1. Background
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) characterized by ulcers and inflammation of the intestinal mucosa. Clinical features include mucous diarrhea with blood, weight loss, abdominal pain, anemia, and fatigue. It is a chronic, recurring, and progressive condition (1, 2). According to a recent systematic study, the highest prevalence rates of UC are found in North America (0.29%) and Europe (0.51%). Over the past 20 years, the incidence of UC in Iran has increased (3, 4). The etiology of UC is complex and not fully understood. Epidemiological research indicates that hereditary and environmental factors play significant roles in inflammatory diseases. Pathogenic characteristics include immunologic abnormalities (5), aberrant gastrointestinal microbiota, oxidant/antioxidant imbalance (6), increased pro-inflammatory cytokines, and deficiencies in mucosal integrity (7). Typically, individuals are diagnosed with IBD before the third decade of life, with possible recurrence between the ages of 50 and 80 (8). Ulcerative colitis affects the large intestine, damaging the intestinal lining, with symptoms including tenesmus, bloody diarrhea, and abdominal pain that subsides after a bowel movement (8-10).
Ulcerative colitis is managed using corticosteroids, aminosalicylates, immunosuppressants, and antibiotics. Antioxidants help alleviate the condition; however, synthetic antioxidants can be carcinogenic, making herbal antioxidants a recommended alternative (11-13). Recent data suggest that oxidative stress, through mechanisms such as increased reactive oxygen species (ROS) formation, immune cell infiltration, and elevation of inflammatory cytokines, is a major factor in the development of intestinal inflammation (14, 15). Studies also show a link between elevated pro-inflammatory cytokines in chronic UC and high serum and intestinal mucosal concentrations of nitric oxide (NO) (16). Tumor necrosis factor-alpha (TNF-α), found in high concentrations in the blood, feces, and inflamed intestinal mucosa of patients with IBD, is implicated in the pathogenesis of UC and Crohn's disease (17). Although acetic acid (AA) is used rectally to induce UC, its exact mechanism of action remains unknown, but it is thought to involve ROS-induced tissue damage and apoptosis (18, 19).
Investigating the efficacy of natural products in treating IBD is prudent, given their lower toxicity, appropriate efficacy, and common use as traditional treatments (20). Plant-derived natural compounds have been effective in managing conditions such as cancer and inflammatory illnesses (21, 22). Flavonoids, which are widely distributed in fruits and vegetables, represent a class of plant components that are effective as anti-inflammatories (21). Rutin, a glycoside consisting of a rutinose disaccharide and quercetin flavonol, is found in asparagus, apple, cherry, apricot, grape, passiflora, plum, orange, tea plants, wine, and primarily in buckwheat (Fagopyrum esculentum) (23, 24). This study aims to investigate the anti-inflammatory and antioxidant effects of rutin on IBD-induced rats. It builds on previous studies demonstrating its anti-cancer, anti-cardiovascular disease, and anti-neurological disorder properties (25-27).
Rutin is well-studied for its ability to act as an anti-inflammatory agent by scavenging free radicals and inhibiting TNF-α generation. It inhibits phospholipase, preventing arachidonic acid from converting into inflammatory mediators such as prostaglandins and leukotrienes. Flavonoids, including rutin, exhibit poor absorption in the digestive tract. In vivo studies have shown that rutin undergoes a first-pass effect, converting it into metabolites such as 3,4-dihydroxyphenylacetic acid, 3-methoxy-4-hydroxyphenylacetic acid, and m-hydroxyphenylacetic acid, which are subsequently excreted in urine (28). Rutin is removed from the body within 48 hours of oral or intraperitoneal treatment (29).
2. Objectives
3. Methods
3.1. Chemicals
The chemicals used in this study were sourced from Sigma-Aldrich (GmbH, Munich, Germany). Acetic acid was supplied by MERK, and TNF-α ELISA kits, specific for rats, were acquired from Enzo Life Science, Lorrach, Germany.
3.2. Fabrication of Nanoparticles Laden with Rutin
Chitosan nanoparticles were created with minor adjustments using the ionic gelation method reported by Gravandi et al. (33). Rutin-loaded nanoparticles were synthesized in different chitosan: Rutin (w/w) ratios: 7.5:1, 15:1, and 30:1. Specifically, 60 mg of chitosan powder was dissolved in 2 mL of 3% w/v AA, followed by the addition of 8 mL of 70% ethanol containing rutin. The solution was then added to the acidic chitosan solution and stirred for three hours to completely dissolve the rutin, including 15 minutes in an ultrasonic bath. Six mL of 0.2% (w/v) tripolyphosphate (TPP) solution was added dropwise to the chitosan-rutin mixture, stirring for 30 minutes. The nanoparticles were ultimately dried using a spray dryer with an inlet temperature of 110°C and an airflow rate of 70%.
3.3. Nanoparticle Characterization
In our previous study (33), the rutin-loaded chitosan nanoparticles were systematically characterized using dynamic light scattering (DLS), scanning electron microscopy (SEM), and Fourier-transform infrared spectroscopy (FTIR). These techniques confirmed the nanoparticles' uniformity and stability, which are crucial for understanding their interaction with the biological environment, including any effects on microbial populations (33).
3.4. Cytotoxicity and Biocompatibility Assessment
Using the MTT assay, we evaluated the cytotoxic effects of the nanoparticles, confirming their biocompatibility and safe profile in biological environments, as reported in our previous study (33). This step is essential as it indicates that the nanoparticles do not adversely affect cell viability, indirectly suggesting minimal disruption to the gut microbiota (33).
3.5. Animals
Thirty adult male Wistar rats, weighing between 180 and 220 grams and aged 8 to 9 weeks, were housed under standard conditions in the animal facility of the Faculty of Pharmacy at Kermanshah University of Medical Sciences. Conditions included a 12-hour light/dark cycle, free access to standard food and tap water, a temperature of 22 ± 0.1°C, and a humidity of 50 - 55%. Each cage housed five rats. The study protocol was approved by the Kermanshah University of Medical Sciences Ethics Committee (IR.KUMS.AEC.1401.031).
