1. Background
Staphylococcus aureus is a gram-positive bacterium responsible for a broad range of clinical diseases. It has become notorious for causing infections in both hospitals and the community (1). Chronic biofilm-related infections triggered by S. aureus predominantly drive major increases in morbidity and mortality, notably when linked with medical devices (2). Accordingly, many studies have been conducted to understand the molecular basis affecting S. aureus biofilm formation and the mechanisms of antibiotic resistance (3). Treatment has become challenging due to the emergence of multidrug-resistant strains (4). One strategy to suppress the drug resistance of biofilms is to exploit new drug delivery technologies (5). Recently, many attempts have been made to use nanoparticles as carriers due to their unique behavior (6, 7). Additionally, these systems are suitable for the delivery of natural materials (8). These nano-engineered technologies, with their outstanding properties, enable them to overcome hurdles faced by conventional antimicrobials and, with appropriate formulation, can be considered drug delivery agents to biofilms (9). Lipid-based nanocarriers such as solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) have been developed, indicating improved properties such as increased drug loading capacity and physical stability (10). These nanocarriers aim to overcome the limitations of other colloidal carriers, including emulsions, liposomes, and polymeric nanoparticles (11, 12). Piperine, the main bioactive constituent of black pepper, exhibits a plethora of beneficial effects, including antioxidant, anti-tumor, anti-inflammatory, anti-anxiety, liver-protective, anti-depression, and antibacterial effects (13). However, its therapeutic application is limited by poor solubility and bioavailability. Consequently, various formulation strategies with different techniques have been exploited to improve piperine's physicochemical, pharmacokinetic, and pharmacodynamic properties (14). Recent investigations show that nanoencapsulation and nanoparticles of piperine products enhance the bioavailability of piperine through local and oral administration (15). The antibacterial activity of black pepper against gram-positive bacteria, such as S. aureus, reported by some studies, corroborates that this spice can be a powerful agent against infectious diseases and pathogens caused by this microorganism (16, 17). Meanwhile, liquid lipids, as an essential component of NLCs, should be examined to achieve possible synergistic performance. Liquid lipids are composed of fatty acids, triglycerides, monoglycerides, etc., and oils of natural sources extracted from various sources with therapeutic properties can be loaded into the matrix of NLCs (18, 19). Lavender (Lavandula spp.) is recognized as one of the medicinal plants for stress control and belongs to the Lamiaceae family (20). Lavender essential oil comprises several antimicrobial substances, such as eucalyptol, linalool, terpinen-4-ol, and α-terpineol (21), with linalool being the potent active ingredient against a broad range of microorganisms (21). It has been revealed that lavender oil has effective performance on species of bacteria that are even resistant to antibiotics, such as methicillin-resistant S. aureus (MRSA), vancomycin-resistant Enterococcus (VRE), Escherichia coli, Pseudomonas aeruginosa, and Propionibacterium acnes (22). Various investigations have elucidated the presence of a moderate synergistic effect between essential oils and pharmaceutical agents, notably antibiotics, which is predominantly attributable to the membrane interactions of the constituents of essential oils; in this regard, essential oils present a compelling alternative for mitigating antibiotic utilization (23). Considering that no piperine/lavender oil combination has been reported to date, the aim of this study was to examine in vitro interactions between lavender oil encapsulated within NLCs and piperine.
2. Objectives
In this study, NLCs incorporating piperine and lavender oil were prepared by hot homogenization and ultrasound, and their antibacterial effect was investigated on S. aureus biofilm.
3. Methods
Lavender oil and piperine powder were purchased from Barij Essence (Iran) and Gol Exir Pars (Iran), respectively. Stearic acid, Tween, and Span 80 were obtained from Merck (Germany). All culture media, including nutrient broth (NB), Mueller Hinton broth (MHb), blood agar (BA), tryptic soy broth (TSB), BaSO4, and H2SO4, were supplied by Merck (Germany). The S. aureus strain, in the form of lyophilized vials, was provided by Arian Mehr Center (Iran). Dimethyl sulfoxide (DMSO) was purchased from Dae Jung (Korea). Gentamycin was provided by Zahravi Pharmaceutical Company (Iran).
