1. Background
The rose is one of the most common genera in the Rosaceae family (1). There are more than 120 species of roses distributed worldwide (2, 3). They are usually used as cut or garden flowers for their beauty. In addition, roses have been used in the food, perfume, and cosmetics industries (4, 5), as well as in the pharmaceutical industry for many years (6). So far, medicinal properties such as sedative, anti-stress, hematopoietic, and anti-fever effects have been reported for roses. The plants are useful remedies for stomach, liver, and intestine problems. Additionally, roses have suitable therapeutic functions on the skin, along with inflammatory diseases (4).
Rosa foetida is known as a common species in Iran, particularly distributed in the west, northwest, and center of the country (7). Rosa foetida has yellow petals due to the presence of carotenoids in the plant part (8). The R. foetida shrub is 80 to 120 centimeters in height and has strong branches that are upright or curved with a strong razor. In Iran, the plant is known by the local names of "Gol-e-Zard," "yellow rose," and "yellow Nastran" (7). In Europe, it is known as the Austrian briar (9). Rosa foetida grows wild in the mountains of Kurdistan in Iran. The decoction of plant petals is used in Iranian traditional medicine to treat stomach aches and as an anti-diarrhea remedy (2, 5). Rosa foetida is an efficient drug to treat kidney stones, kidney diseases, and ducts (10, 11). It is also used in the treatment of heart and skin diseases (12).
2. Objectives
In the present study, we focus on the extraction of the essential oil of R. foetida and the identification of its chemical composition, as well as the evaluation of the anticancer activity of the plant's essential oil against oral malignancy cell lines using the MTT assay for the first time. Additionally, we evaluate the antioxidant activity of the extract of R. foetida.
3. Methods
3.1. Plant Material
The dried flowers of R. foetida were purchased from a reputable medicinal plants market in Mashhad. This plant originated from Mahallat, a city in Markazi province located in central Iran.
3.2. Essential Oil Extraction
A total of 150 g of dried flowers of R. foetida was extracted by the water distillation method using a Clevenger apparatus for 3 hours. The extraction was repeated four times. The essential oils were combined and kept in a vial. The obtained essential oil was dried over sodium sulfate and then stored in a small glass container in the refrigerator until analysis and biological evaluations (13).
3.3. Analysis of the Essential Oil
The gas chromatographic device Agilent 6890 was used to identify the chemical composition. A BPX5 type column was used. The essential oil was diluted with n-hexane at a ratio of 1 to 10. Then, 1 µL of the solution was injected into the instrument. The sample was injected with a split ratio of 1:35. The oven temperature program was set with an initial temperature of 50°C for 5 minutes, a thermal gradient of 3°C per minute to increase to 240°C, and 15°C per minute to reach 330°C, held for 3 minutes. The injection chamber temperature was 250°C. Helium was the carrier gas with a flow rate of 0.5 mL per minute. The Agilent 5973 was the mass spectrometer with an ionization voltage of 70 eV, using the EI ionization method, and an ionization source temperature of 220°C. The mass scan range was set from 40 to 500 amu. Chemstation software was used. To identify the chemical composition, a comparison of mass spectra fragmentation patterns with those of known compounds from reference books, articles, and information available in the computer library was applied (14, 15).
