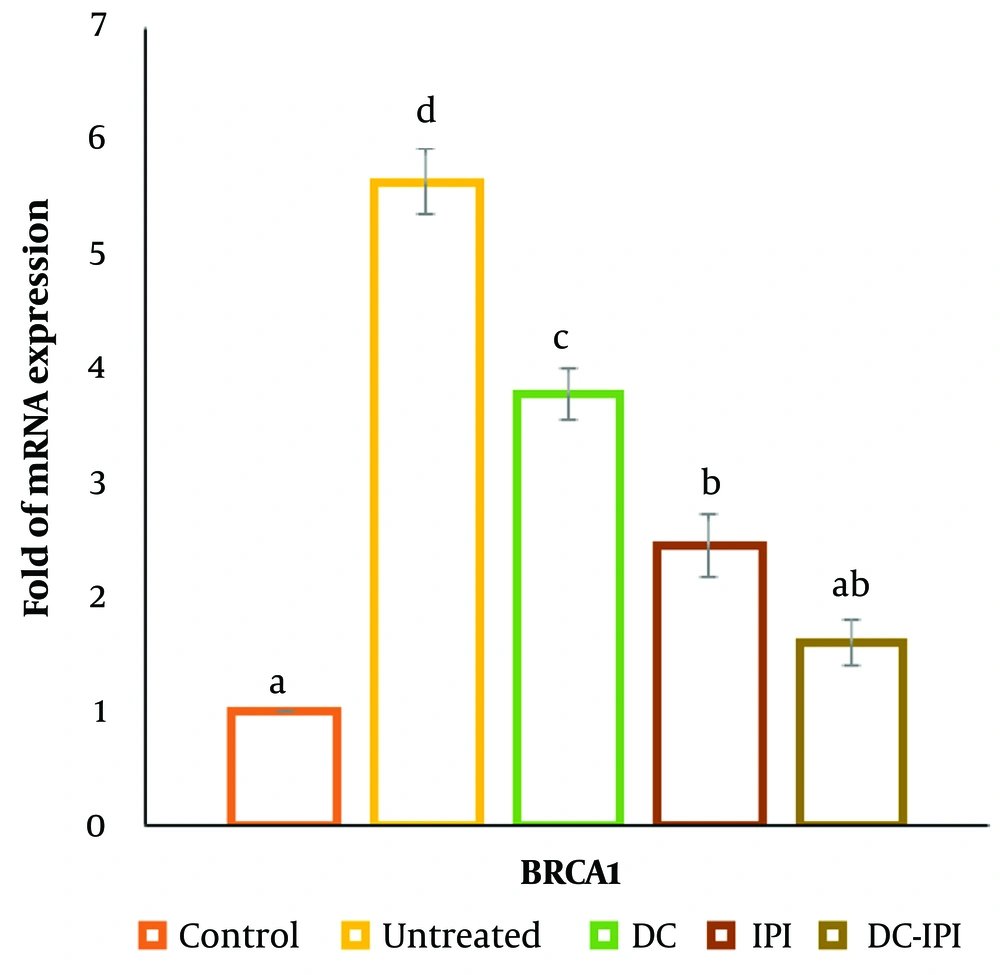
4.6. Fold of BRCA1 mRNA Expression
The data presented in Figure 9 demonstrates that the mRNA expression level of BRCA1 was significantly elevated in the breast cancer cohort compared to the control group. However, following treatment with DC-IPI, a marked reduction in BRCA1 mRNA levels was observed, indicating the potential impact of DC-IPI in modulating BRCA1 expression.
5. Discussion
The BRCA1 gene encodes the breast cancer type 1 susceptibility protein, a tumor suppressor, in humans. Orthologs of BRCA1 are commonly found in various vertebrate species, while invertebrates may carry more distantly related genes (10). BRCA1 plays a critical role in DNA repair and cell cycle regulation. Along with BRCA2, it is involved in the repair of damaged DNA or the initiation of apoptosis when repair is not possible (10, 11). Both proteins are integral to the repair of chromosomal damage, particularly in fixing DNA double-strand breaks, which is essential for maintaining genomic stability. Mutations in BRCA1 or BRCA2 impair the DNA repair mechanisms, thereby increasing the risk of breast cancer development (11).