3.6. Induction
Acetic acid was used to induce UC, replicating acute inflammatory conditions (34, 35). To anesthetize the rats, intraperitoneal injections of xylazine (1 mg/100 g) and ketamine (4 mg/100 g) were administered, following a 24-hour fast with water only. A medical-grade polyurethane catheter (2 mm outer diameter) was inserted into the anus and advanced 8 cm. All rats, except the control group, received an intra-colonic infusion of 2 mL of 4% AA. Animal welfare was fully considered during the induction process.
3.7. Experimental Design
The protocol involved administering the medication for seven days, inducing the illness with AA on the eighth day, and then resuming the medication for three days (36). Six groups of rats were randomly selected as follows: The normal group received 1 mg of saline by gavage daily; the induction group received an intra-colonic infusion of 2 mL of 4% AA to induce colitis; the sulfasalazine group received 100 mg/kg of sulfasalazine to treat colitis induced by 2 mL of 4% AA; the 100 mg/kg nanorutin group received 100 mg/kg of nanorutin by gavage once a day to treat colitis induced by 2 mL of 4% AA; the 150 mg/kg nanorutin group received 150 mg/kg of nanorutin by gavage once a day to treat colitis induced by 2 mL of 4% AA; and the 200 mg/kg nanorutin group received 200 mg/kg of nanorutin by gavage once a day to treat colitis induced by 2 mL of 4% AA.
3.8. Macroscopic Evaluation of Intestinal Injury
The macroscopic evaluation of intestinal injury was conducted according to the definitions provided by another study (36), as detailed in Table 1.
| Scores | Descriptions |
|---|---|
| 0 | Normal appearance and lack of harm |
| 1 | Localized hyperemia devoid of ulcers |
| 2 | Linear ulceration with minimal inflammation |
| 3 | One location of inflammation with linear ulceration |
| 4 | Multiple ulcerated spots extending more than one centimeter along the colon's length |
| 5 - 8 | Damage extending more than two centimeters along the colon's length; the score increases by one for every additional centimeter of involvement |
3.9. Microscopic Assessment of Colonic Damage
For histopathological evaluation, appropriate tissue samples were collected from the colon segments and then fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm thickness, and stained with hematoxylin-eosin for light microscopic examination. The description and scoring of lesions were performed according to the grading criteria explained previously (Table 2).
| Lesions | Scores |
|---|---|
| Architectural distortion of crypts | (0) absent; (1) mild; (2) moderate; (3) severe |
| Colonic inflammation | (0) absent; (1) restricted to the mucosa; (2) mucosa and submucosa; (3) traversal of the entire length of the colon wall |
| Cryptitis | (0) absent; (1) mild; (2) moderate; (3) severe |
| Surface ulceration and necrosis | (0) absent; (1) mild; (2) moderate; (3) severe |
| Glandular atrophy | (0) absent; (1) mild; (2) moderate; (3) severe |
| Submucosal edema and hyperemia | (0) absent; (1) mild; (2) moderate; (3) severe |
a Mild: Present in less than 10% of examined tissue; moderate: Present in 10% - 50% of examined tissue; severe: Present in over 50% of examined tissue.
3.10. Biochemical Analyses
3.10.1. Glutathione Assay
Elman's technique was used to quantify the reduced glutathione (GSH) level. Each well received 60 μL of the sample and 50 μL of phosphate buffer (pH 7.4). After adding 100 μL of DTNP reagent, the wells were incubated for ten minutes at 37°C. The thiol group of GSH combines with the DTNP reagent to break the disulfide link and form the TNB2- ion, resulting in a yellow appearance due to TNB2- ions. After incubation, the samples' absorbance was measured using an ELISA reader at a wavelength of 412 nm (36).
3.10.2. Nitric Oxide Assay
Since NO is unstable, its stable metabolites, nitrate, and nitrite, were detected using the GRACE quantitative colorimetric approach, which involves a deproteinization step. In this reaction, sulfanilamide and nitrite combine to generate an unstable salt. Sulfanilamide is mixed with another substance, such as N-naphthyl-ethylenediamine dihydrochloride, to determine the amount of nitrite in the mixture by measuring the resultant purple color spectrum.
3.10.3. Superoxide Dismutase Assay
Superoxide dismutase (SOD) helps prevent tissue damage caused by negative oxygen radicals. Due to the short half-life and radical nature of oxygen, detecting SOD directly is challenging. As per Marknuld's definition, SOD activity was assessed by evaluating the enzyme's capacity to prevent pyrogallol autoxidation. Auto-oxidation and illumination at a wavelength of 420 nm were measured using a spectrophotometer. One enzyme unit was defined as the amount of protein that inhibits 50% of pyrogallol autoxidation in one minute.
3.11. Measurement of Tumor Necrosis Factor-Alpha
Tumor necrosis factor-alpha levels in colon tissue were assessed using an ELISA kit. The absorbance of the final solution was measured at two different wavelengths: An initial wavelength of 450 nm and a reference wavelength of 600 nm.
3.12. Statistical Analysis
Multiple comparison analyses were conducted using SPSS software and one-way ANOVA with the Newman-Keuls post hoc test. All data were expressed as mean ± standard deviation (mean ± SD).