3.1. Nanostructured Lipid Carriers Preparation
To synthesize NLCs, a hot homogenization and ultrasound method was employed. In this procedure, lipids were heated 5 - 10°C above the melting point of the solid lipid. The lipophilic materials, including piperine, stearic acid, Span 80, and lavender oil, were weighed and placed into a tube. An aqueous solution of poloxamer 188 was prepared in another tube. Both tubes were then placed separately in a Bain-Marie (Memmert, Germany) to heat and melt the contents. After reaching isothermal conditions, the aqueous phase was added to the lipid phase, and the resulting pre-emulsion was homogenized using an Ultratorx homogenizer (Heidolph, Germany) at 11,500 rpm for 3 minutes. Following the disruption of emulsion particles, ultrasonic waves were applied for 3 minutes with 70% on and 30% off cycles. After sonication, the dispersion was left overnight at room temperature to cool and facilitate the formation of NLCs. Blank nanoparticles were also prepared using the same method described above, without the addition of piperine (24).
3.2. Determination of Particle Size and Zeta Potential
To assess the shape and size of the obtained NLCs, electron microscopy (Philips VP-1450, Netherlands) and a particle size analyzer (Malvern, UK) were used. For this purpose, 10 μL of the freshly prepared sample at room temperature was added to a microtube (1.5 mL volume) along with 990 μL of deionized water, achieving a 1:100 dilution ratio (25).
3.3. Determination of Encapsulation Efficiency
The encapsulation efficiency of piperine in nanocarriers was assessed using an indirect method involving centrifugation (Hettich, Germany) and filtration (Amicon, cut-off = 14 kDa). In brief, the filter device was filled with the nanosuspension of NLCs and centrifuged at 6000 × g for 34 minutes. The encapsulated piperine was retained in the filter device, while the unencapsulated piperine passed through the filter. The ultra-filtrate solution was subsequently analyzed to quantify the amount of unencapsulated piperine. Standard solutions of piperine were prepared at concentrations of 1, 2, 5, 10, and 20 μg/mL, and their absorbance was measured at 342 nm, the maximum wavelength for piperine, using UV-Vis spectroscopy. The encapsulation efficiency was then calculated using the following formula (26):
Where Ts and Tt represent the initial amount of the active ingredient and the amount of the effective substance detected in the supernatant after separation of the aqueous and lipid phases, respectively.
3.4. Investigation of Morphology of Lipid Nanoparticles
To examine the shape and size of the obtained lipid nanoparticles, electron microscopy (Philips VP-1450, Netherlands) was employed. The sample was diluted 15-fold with distilled water, and 20 μL of the diluted sample was placed on carbon-coated grids. After 30 seconds, the excess liquid was removed. Subsequently, 20 μL of a 2% uranyl acetate solution was added to the grids and left for 30 seconds, followed by drying with filter paper. Finally, the prepared samples were observed under the electron microscope (27, 28).
3.5. Fourier-Transform Infrared Spectroscopy
Fourier-transform infrared (FTIR) spectra were acquired using the KBr pellet method. An FTIR spectrophotometer (IR Prestige, Shimadzu, Japan) was employed with a resolution of 16 cm-1, 10 scans, and a spectral range of 4000 to 500 cm-1 at ambient temperature. To prepare the samples, 10 mg of each sample was crushed with KBr to obtain a uniform mixture. The NLC samples were first freeze-dried (Chaist, Germany) to convert them into a solid form. Subsequently, the solid samples were mixed with KBr to prepare KBr disks (27).
3.6. Preparation of 0.5 McFarland Standard
A barium sulfate (BaSO4) standard, known as the McFarland solution, was used to standardize the concentration of bacterial inoculation and to conduct microbial sensitivity tests. To prepare the target BaSO4 solution, 0.5 mL of barium chloride (BaCl2) (0.048 M) was added to 99.5 mL of sulfuric acid (H2SO4) (0.18 M), and the resulting solution was mixed thoroughly. The precise density of the standard turbidity was measured using a spectrophotometer (PerkinElmer, Germany) at a wavelength of 625 nm, with an expected absorbance between 0.08 and 0.13; the observed optical absorbance was 0.09 λ. Six mL of BaSO4 was poured into tubes with lids matching the size of the bacterial suspension tubes. The tubes were sealed tightly and stored in the dark at room temperature. A 0.5 McFarland standard solution has a specific turbidity (equal to 1.5 × 108) CFU/mL of the organism. Subsequently, two or three bacterial colonies were transferred to tubes containing 3 mL of sterile physiological saline (distilled water or NB liquid medium) after 48 hours of bacterial culture. Finally, they were monitored using a spectrophotometer, with an expected absorbance value between 0.08 and 1 at a wavelength of 625 nm.