BRCA1 and BRCA2 are categorized as tumor suppressor genes and are often referred to as "breast cancer susceptibility genes." While their normal alleles function to suppress tumor formation, mutations in these genes compromise their tumor-suppressive capabilities, leading to a significantly elevated risk of developing breast cancer (10, 11).
Degnim et al. conducted a study characterizing immune cell composition in benign breast disease (BBD) samples compared to normal breast tissues. Their findings revealed higher densities of CD8+ T-lymphocytes, CD20+ B-lymphocytes, and dendritic cells (CD11c) in benign breast disease samples than in normal tissues (10). Similarly, Ogony et al. investigated immune cell densities in individuals carrying BRCA1/2 mutations and found that these individuals had significantly higher immune cell densities than normal donors, suggesting an activated immune response (11). However, it remains unclear whether this elevated immune cell density represents tumor-suppressive immunosurveillance or if it reflects a state of chronic inflammation that may contribute to tumor growth (11).
Recently, the use of immunotherapy techniques for cancer treatment has gained significant attention, as these approaches stimulate antitumor responses within the host while preserving healthy cells. Unlike antigens from bacteria and other harmful agents, tumor antigens originate from the body’s own cells, often leading to immune tolerance towards them (38). Compared to melanoma and renal cell carcinoma (RCC), which show a higher responsiveness to immunotherapies, breast cancer has historically shown low immunogenicity, as its incidence does not increase among individuals who have undergone immunosuppressive treatments. Additionally, the tumor microenvironment releases immunosuppressive factors that weaken the immune response, making it difficult to deliver antigens effectively (39). However, even in tumor types not typically considered responsive to immunotherapy, immunogenicity can be induced through proper immune system activation. As a result, immunotherapy is increasingly recognized as a critical component in breast cancer management (39). Among the different immunotherapy strategies, vaccination stands out for its ability to trigger a long-lasting protective response through the development of immune memory. This not only aids in eliminating the tumor but also plays a key role in preventing its recurrence. Various combinations, such as whole-cell vaccines, peptide vaccines, and dendritic cell vaccines, have been used in immunotherapy (40).
Early research on cell-based autologous vaccines focused on using inactivated tumor cells. However, the practical effectiveness of these whole-cell cancer vaccines was limited. In one early clinical trial, tumor cells obtained from the patient were irradiated and then reintroduced into the patient along with the bacillus Calmette-Guerin (BCG) adjuvant after tumor removal (18). Tumor cells act as a significant source of antigens and epitopes for TCD4+ and TCD8+ lymphocytes. This approach has the advantage of eliciting an immune response against all antigens within the tumor cells, and its autologous nature allows the use of tumor-specific antigens for vaccination. A major drawback is the need to obtain a large quantity of tumor tissue (18-20). One potential solution to this issue is the use of one or more cell lines to create allogeneic cell extracts for patients with similar tumors. However, a key limitation of whole-cell extract vaccines is that, while they provide a plentiful supply of antigens, they often lack the necessary signals to recruit and activate immune cells (19, 20).
Peptide vaccines are based on the recognition of tumor antigen peptides by T-lymphocytes, which are associated with MHC complex molecules. To achieve this, peptides from known tumor antigens are synthesized and included in the vaccine formulation. The limitations of this type of vaccine include, first, the restriction of peptide presentation to a specific class of MHC molecules, and second, the absence of a clinical response in some patients, despite the induction of antigen-specific T-lymphocytes (18).
Dendritic cell vaccines utilize these cells to stimulate a T-lymphocyte response specific to the antigen. Dendritic cells are particularly effective in vaccination due to their role as specialized antigen-presenting cells in the immune system (25, 38). Three key attributes make them ideal for triggering antitumor T cell responses: (1) they can present antigens; (2) they detect external signals and act as potent stimulators of T-cells; and (3) they influence the polarization of the T-helper response, shaping the immune response (38). For cancer vaccines to be effective, antigens must be captured by dendritic cells (DCs). This can be done through ex vivo pulsing, where dendritic cells are loaded with antigens outside the body, or by targeting DCs within the body via in vivo methods (25). A notable example of dendritic cell therapy is a vaccine for advanced castration-resistant prostate cancer, which was the first dendritic cell therapeutic vaccine approved by the U.S. FDA, marking a new era in cancer immunotherapy (25, 38).