4. Results
4.1. Macroscopic Evaluation of Colonic Damage
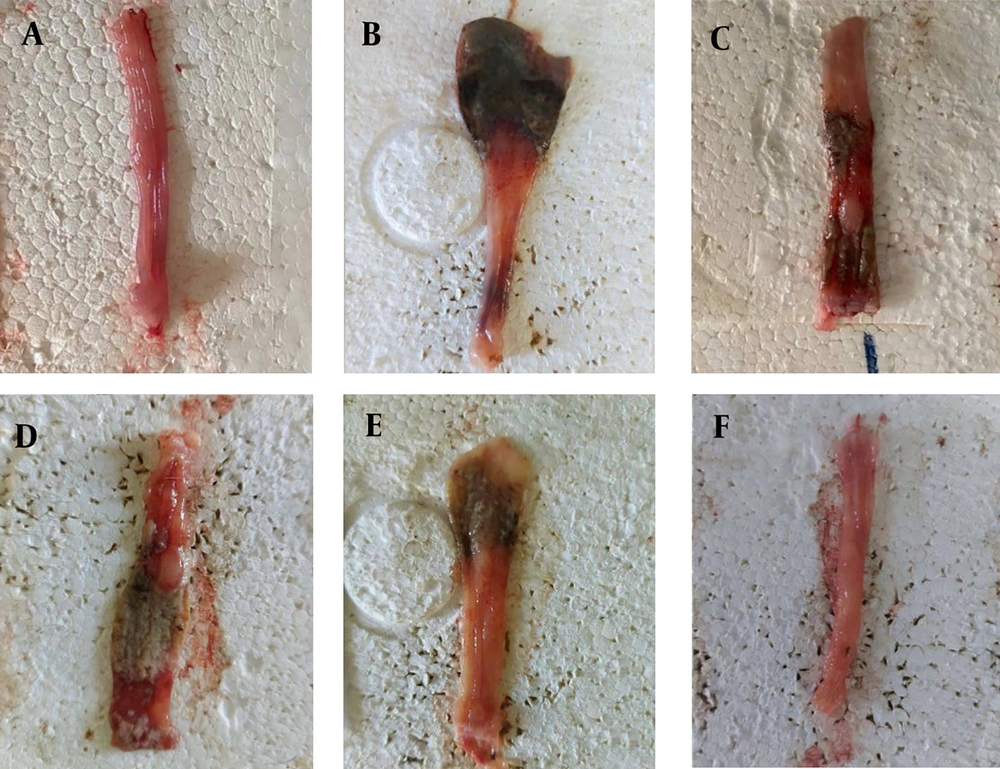
Macroscopic evaluations of colonic damage were conducted to assess the protective effect of rutin nanoformulation. The evaluation aimed to compare the extent of colonic damage across different treatment groups. As shown in Table 3, the normal group exhibited no colonic damage, indicated by a mean score of 0.00 ± 0.00. In contrast, the control group, which did not receive any treatment, showed significant colonic damage with a mean score of 4.00 ± 0.81 (***). Treatment with mesalazine (300 mg/kg) significantly reduced colonic damage, reflected by the lower mean score of 0.43 ± 0.23 (***, ###). The groups treated with nanorutin at doses of 100, 150, and 200 mg/kg showed dose-dependent reductions in colonic damage. The 200 mg/kg nanorutin group exhibited the greatest protective effect, with a mean score of 0.75 ± 0.35 (*, ###, @, &). The findings, illustrated in Figure 1, suggest that rutin nanoformulation, particularly at higher doses, effectively reduces colonic damage in a rat model of AA-induced UC.
| Groups | Mean ± SD |
|---|---|
| Sham | 0.00 ± 0.00 |
| Negative control | 4.00 ± 0.81 *** |
| 300 mg/kg mesalazin | 0.43 ± 0.23 ***, ### |
| 100 mg/kg nanorutin | 2.37 ± 0.75 **, ##, @@@ |
| 150 mg/kg nanorutin | 1.62 ± 0.25 **, ##, @@, $ |
| 200 mg/kg nanorutin | 0.75 ± 0.35 *, ###, @, & |
a *: Compared to sham group (*: P < 0.05), (**: P < 0.01,***: P < 0.001); #: Compared to negative control (##: P < 0.01), (###: P < 0.001); @: Compared to mesalazine as a positive control (@: P < 0.05, @@: P < 0.01, @@@: P < 0.001; $: Compared to the nanorutin 100 mg/kg group ($: P < 0.05,$$$: P < 0.001); &: Compared to the nanorutin 150 mg/kg group (&: P < 0.05).
Macroscopic data collected from the colons of rats. A, demonstrates the rat colon's typical appearance. The colon of the negative control group, represented by B, shows the greatest damage. The group depicted in C was administered mesalazine at 300 mg/kg. The colons of three groups of rats - D, E, and F - received doses of nanorutin of 100, 150, and 200 mg, respectively.
4.2. Biochemical Analyses
4.2.1. Statistical Analysis Explanation
To assess the statistical significance of this section, the following thresholds were used: P-value < 0.05 (*), P-value < 0.01 (**), and P-value < 0.001 (***). These comparisons were made using one-way ANOVA followed by a post hoc Newman-Keuls multiple comparison test (37). The symbols *, #, @, $, and & denote comparisons against various control groups as specified in the figure legends.