3.4. Anticancer Activities (MTT Test)
MTT is a colorimetric technique. Due to the ability of living cells to perform oxidative metabolism, the MTT dye is reduced, resulting in a color change from yellow to blue. This test determines the number of living cells (16, 17). In this research, the following cell lines were used to evaluate the anti-human oral squamous cell carcinoma and cytotoxicity effects of the essential oil of R. foetida using the MTT method:
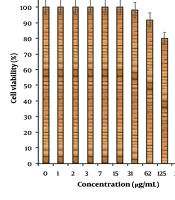
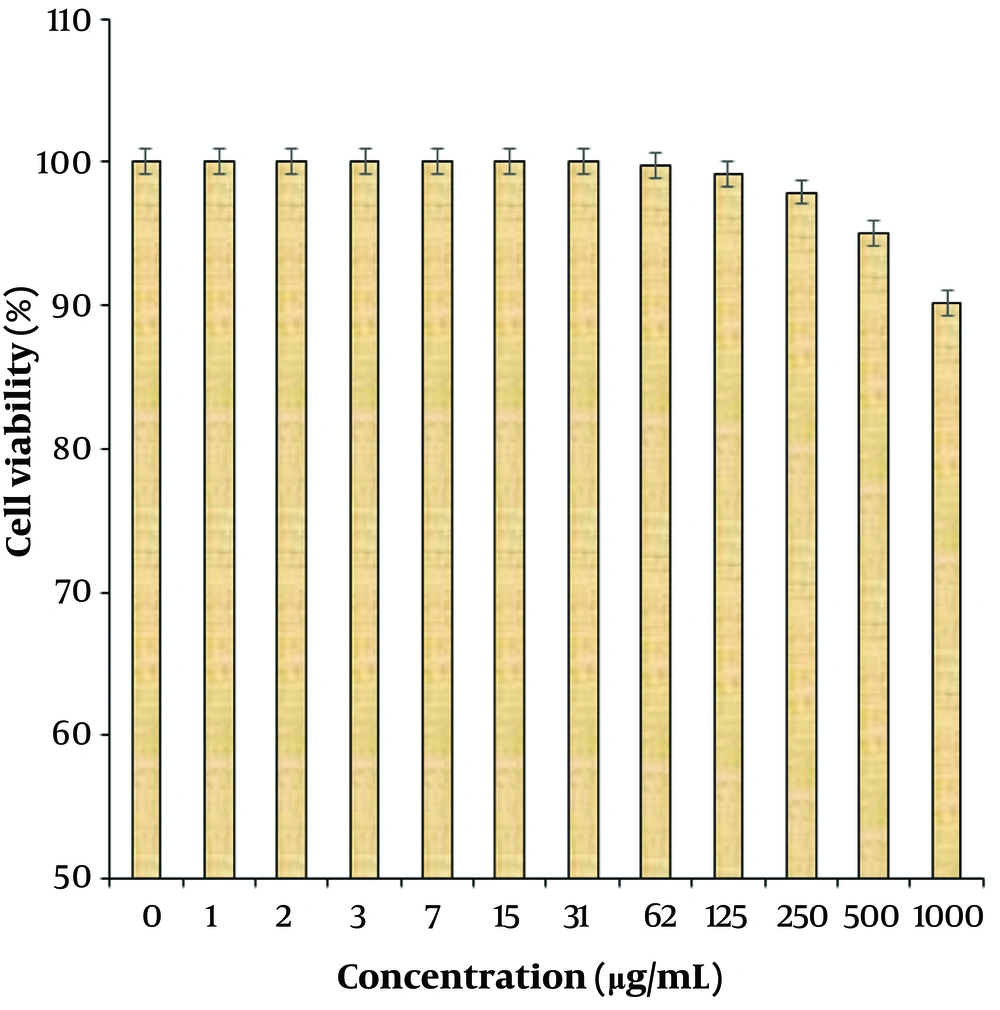
(1) Normal cell line: HUVEC.
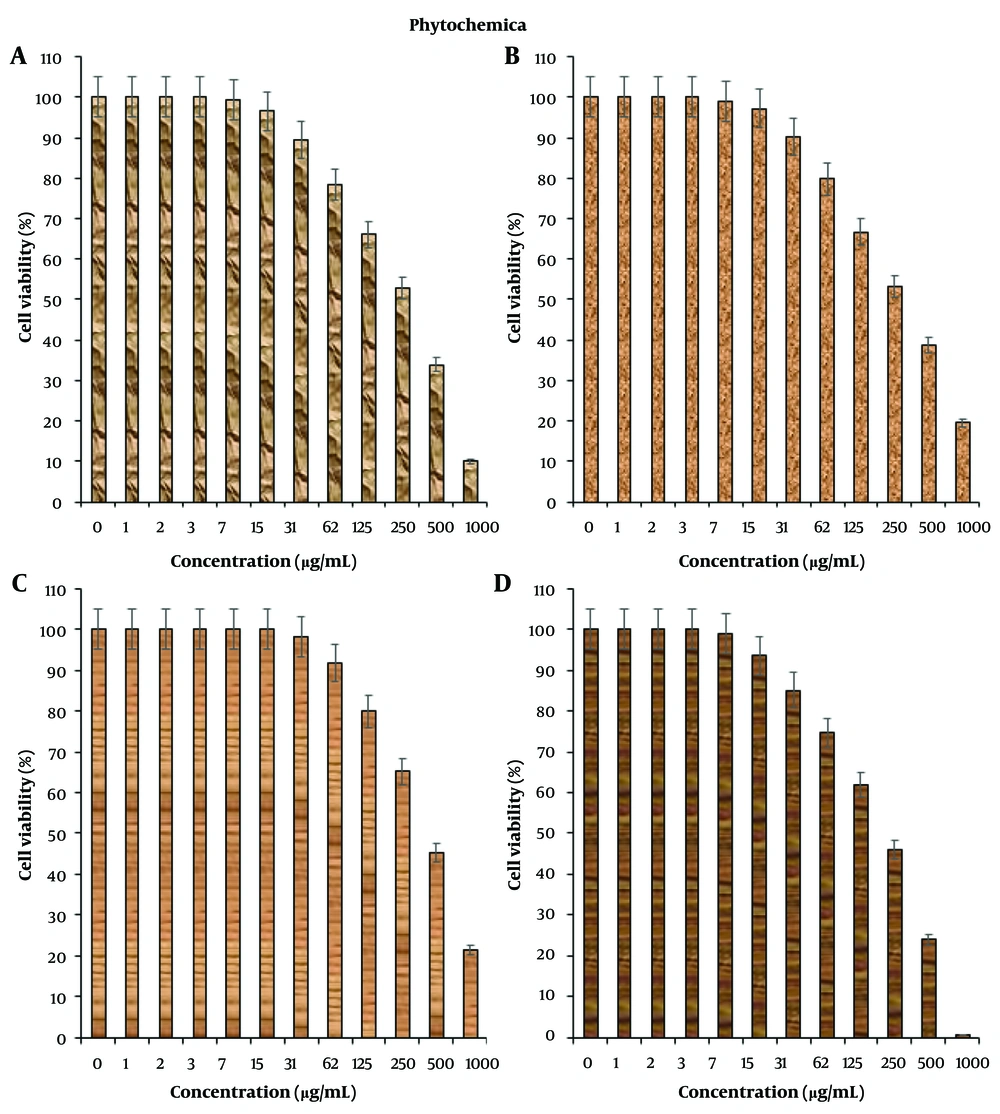
(2) Human oral squamous cell carcinoma cell lines: HSC-2, HSC-3, HSC-4, and Ca9-22.
Cell viability was calculated using the following equation:
At least three independent replications were performed for each data set, and the results were presented as mean ± SD. Data statistical analysis was conducted using SPSS software version 19, employing one-way ANOVA and Duncan’s test. Significance was considered at the level of P ≤ 0.05.
3.5. Preparation of Rosa foetida Extract
A total of 40 g of dried flowers of R. foetida were immersed in 70% ethanol for 36 hours. After filtering, the extract was concentrated at 45°C under low pressure using a rotary evaporator (Buchi Rotavapor R-114). The concentrated crude extract was then dried on a glass plate at room temperature for 72 hours under a hood. The dried extract was kept in a refrigerator before the antioxidant evaluation.
3.6. Determination of the Amount of Total Phenolic Content
The total phenolic content (TPC) was determined by the Folin-Ciocalteu method with some modifications (16, 17). The TPC was calculated based on the standard curve of Gallic acid (5 - 250 µg/mL). The results were expressed as mg of Gallic acid equivalents per gram of dried extract (GAE).
3.7. Determination of the Amount of Total Flavonoid Content
The total flavonoid content (TFC) was measured using the aluminum chloride method (18). The TFC was calculated based on the standard curve of rutin (5 - 250 µg/mL). The results were expressed as mg of rutin equivalents per gram of dried extract.
3.8. DPPH Radical Scavenging Activity
The antioxidant activity of the extract was measured based on the scavenging of DPPH, a stable free radical (16, 17). The mean values were calculated using the following equation. Butylated hydroxytoluene (BHT) was used as a positive control.
In this equation, Ac is the absorbance of the control (DPPH solution and solvent), and As is the absorbance of the sample (DPPH solution and extract solution).