Ex vivo pulsing involves loading autologous dendritic cells with specific antigens and maturing them in optimal conditions outside the body. These matured cells are then administered to the patient to trigger a protective immune response (25, 38). Unlike in vivo targeting, ex vivo pulsing offers reduced risk, increased efficacy, and fewer technical challenges. One benefit of this method is that dendritic cells are extracted from the immunosuppressive tumor microenvironment, allowing them to mature into distinct subsets influenced by cytokines in the culture medium. This approach enhances dendritic cell maturation and increases co-stimulatory molecule expression, improving T-lymphocyte priming, which is often impaired in cancer patients (25). Pulsing dendritic cells with cancer antigens is a crucial step in preparing dendritic cell vaccines (25-28). Dendritic cells are typically isolated using whole-cell antigens from ultrasonicated or freeze-thawed cancer specimens (38). These antigens can also come from tumor cell lysates, synthetic peptides, DNA or RNA from cancer cells, or exosomes from tumor cells. Once prepared, the dendritic cells are reintroduced into the patient, with or without adjuvants (25, 38).
This research will utilize whole tumor cell extract to pulse dendritic cells. The ex vivo pulsing method, using whole tumor cell extract, has been employed in studies targeting breast, ovarian, prostate, melanoma, RCC, and glioblastoma (38). For tumors that do not express specific antigens, such as certain breast tumors, whole tumor cells or extracts containing the entire protein composition of lysed cells can eliminate the need for tumor antigen purification. This approach also helps prevent the loss of crucial antigens or mutations in epitopes needed for T-cell recognition, reducing the risk of tumor immune evasion (25, 38). The presence of multiple antigens in whole cell extracts is an advantage; however, competition among antigens for uptake and processing by dendritic cells may negatively affect T-lymphocyte activation (25). Additionally, stress from cell lysis may activate immune responses through the release of heat shock proteins (HSPs) from necrotic cells, enhancing the recognition and uptake of dying cells by dendritic cells. Tumor peptides associated with HSPs may also undergo recycling for more efficient antigen presentation (41-43). Studies indicate that patients vaccinated with whole tumor cell extracts show a significantly improved reduction in tumor size compared to those vaccinated with specific tumor antigens (25, 38).
This research will utilize the 4T1 Balb/c breast cancer cell line, known for its high invasiveness. This cell line, derived from a spontaneous tumor in BALB/c mice, exhibits characteristics that make it suitable for studying human breast cancer (44, 45). Tumor cells are located in the mammary glands, allowing the primary tumor to develop in its natural anatomical position. Like human breast cancer, 4T1 breast cancer metastasizes spontaneously from the primary tumor (44). The progression of 4T1 metastasis to lymph nodes and organs, such as the liver, lungs, brain, and bones, closely mirrors human breast cancer (45-48). In this study, whole tumor cell extracts from the 4T1 cell line will be used to pulse dendritic cells. These pulsed dendritic cells will be administered to mice with breast cancer to evaluate the impact of dendritic cell-based therapy on immune function and the tumor's response to treatment (44, 47).
5.1. Conclusions
In conclusion, our data indicate that the integration of dendritic cells with an IPI-549 (Eganelisib)-based vaccine, formulated with a dendritic cell-specific targeting ligand, tumor cell lysate, and adjuvants that stimulate dendritic cells, has the potential to elicit tumor-specific T-cell responses, reduce metastasis and tumor growth, and improve the survival rates of tumor-bearing mice. This DC-IPI delivery system shows promise for the treatment of various cancers by effectively co-delivering tumor antigens and adjuvants to dendritic cells, which then present these antigens to T-cells through cross-presentation. Additionally, it stimulated robust tumor-specific humoral and cellular immune responses, creating new opportunities to address challenges in cancer immunotherapy, particularly through the application of nanotechnology.
.png)