3.7. Preparation of Bacteria
In accordance with the guidelines, S. aureus (12600) was handled under sterile conditions within a microbiology hood (Envair, UK). The vials were carefully scratched and broken using a diamond pen. Sterile NB culture medium (2 mL) was introduced into each vial using a syringe. The lyophilized microbial powder was thoroughly dissolved and homogenized. The resulting suspension was inoculated into sterile BA culture medium, NB, and plates. The cultures were incubated at 37°C for 48 hours.
3.8. Evaluation of Antimicrobial Activity by Cup Plate Method
A microbial suspension with a turbidity equivalent to a 0.5 McFarland standard contains 1.5 × 108 CFU/mL of bacteria. This suspension was uniformly spread on the freshly prepared surface of Müller-Hinton plates using a sterile cotton swab. Seven wells were then created on the culture medium of each plate using a sterile Pasteur pipette. Twenty microliters of sterile Müller-Hinton broth (MHb) culture medium, maintained at 45°C, was used to cover the bottom surface of each well. Free piperine, dissolved in 10% methanol, was added to the wells in a volume of 100 μL. The plates were then sealed and incubated at 37°C for 24 hours.
3.9. Measurement of Minimum Inhibitory Concentration by Microdilution Technique
The MIC test was conducted in accordance with our previously published works (12). Compounds containing free piperine, NLCs with piperine (NLCs-Pip), and NLCs without piperine were dissolved in 10% methanol. A volume of 100 μL of each sample was used for the test. The results were recorded after a 24-hour incubation period for each bacterium at the specified temperature. The presence of turbidity compared to the control indicates bacterial growth, whereas clarity signifies the inhibition of bacterial proliferation (29).
3.10. Assessment of Minimum Bactericidal Concentration
The MBC was evaluated by culturing all clear wells in TSB medium and incubating the media for 24 hours for all investigated bacteria at 37°C. The assays were performed in at least three distinct replicates (29).
3.11. Microtiter Plate Assay to Assess Biofilm Elimination Potential
A microbial suspension was prepared to match a 0.5 McFarland standard after culturing (18 - 20 hours) and activating the bacteria. A volume of 100 μL of TSB culture medium was added to a 96-well plate, followed by formulations (100 μL) containing free piperine, gentamicin, NLCs containing piperine (NLC-Pip), and NLCs, along with 10 μL of microbial suspension. After inoculation, the plates were covered and incubated overnight at 37°C. In addition to the treated wells, control columns containing untreated biofilm and control columns containing sterile broth medium were included in each plate. The wells were washed and stained with 2% crystal violet (200 μL) for 5 minutes. Subsequently, the wells were washed, and 33% glacial acetic acid (200 μL) was added. Finally, the plates were incubated for 15 minutes, and the absorbance was recorded at 450 - 620 nm.
3.12. Statistical Analysis
The results were statistically analyzed using one-way analysis of variance (ANOVA) with GraphPad Prism (version 6.00 for Windows; GraphPad Software, La Jolla, CA, USA). Differences were considered significant when the P-value was less than 0.05. All tests were performed in triplicate (n = 3).