4.2.2. Glutathione Assay
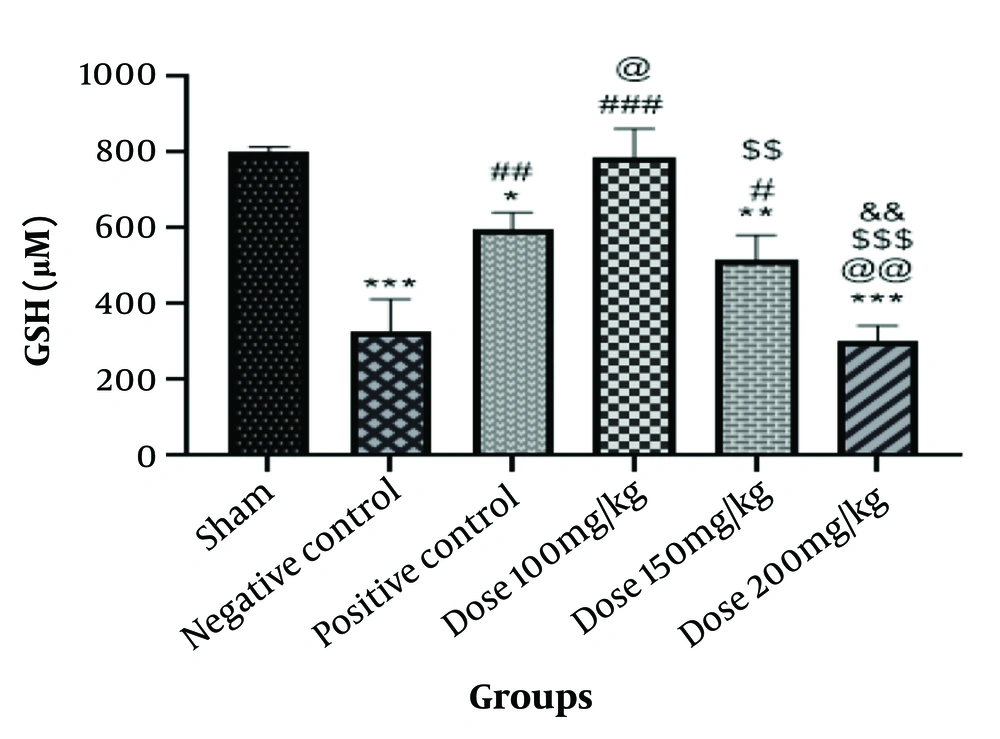
In addition to preventing unnecessary oxidations, GSH acts as an antioxidant to eliminate reactive radicals. As shown in Figure 2, the amount of GSH is significantly lower in the negative control group than in the sham group (P < 0.001). The graph data indicates a significant decrease in GSH levels in the positive control group compared to the sham group (P < 0.05) and a noticeable increase in GSH levels in the positive control group when compared to the negative control group (P < 0.01). The GSH level in the group of rats given 100 mg/kg of the medication was significantly higher than in the negative control group (P < 0.001), and it was also different from the sham group. In rats in the 100 mg/kg drug group, the levels increased somewhat compared to the positive control group (P < 0.05). Glutathione levels were significantly higher in the group given 150 mg/kg compared to the negative control and the group that received 200 mg/kg (P < 0.01, P < 0.05). When rats in the 200 mg/kg drug group were compared to the sham group, the group receiving 100 mg/kg drug, the positive control group, and others, there was a substantial decrease (P < 0.001). The group that was given the medication at a dose of 150 mg/kg showed a relative decrease (P < 0.01) (Figure 2).
Information from the glutathione (GSH) test. The * is used as a benchmark against the fictitious group. The # is employed in contrast to the negative control. When compared with the positive control group, @ is utilized. The $ is used in contrast to the group that took the medication at a dose of 100 mg/kg. The & is utilized compared to the group given 150 mg/kg of medication. * P-value < 0.05; ** P-value < 0.01; and *** P-value < 0.001. The same pattern, based on the asterisk (*), applies to the other symbols.
4.2.3. Nitrite Assay
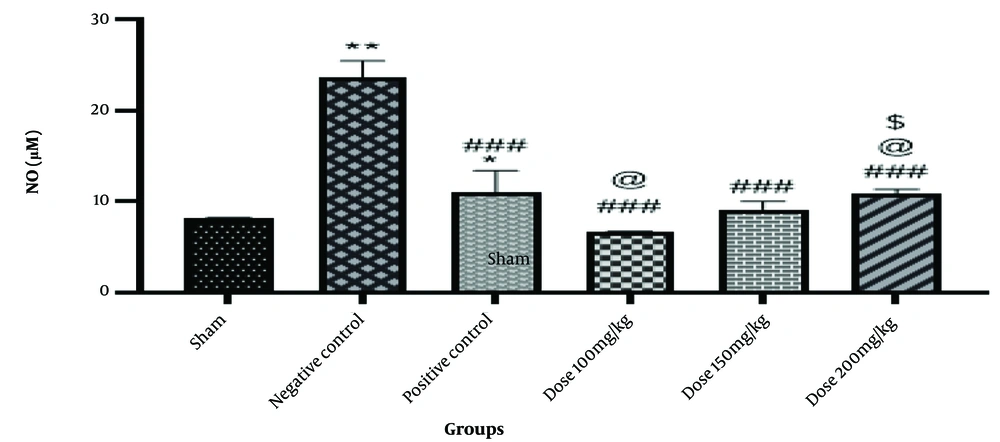
Free radicals like NO, which have a brief half-life and are involved in physiological and pathological processes, are crucial for preserving certain bodily functions. The graph's data indicates that the negative control group's NO level was significantly higher than the sham group's (P < 0.01). Nitric oxide levels in the positive control group are lower than in the negative control group (P < 0.001), with a slight rise in NO levels compared to the sham group (P < 0.05). The amount of NO in the group that received a dose of 100 mg/kg decreased significantly (P < 0.001) when compared to the negative control group, and it also decreased somewhat (P < 0.05) when compared to the positive control group. Nitric oxide levels decreased in the group that took 150 mg of the medication compared to the negative control group (P < 0.001). Nitric oxide levels also significantly reduced in the drug-treated group (200 mg/kg) compared to the negative control group (P < 0.001), as well as in the groups that received 100 mg/kg of the drug and the positive control group (P < 0.05) (Figure 3).
Information from the nitric oxide (NO) test. The * is used as a benchmark against the fictitious group. The # is employed in contrast to the negative control. When compared with the positive control group, @ is utilized. The $ is used in contrast to the group that took the medication at a dose of 100 mg/kg. The & is utilized compared to the group given 150 mg/kg of medication. * P-value < 0.05; ** P-value < 0.01; and *** P-value < 0.001. The same pattern, based on the asterisk (*), applies to the other symbols.