4. Results
4.1. Chemical Composition of Essential Oil
Rosa foetida essential oil was pale yellow. A total of 24 components were identified, accounting for 87.78% of the R. foetida essential oil. The compounds are listed in Table 1. The major constituents of the oil were heneicosane (27.72%), tricosane (24.30%), and nonadecane (14.75%). Rosa foetida oil also contained two phenols, thymol (1.77%) and carvacrol (2.05%), and two alcohols, trans-nerolidol (1.37%) and L-menthol (0.3%).
| Compound Name | RT Min. | RI b | RI c | Area (%) | Class of Compound |
|---|---|---|---|---|---|
| 1. Octane | 5.59 | 801 | 800 | 0.35 | HC |
| 2. Limonene | 16.44 | 1035 | 1024 | 3.10 | MH |
| 3. l-Menthol | 24.27 | 1190 | 1178 | 0.30 | OM |
| 4. Dodecane | 24.85 | 1202 | 1200 | 0.31 | HC |
| 5. Cumaldehyde | 27.64 | 1260 | 1238 | 0.60 | OM |
| 6. 3,5-dimethoxytoluene | 28.63 | 1282 | 1282 | 0.64 | OH |
| 7. Thymol | 29.83 | 1307 | 1289 | 1.77 | OM |
| 8. Carvacrol | 30.21 | 1316 | 1296 | 2.05 | OM |
| 9. β-damascenone | 33.49 | 1390 | 1386 | 0.35 | OM |
| 10. Tetradecane | 33.98 | 1401 | 1400 | 0.32 | HC |
| 11. Geranyl acetone | 36.33 | 1457 | 1453 | 0.49 | OM |
| 12. Curcumene-α | 37.70 | 1490 | 1479 | 1.07 | SH |
| 13. β-bisabolene | 38.72 | 1515 | 1505 | 0.45 | SH |
| 14. Trans-nerolidol | 40.87 | 1569 | 1561 | 1.37 | OS |
| 15. Lauric acid | 41.18 | 1577 | 1565 | 0.11 | FA |
| 16. Heptadecane | 45.87 | 1701 | 1700 | 0.71 | HC |
| 17. Octadecane | 49.43 | 1801 | 1800 | 0.41 | HC |
| 18. Nonadecane | 52.84 | 1902 | 1900 | 14.75 | HC |
| 19. Eicosane | 56.08 | 2001 | 2000 | 3.45 | HC |
| 20. Heneicosane | 59.19 | 2052 | 2100 | 27.72 | HC |
| 21. Docosane | 62.14 | 2201 | 2200 | 1.31 | HC |
| 22. Tricosane | 65.00 | 2252 | 2300 | 24.30 | HC |
| 23. Tetracosane | 67.72 | 2402 | 2400 | 1.85 | HC |
a Area (%) of class of compound: [Hydrocarbons (HC): 75.48; monoterpene hydrocarbons (MH): 3.10; oxygenated monoterpene (OM): 5.56; oxygenated hydrocarbons (OH): 0.64; sesquiterpene hydrocarbons (SH): 1.52; oxygenated sesquiterpene (OS): 1.37; fatty acid (FA): 0.11].
b Kovats retention index.
c Kovats retention index from the literature.
4.2. Anticancer Activities
In this study, cells treated with different concentrations of R. foetida essential oil were assessed by MTT assay for 48 hours to evaluate the cytotoxicity properties on normal (HUVEC) and human oral malignancy cell lines, including HSC-2, HSC-3, HSC-4, and Ca9-22. The viability of the human oral malignancy cell lines was reduced dose-dependently in the presence of R. foetida essential oil. The IC50 of R. foetida essential oil was 288, 307, 440, and 218 µg/mL against HSC-2, HSC-3, HSC-4, and Ca9-22 cell lines, respectively (Figures 1, 2). The absorbance rate was evaluated at 570 nm, which indicated viability on the normal cell line (HUVEC) even up to 1000 µg/mL for R. foetida essential oil (Figures 1, 2).
4.3. Antioxidant Evaluation of Rosa foetida Extract
According to the obtained results, 164 ± 0.67 mg GAE/g and 75.5 ± 0.17 mg RU/g were calculated for the TPC and TFC of R. foetida extract, respectively. The plant extract scavenged DPPH with an IC50 of 6.54 ± 0.2 µg/mL, whereas the IC50 of BHT, as the selected standard, was 14.25 ± 1.18 µg/mL.