4. Results
4.1. Experimental Design
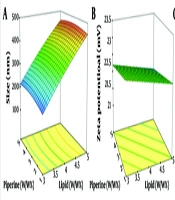
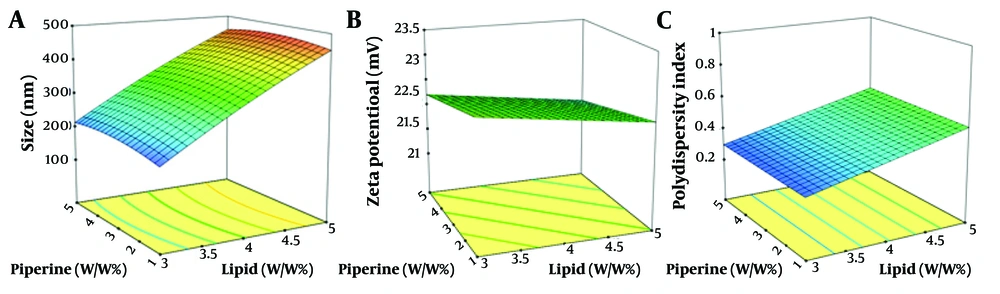
The central composite design was employed using Design-Expert software (version 10.0.3; Stat-Ease Inc., Minneapolis, MN, USA) to investigate the influence of various factors, including lipid percentage, lipid ratio, surfactant percentage, feed ratio of surfactants, and the percentage of piperine as the medicinal substance. The levels of these parameters are listed in Table 1. The outcomes of 26 runs, detailing the structural results of the formulations, including particle size, zeta potential, and Polydispersity Index (PDI), as determined by dynamic light scattering (DLS), are reported in Table 2.
| Levels | -1 | 0 | +1 |
|---|---|---|---|
| Lipid (w/w %) | 3 | 4 | 5 |
| Lipid ratio (stearic acid/lavender oil) | 3 | 4 | 5 |
| Surfactant (w/w %) | 1 | 2 | 3 |
| Feed ratio of surfactants | 0.5 | 1.25 | 2 |
| Drug (w/w %) | 1 | 3 | 5 |
| Run | Lipid (w/w %) | Lipid Ratio b | Surfactant (w/w %) | Feed Ratio of Surfactant (%) c | Piperine (w/w %) | Size (nm) | Zeta Potential (mV) | Polydispersity |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.00 | 4.00 | 2.00 | 1.25 | 5.00 | 344 ± 8.22 | -23.2 ± 6.91 | 0.401 ± 0.054 |
| 2 | 4.00 | 4.00 | 2.00 | 1.25 | 3.00 | 357 ± 7.34 | -21.7 ± 7.32 | 0.411 ± 0.061 |
| 3 | 4.00 | 4.00 | 2.00 | 1.25 | 1.00 | 343 ± 6.51 | -22.8 ± 5.32 | 0.390 ± 0.032 |
| 4 | 4.00 | 4.00 | 2.00 | 1.25 | 3.00 | 359 ± 6.87 | -21.3 ± 3.28 | 0.390 ± 0.045 |
| 5 | 4.00 | 4.00 | 3.00 | 1.25 | 3.00 | 318 ± 3.82 | -22.3 ± 4.38 | 0.321 ± 0.081 |
| 6 | 3.00 | 4.00 | 2.00 | 1.25 | 3.00 | 223 ± 6.21 | -23.4 ± 2.87 | 0.290 ± 0.032 |
| 7 | 4.00 | 3.00 | 2.00 | 1.25 | 3.00 | 322 ± 5.75 | -21.8 ± 5.37 | 0.332 ± 0.058 |
| 8 | 4.00 | 4.00 | 2.00 | 0.50 | 3.00 | 344 ± 4.37 | -21.2 ± 4.61 | 0.391 ± 0.067 |
| 9 | 5.00 | 5.00 | 1.00 | 2.00 | 1.00 | 484 ± 9.11 | -22.3 ± 3.65 | 0.530 ± 0.028 |
| 10 | 4.00 | 5.00 | 2.00 | 1.25 | 3.00 | 384 ± 8.15 | -23.2 ± 9.10 | 0.933 ± 0.034 |
| 11 | 4.00 | 4.00 | 2.00 | 2.00 | 3.00 | 370 ± 7.21 | -21.5 ± 8.23 | 0.315 ± 0.051 |
| 12 | 3.00 | 5.00 | 3.00 | 2.00 | 1.00 | 187 ± 4.14 | -23.1 ± 5.38 | 0.241 ± 0.068 |
| 13 | 5.00 | 5.00 | 3.00 | 0.50 | 1.00 | 454 ± 4.21 | -21.9 ± 5.39 | 0.490 ± 0.092 |
| 14 | 3.00 | 5.00 | 1.00 | 2.00 | 5.00 | 245 ± 3.78 | -22.1 ± 3.84 | 0.290 ± 0.085 |
| 15 | 4.00 | 4.00 | 2.00 | 1.25 | 3.00 | 353 ± 2.91 | -21.2 ± 4.37 | 0.392 ± 0.075 |
| 16 | 5.00 | 3.00 | 1.00 | 2.00 | 5.00 | 465 ± 2.15 | -22.5 ± 4.93 | 0.510 ± 0.073 |
| 17 | 5.00 | 5.00 | 1.00 | 0.50 | 5.00 | 473 ± 8.17 | -21.9 ± 5.81 | 0.503 ± 0.019 |
| 18 | 3.00 | 3.00 | 1.00 | 0.50 | 1.00 | 204 ± 9.2 | -22.8 ± 8.94 | 0.315 ± 0.082 |
| 19 | 4.00 | 4.00 | 2.00 | 1.25 | 3.00 | 360 ± 8.72 | -22.2 ± 7.34 | 0.395 ± 0.065 |
| 20 | 3.00 | 5.00 | 3.00 | 0.50 | 5.00 | 210 ± 6.94 | -21.8 ± 5.87 | 0.295 ± 0.028 |