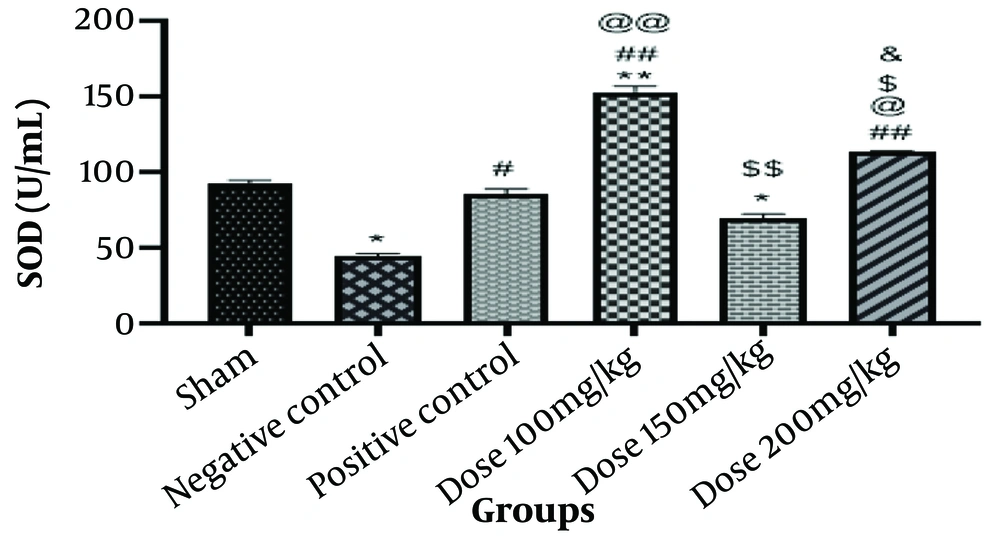
4.2.4. Superoxide Dismutase Assay
Superoxide dismutase is a crucial component of the antioxidant defense system, helping to prevent tissue damage in illnesses where negative oxygen radicals may be the cause. According to Figure 3, the negative control group's SOD level was significantly lower than that of the sham group (P < 0.05). A discernible rise in SOD level (P < 0.05) was observed in the positive control group compared to the negative control group. The level of SOD in the rats receiving 100 mg/kg of the medication was significantly higher in the treated group than in the sham group (P < 0.01) and also considerably higher than in the negative control group (P < 0.01). This group exhibited a highly significant rise in SOD (P < 0.01) compared to the positive control group. The SOD level was slightly lower in the 150 mg/kg group of rats than in the sham group (P < 0.05). The SOD level was substantially lower in this group's findings compared to the group that received a medication dose of 100 mg/kg (P < 0.01).
The findings showed that compared to the negative control group, a group of rats given 200 mg/kg of the medication had a significantly higher SOD level (P < 0.01). This group exhibited a substantial rise in SOD levels (P < 0.05) when compared to the positive control group and a clear drop in SOD levels when compared to the group that received a dose of 100 mg/kg. The results indicate a significant rise in SOD level (P < 0.05) when comparing this group to the group that received a medication dose of 150 mg/kg (Figure 4).
Information from the superoxide dismutase (SOD) assay. The * is used as a benchmark against the fictitious group. The # is employed in contrast to the negative control. When compared with the positive control group, @ is utilized. The $ is used in contrast to the group that took the medication at a dose of 100 mg/kg. The & is utilized compared to the group given 150 mg/kg of medication. * P-value < 0.05; ** P-value < 0.01; and *** P-value < 0.001. The same pattern, based on the asterisk (*), applies to the other symbols.
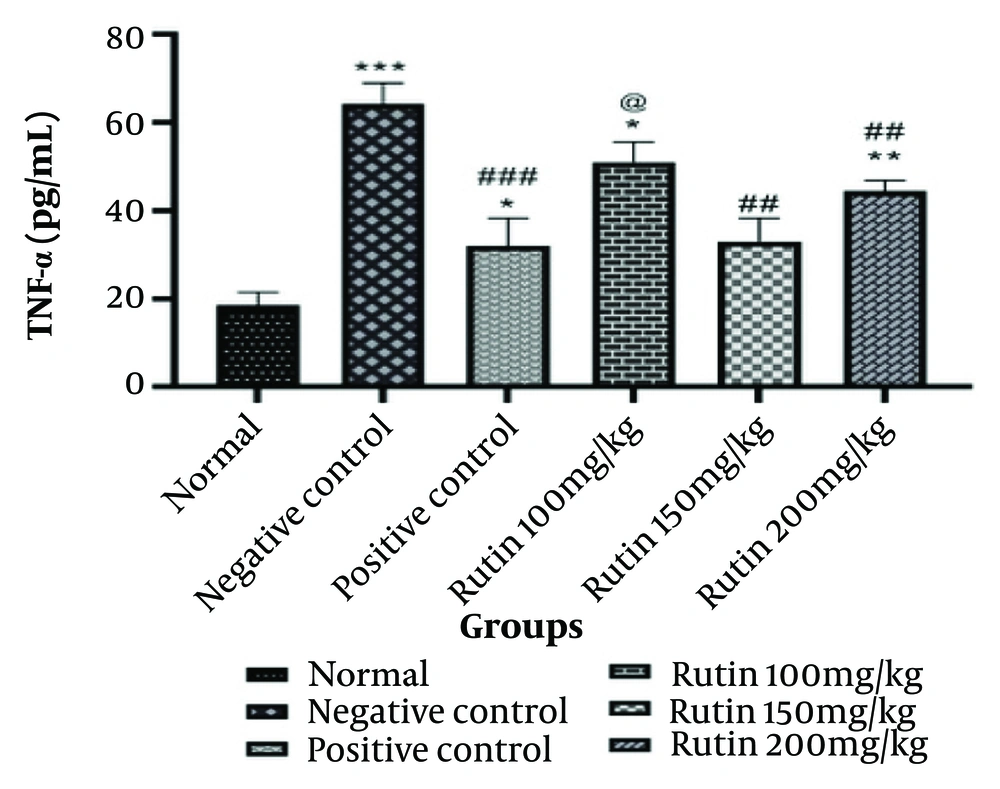
4.3. Measurement of Tumor Necrosis Factor-Alpha
Tumor necrosis factor-alpha is one of the most significant mediators of inflammation, produced by various hematopoietic, non-hematopoietic, and malignant cell types. Its primary function is to regulate the immune system. As demonstrated by the data in Figure 4, the negative control group's TNF-alpha level is significantly higher than that of the sham group (P < 0.001). There is a noticeable rise in TNF-alpha levels in the positive control group when compared to the sham group (P < 0.05) and a noticeable drop in TNF-alpha levels when compared to the negative control group (P < 0.001). The level of TNF-alpha was significantly higher in the group that received the 100 mg/kg dose than in the sham group (P < 0.05) and also considerably higher than in the positive control group (P < 0.05). A significant reduction in TNF-alpha levels was noted in the rats administered 150 mg/kg of the medication compared to the negative control group (P < 0.01). There was a substantial increase in TNF-alpha levels in the group that received a dose of 200 mg/kg of the medicine compared to the sham group (P < 0.01) and a significant decrease in TNF-alpha levels compared to the negative control group (P < 0.01) (Figure 5).