5. Discussion
5.1. Chemical Composition
So far, the chemical compositions of essential oils from various rose species have been reported. According to the reports, citronellol and geraniol have been identified as the main components of rose essential oils (19-21). These compounds are primarily responsible for the pleasant aroma of rose species. For R. foetida, previous research reveals that the major components differ because the flowers of R. foetida are odorless (2). Nonadecane, n-heptadecane, and n-dodecanoic acid have been reported as the main compounds of R. foetida essential oil from the west of Iran (2). In another study, n-nonadecane, n-heneicosane, 1-hexadecanol, n-tetradecane, and Z-octadecadienoic acid were identified as the main components of the plant's essential oil (22). These results are similar to the major compounds of R. damascena, which was dominated by nonadecane, heneicosane, and docosane (23).
5.2. Anticancer Activities
The study of the biological activity of essential oils is one of the intriguing aspects of the field of medicinal plants. Many research groups have evaluated the anticancer and anti-inflammatory activities of essential oils. Essential oils may have antioxidant effects due to their capacity to interfere with mitochondrial function, making them potentially effective anti-tumor agents. Many radical-producing agents are used in the treatment of cancer tumors. In this context, the production of radicals and the toxic or mutagenic side effects that damage healthy tissues can be well controlled (24). Plant extracts as pharmaceutical carriers can address some of the current drug delivery limitations in cancer treatment. It seems that the anti-human oral squamous cell carcinoma effect of recent essential oils is due to their antioxidant effects (25, 26). Recent studies have confirmed that some components play a significant role in anticancer, antioxidant, and anti-inflammatory activities. These components mostly include aromatic ones such as eugenol, thymol, carvacrol, and limonene, suggesting that plants containing these components can exhibit anticancer, antioxidant, and anti-inflammatory activities. Based on the results of the MTT assay and identified components, the anticancer activity of R. foetida is likely related to the presence of these components in the essential oil (27-29).
5.3. Antioxidant Activities
Phenolic compounds are known as one of the most important groups of natural compounds found in plants. These compounds have several health-promoting effects, such as anti-platelet aggregation, anti-tumor activity, protection against pathogens, and inhibition of certain processes (18, 30). Shameh et al. evaluated the TPC of six different species of rose in Iran, including R. canina, R. moscata, R. damascena, R. webbiana, and R. hemisphaerica (31). According to the reported results, the TPC of R. foetida was higher than that of R. damascena and R. webbiana (32, 33). However, R. canina and R. pimpinellifolia were rich in TPC (1).
Flavonoids are widely distributed in the plant kingdom worldwide and are perhaps the most important natural phenols (18). Previous studies have reported the presence of flavonoids in rose species. According to reported results, the TFC from R. moscata was higher than that of R. foetida based on the quercetin standard curve (31). On the other hand, R. damascena was poorer than R. foetida in TFC (33). Zheng et al. reported the TFC of five edible rose flowers in China, namely R. centifolia, R. chinensis, R. gallica, R. rugosa, and R. rugosa, with less TFC in comparison to R. foetida (30).
The evaluation of radical scavenging activity (RSA) is one of the most common methods to measure the antioxidant properties of plant extracts. By oxidizing, antioxidants eliminate free radical mediators and prevent other radical reactions. Plants with richer antioxidant compounds can protect cells from oxidative damage (34, 35). Previous studies have reported the RSA of different rose species. R. canina, R. pimpinellifolia, and R. damascena were more potent than R. foetida (1, 35). However, R. damascena, R. rugosa, R. centifolia, R. chinensis, R. gallica, and R. rugosa were weaker in comparison to R. foetida (32, 36).
5.4. Conclusion
In recent years, herbal medicine as an effective remedy to treat various diseases has gained more attention worldwide. The high side effects of chemical drugs may be the most important reason for this increased interest in medicinal plants. In the present research, we have reported the chemical compositions of R. foetida, a popular plant in the Middle East. The essential oil of the plant flowers was rich in heneicosane, tricosane, and nonadecane. The viability of oral squamous cancer cell lines was reduced dose-dependently in the presence of R. foetida essential oil. Conversely, the essential oil did not show any toxicity against the normal cell line of HUVEC. The plant extract demonstrated high antioxidant activity by scavenging the free radical DPPH. The results revealed that R. foetida can be considered a potent antioxidant and anticancer agent for treating oral squamous cancer; however, more experiments, including in vivo assays, should be conducted.