| 21 | 4.00 | 4.00 | 1.00 | 1.25 | 3.00 | 394 ± 4.31 | -22.7 ± 9.45 | 0.405 ± 0.053 |
| 22 | 5.00 | 3.00 | 3.00 | 0.50 | 5.00 | 437 ± 5.53 | -21.3 ± 8.35 | 0.483 ± 0.038 |
| 23 | 5.00 | 3.00 | 3.00 | 2.00 | 1.00 | 424±5.27 | -22.4 ± 7.11 | 0.495 ± 0.042 |
| 24 | 3.00 | 3.00 | 3.00 | 2.00 | 5.00 | 185 ± 4.38 | -22.1 ± 8.15 | 0.280 ± 0.058 |
| 25 | 4.00 | 4.00 | 2.00 | 1.25 | 3.00 | 356 ± 7.21 | -21.9 ± 6.21 | 0.390 ± 0.092 |
| 26 | 5.00 | 4.00 | 2.00 | 1.25 | 3.00 | 472 ± 5.33 | -22.7 ± 6.52 | 0.580 ± 0.037 |
a Values are expressed as mean ± SD unless otherwise indicated.
b Stearic acid/lavender oil.
c Span 80/Tween.
The size of nanoparticles plays a crucial role in their properties, including stability, biological activity, and appearance. Smaller nanoparticles have a higher surface-to-volume ratio, which can enhance their effectiveness and make them beneficial alternatives. The results of this study confirmed that the particle size ranged from 185 to 484 nm (Figure 1A).
The zeta potential serves as an indicator of the electrical characteristics of the surface, reflecting the extent of charge accumulation within the immobile layer and the degree of adsorption of counter ions onto the particle surface. Consequently, the magnitude of the zeta potential determines the degree of electrostatic stability, which is measured through electrophoretic mobility. A decrease in zeta potential can lead to particle aggregation. The zeta potential of all formulations ranged from -21.2 to -23.4 (Figure 1B). The standard range for the PDI is zero to one. Figure 1C shows the response surface plot of the PDI, ranging from 0.28 to 0.93. All data were within this range, with values closer to zero being preferred for a better formulation.
Figure 1A - C illustrate the response surface plots of particle size, zeta potential, and PDI with respect to lipid and piperine content, respectively. It can be concluded that increasing lipid and piperine content results in increased PDI values, while only a small change in zeta potential was observed. The most preferable formulation (No. 12), with a piperine concentration of 1%, a lipid concentration of 3%, a surfactant ratio of 2%, and a surfactant concentration of 3%, was selected and repeated in triplicate to confirm the predicted results. The empirical results for the formulation were 179.8 nm, 0.416, and -23.8 mV for size, PDI, and zeta potential, respectively, while the corresponding predicted values were 181.2 nm, 0.417, and -22.9 mV. The two sets of data were compared, and the average relative errors were calculated (Table 3). The obtained data demonstrated that the optimization process of the formulation was effective.
| Variables | Experiment Value | Predicted Value | Error (%) |
|---|---|---|---|
| Size (nm) | 187 | 179 | 4.2 |
| PDI | 0.241 | 0.234 | 2.9 |
| Zeta potential (mV) | -23.1 | -23.4 | 1.2 |
Abbreviation: PDI, Polydispersity Index.