Information from measurement of tumor necrosis factor-alpha (TNF-α). The * is used as a benchmark against the fictitious group. The # is employed in contrast to the negative control. When compared with the positive control group, @ is utilized. The $ is used in contrast to the group that took the medication at a dose of 100 mg/kg. The & is utilized compared to the group given 150 mg/kg of medication. * P-value < 0.05; ** P-value < 0.01; and *** P-value < 0.001. The same pattern, based on the asterisk (*), applies to the other symbols.
4.4. Evaluation of Intestinal Injury Under a Microscope
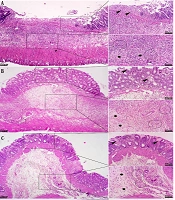
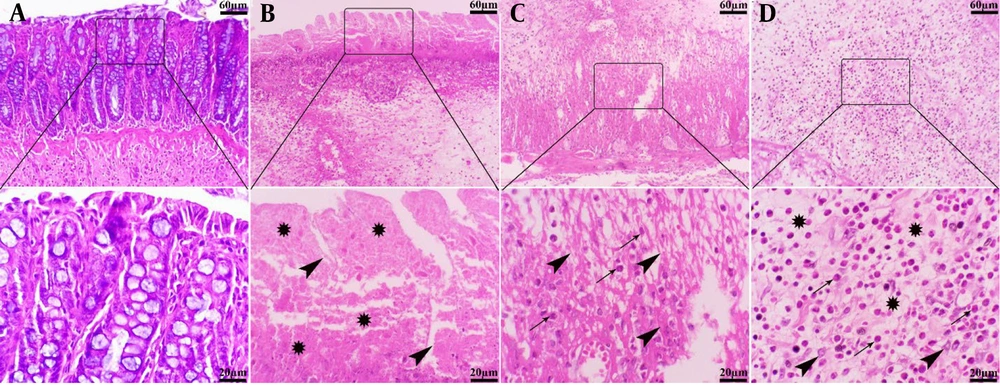
The colons of animals in the control group exhibited normal histological structure, characterized by a well-organized mucosal layer with simple tubular colonic crypts lined by intact epithelium extending down to the muscularis mucosae, submucosa with minimal infiltration of inflammatory cells, and muscularis and serosa layers. Conversely, the intracolonic application of AA induced varying degrees of tissue damage, ranging from mild to severe in the experimental groups. The mucosa showed extensive ulceration and desquamation, with lesions penetrating into the muscularis mucosa. Severe necrosis of the mucosal layer, disruption of crypt architecture with depletion of goblet cells, formation of crypt abscesses, and significant submucosal edema were evident. Additionally, this group displayed pronounced hemorrhages and dense infiltration of inflammatory cells, predominantly neutrophils and plasma cells, throughout the mucosa, submucosa, muscularis, and serosa layers. Although lesion depth extended to the serosal layer, no signs of fibrosis were detected (Figure 6).
Histopathological lesion in the colon tissue of normal and acetic acid (AA) groups (H&E staining). A, normal group: Normal architecture of colon tissue; B, AA group: Severe ulceration, necrosis (stars) and crypt destruction (arrowheads) in the mucosal layer; C, AA group: Severe necrosis of muscularis layer (arrowheads) with severe infiltration of inflammatory cells (arrows); D, AA group: Severe infiltration of mono- (arrowheads) and polymorphonuclear (arrows) cells in the submucosal layer along with severe edema (stars).
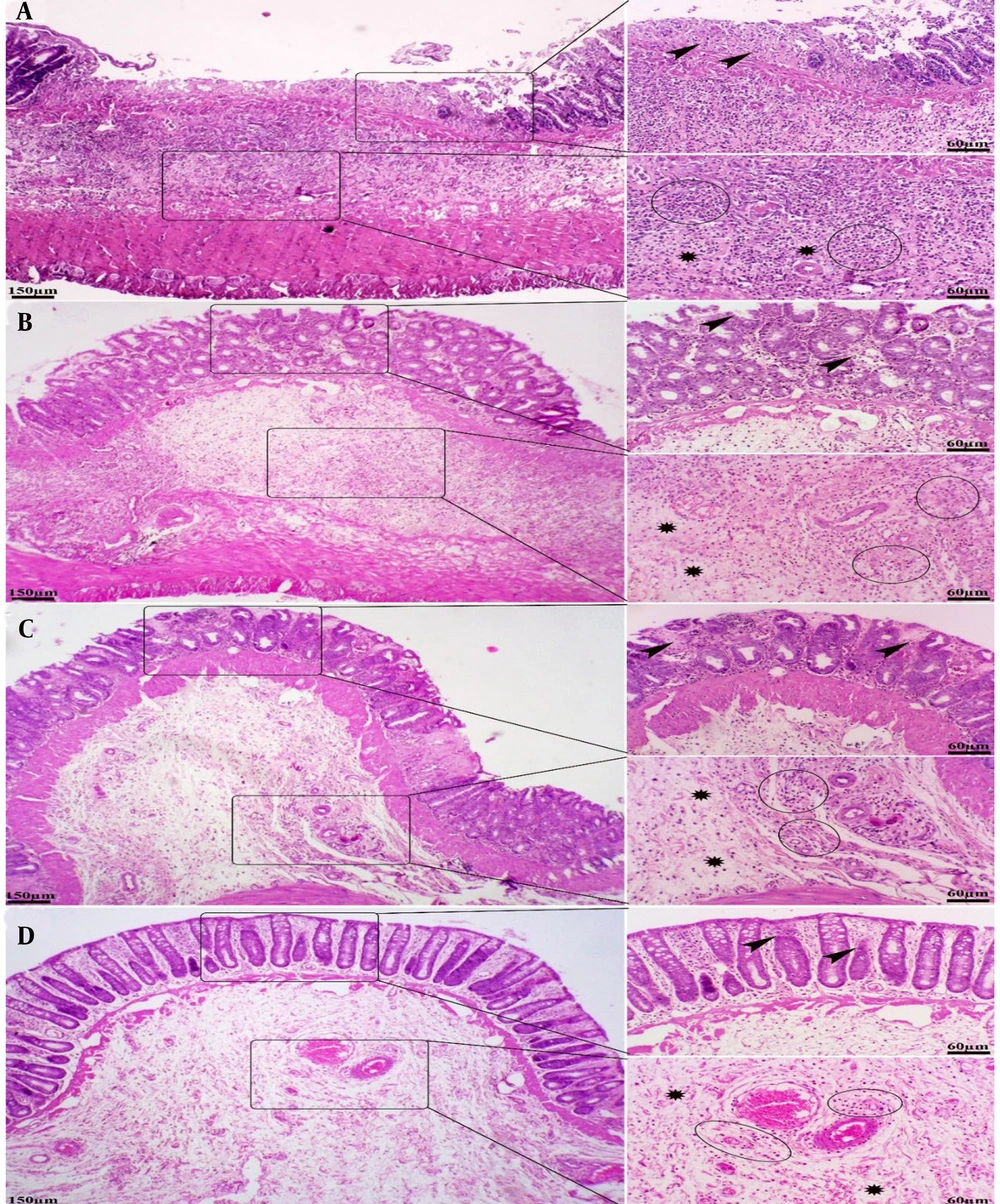
The pattern of lesions in the treated groups mainly resembled those in the AA control group. However, treatment with rutin nanoformulation significantly reduced both the severity and extent of pathological changes in the colon. Specifically, in rats receiving 100 mg/kg of rutin nanoformulation, the colon exhibited moderate to severe focal ulceration and necrosis, accompanied by diffuse moderate inflammatory cell infiltration, severe edema, and hemorrhage within the mucosa, submucosa, and muscularis layers. In the group treated with 150 mg/kg of rutin nanoformulation, there was mild to moderate inflammatory infiltration, moderate focal ulceration and necrosis, and moderate edema and hyperemia in the mucosa, submucosa, and muscularis layers, along with a more organized epithelial structure and crypt architecture. The most pronounced therapeutic effects were observed in the group treated with 200 mg/kg of rutin nanoformulation. At this dose, colon tissues showed minimal inflammatory changes, focal ulceration, mucosal necrosis, and submucosal edema and hyperemia, alongside a greater restoration of crypt architecture and epithelial alignment (Figure 7).
Histopathological lesions in the colon tissue of different treated groups (H&E staining). A, rutin nanoformulation (100 mg/kg) group: Moderate to severe focal ulceration and necrosis (arrowheads), moderate inflammatory cell infiltration (circles), severe edema within the mucosa, submucosa, and muscularis (stars); B, rutin nanoformulation (150 mg/kg) group: Mild to moderate inflammatory infiltration (circles), moderate focal ulceration and necrosis (arrowheads), and moderate edema and hyperemia in the mucosa, submucosa, and muscularis layers (stars); C, rutin nanoformulation (200 mg/kg) group: Focal ulceration, mucosal necrosis (arrowheads), mild inflammation (circles), and submucosal edema and hyperemia (stars), alongside a greater restoration of crypt architecture; D, sulfanilamide group: Minimal mucosal necrosis (arrowheads), mild inflammation (circles), prominent submucosal edema and hyperemia (stars), comparable to the nanoformulation (200 mg/kg) group.
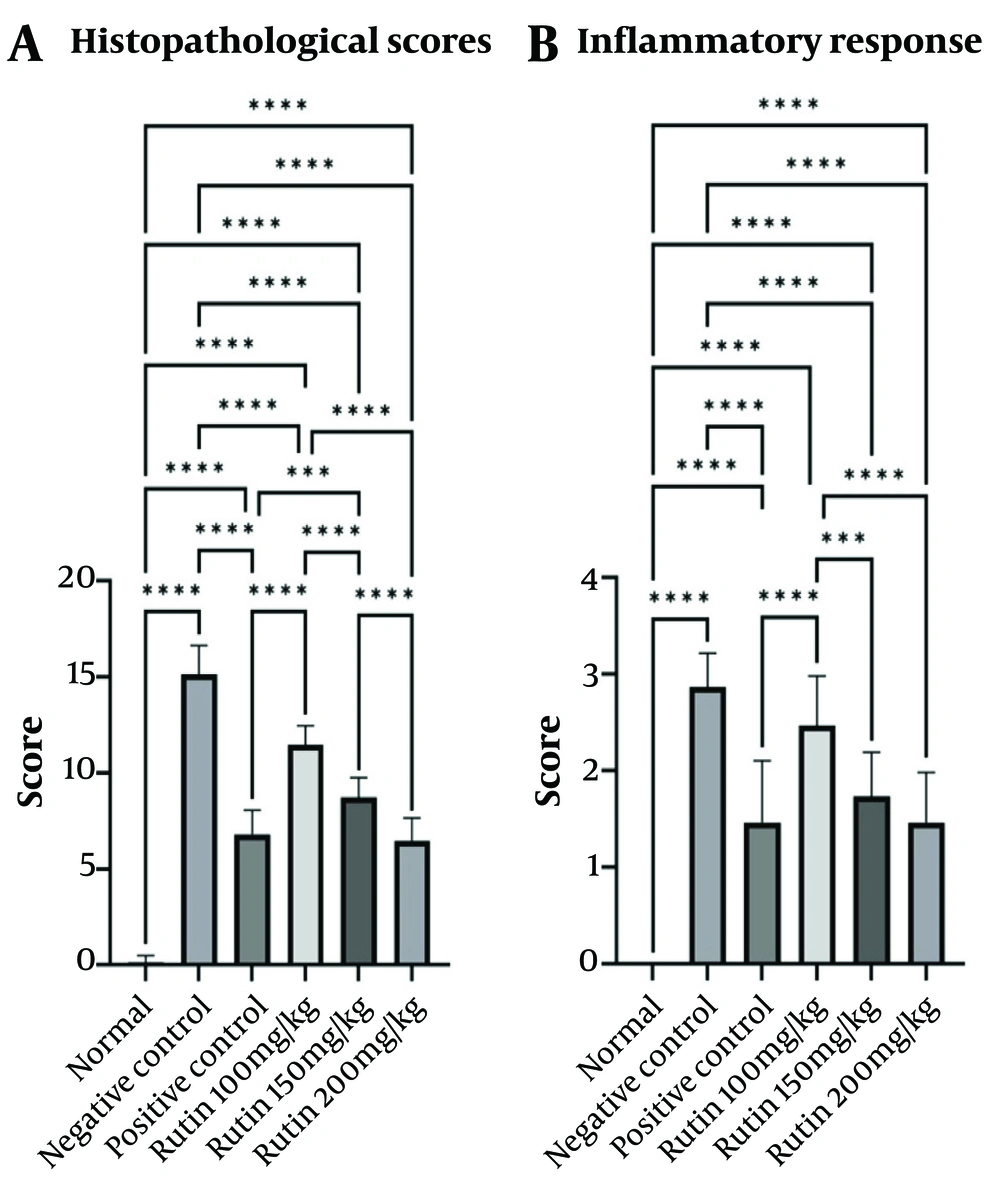
The sulfasalazine-treated group also demonstrated therapeutic benefits in the injured colon, which were comparable to those observed in the groups treated with 200 mg/kg of rutin nanoformulation. Regarding pathological scoring, the administration of rutin nanoformulation significantly reduced the histopathological scores of colonic damage when compared to the AA control group. The highest severity of intestinal lesions was noted in the AA group, followed in descending order by rutin nanoformulation at doses of 100, 150, and 200 mg/kg, respectively (Figure 8).