The following formula is used to calculate the error percentage: The percentage of error = (The results of DLS-The results predicted by software)/(The results of DLS) × 100
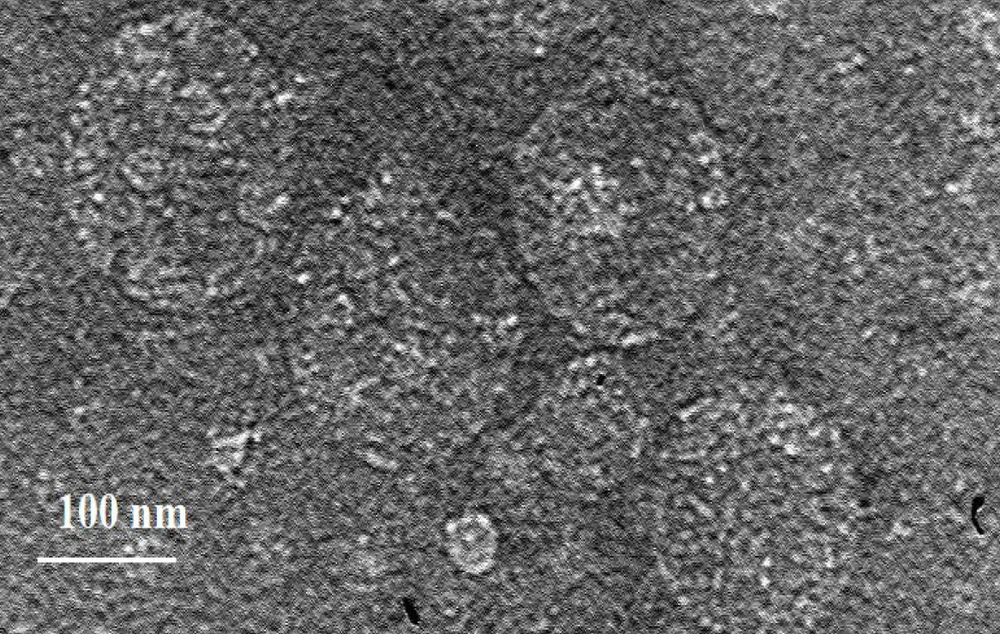
4.2. Transmission Electron Microscopy Image Results
The efficiency of electron microscopes in analyzing the structures of various materials, such as tissues, cell scaffolds, microbial structures, and nanoparticles, is significant due to the limitations of optical microscopes, which cannot observe objects smaller than 100 nm. To assess the morphology of NLCs enriched with piperine and lavender oil, TEM was employed. Figure 2 reveals spherical particles and evidence of phase separation within the particles.
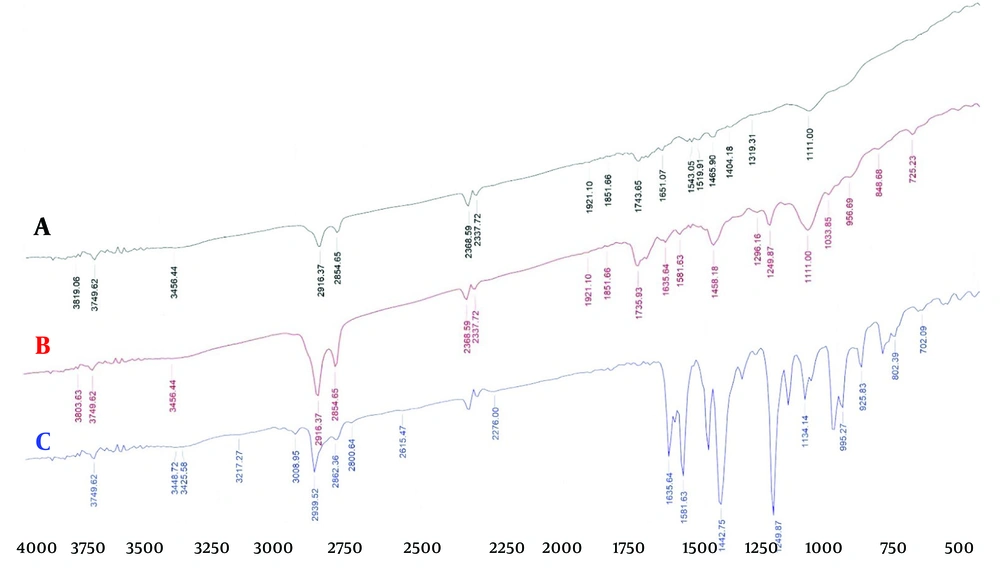
4.3. Fourier-Transform Infrared Spectrum of Piperine
The information obtained by FTIR is displayed in Figure 3A - C. The peaks observed at 1442 cm-1 and 2939 cm-1 indicate bending vibrations (CH2) and aromatic stretching vibrations (H-C), respectively. Additionally, peaks at 1581 cm-1, 1249 cm-1, and 995 cm-1 represent aromatic stretching vibrations (C=C) and asymmetric stretching vibrations related to =C-O-C and CH2 oscillatory vibration. The indicative peak at 1635 cm-1 reveals the tensile vibration of -CO-N as well as the symmetric and asymmetric stretching vibration of C=C.