5. Discussion
Inflammatory bowel disease encompasses chronic conditions such as UC and Crohn's disease, significantly impairing patients' quality of life. The prevalence of IBD has been on the rise, primarily affecting the intestinal mucosa (38). Various factors, including infections, environmental influences, and immunological abnormalities, play a role in colitis etiology (39). Central to the pathophysiology of UC are the pro-inflammatory cytokine TNF-α and interleukins such as IL-1, IL-6, and IL-8. Elevated levels of these cytokines signal inflammatory dysregulation of the intestinal mucosa (40-44). Tumor necrosis factor-alpha, notably upregulated during the inflammatory phase, initiates and sustains intestinal inflammation, promoting the expression of other cytokines and pro-inflammatory mediators (45, 46). This dysregulation can lead to increased production of matrix-degrading enzymes by colon fibroblasts, consequently resulting in a loss of mucosal integrity and ulceration.
In our study, we utilized AA to induce colitis in a rat model, as it effectively increases intestinal mucosal vascular permeability and promotes inflammation and ulceration (46, 47). We investigated the impact of rutin — a flavon glycoside recognized for its potent antioxidant and anti-inflammatory properties — on this model. Rutin enhances antioxidant defenses by increasing GSH levels and activating key antioxidant enzymes such as catalase and SOD, thereby counteracting oxidative stress and reducing tissue damage (48). Moreover, rutin's ability to modulate inflammatory pathways, particularly by inhibiting the NLRP3 inflammasome and the NF-κB signaling pathway, contributes to its protective effects in colitis (48, 49). By lowering the levels of TNF-α and other pro-inflammatory cytokines, rutin interrupts the inflammatory cascade associated with UC (48, 49). Compared to other natural compounds like curcumin and quercetin, rutin exhibits significant therapeutic benefits, although its impact on gut microbiota requires further exploration (50, 51). These results align with previous studies demonstrating rutin's anti-inflammatory and antioxidant properties in treating UC.
Our findings corroborate previous research demonstrating rutin's efficacy against oxidative stress induced by butyl hydroperoxide and its modulation of the Nrf2 and iNOS pathways (52). The protective effects of rutin-loaded nanoparticles were evident in dose-dependent reductions of colonic damage and improved histological outcomes, with significant preservation of colonic tissue and lower inflammatory cell infiltration. This suggests that rutin can maintain intestinal barrier integrity by inhibiting matrix metalloproteinases (MMPs), which can lead to mucosal breakdown (53). Furthermore, regulating gut microbiota by rutin could play a significant role in inflammatory modulation by promoting a healthier microbial balance, further contributing to its therapeutic potential (54).
Despite encouraging results, we acknowledge limitations inherent in our study design, including the use of an animal model that does not fully replicate human UC, a relatively short observation period, and the exclusive use of male rats, potentially introducing gender bias. Additionally, the complex interactions between environmental factors and gut microbiota were not fully explored. Future studies should address these limitations by incorporating diverse animal models, longer durations, sex-specific analyses, and advanced tools like metagenomics to enhance our understanding of the interactions within the gut microbiome and the therapeutic potential of rutin nanoparticles.
The implications of our research suggest that rutin-loaded nanoparticles offer a promising novel therapeutic approach for IBD, presenting a safer and more tolerable alternative to conventional treatments. By targeting inflammation and oxidative stress, rutin can improve patient outcomes, reduce healthcare costs, and enhance accessibility to effective therapies, particularly in resource-limited settings. Integrating such natural compounds into treatment regimens could aid in maintaining remission and reducing the frequency of IBD flare-ups, significantly enhancing patients' quality of life (51, 55).
5.1. Conclusions
This study demonstrated that rutin-loaded chitosan nanoparticles have significant therapeutic potential for treating UC. These nanoparticles effectively mitigated inflammation and oxidative stress by reducing pro-inflammatory cytokines and enhancing antioxidant defenses. Macroscopic and histological evaluations confirmed their role in preserving colonic integrity, while emerging evidence suggested beneficial impacts on gut microbiota. The safety and biocompatibility of rutin nanoparticles were also established, highlighting their potential as a safer, more tolerable alternative to conventional UC treatments. These findings underscore the promise of rutin-loaded nanoparticles in managing UC, with significant implications for public health by potentially reducing healthcare costs and improving patient quality of life. Future research should focus on longer study durations, diverse animal models, sex-specific analyses, and advanced techniques like metagenomics to further investigate the interactions between rutin nanoparticles and the microbiome.