As shown, the FTIR spectrum of pure piperine exhibits sharp and prominent peaks, indicating the presence of various functional groups in the piperine structure. The spectra of the blank sample and the NLC-Pip are very similar, and the peaks in the NLC-Pip spectrum have become significantly smaller, indicating that piperine is entrapped within the nanoparticles.
4.4. Encapsulation Percentage
The loading percentage was estimated at approximately 99% using the indirect method (SD = 4.15%, n = 3). To date, various drugs and natural compounds have been formulated in lipid formulations such as NLCs and SLNs. A key characteristic for achieving high loading efficiency is the solubility of the drug in the lipid matrix. The preparation method is another major factor. Another probable reason for high entrapment efficiency is the use of lavender oil in the NLCs structure, which creates lattice imperfections where the molecules can be inserted (30). The regression equation for piperine was A = 0.083C + 0.084, and a linear correlation was observed in the range of 1 - 20 μg/mL (R² = 0.99).
4.5. Evaluation of Diameter of the Inhibition Zone
The evaluation of the diameter of the inhibition zone indicates that NLC-Pip acts as an inhibitor of bacterial growth. The NLC-Pip produced a diameter of 17 mm, whereas the NLCs without piperine and free piperine exhibited diameters of 0 mm and 19 mm, respectively. These results are summarized in Table 4.
| Formulation | NLC-Pip | NLC Without Piperine | Free Piperine | Gentamicin |
|---|---|---|---|---|
| The diameter of inhibition zone | 17 ± 2.3 | 0.0 ± 0.0 | 19 ± 1.1 | 20 ± 1.8 |
Abbreviations: NLC-Pip, nanostructured lipid carriers containing piperine; NLC, nanostructured lipid carrier.
a Values are reported as mean ± SD.
4.6. Assessment of the Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
Table 5 presents the values of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of lipid nanoparticles on bacteria, expressed in micrograms per milliliter (µg/mL). The results indicate that the MIC for NLC-Pip was 40 µg/mL, while the MBC was 80 µg/mL. Similarly, the MIC and MBC for free piperine were also 40 µg/mL and 80 µg/mL, respectively. These findings demonstrate the inhibition and elimination of bacterial growth.
| Tests | NLC-Pip | Free Piperine |
|---|---|---|
| MIC (µg/mL) | 40 | 40 |
| MBC (µg/mL) | 80 | 80 |
Abbreviation: MIC, minimum inhibitory concentration; MBC, minimum bactericidal concentration; NLC-Pip, nanostructured lipid carriers containing piperine.
4.7. Results of Monitoring Biofilm Inhibition by Nanostructured Lipid Carriers Containing Piperine
Numerous studies have explored the interaction between essential oils and antibacterial agents, including antibiotics. In managing infections, there are several scenarios where the co-administration of two or more antibiotics is necessary (31). Indeed, the use of combined antibiotic therapy has been employed to combat multi-resistant bacterial strains. Given their mechanisms of action, essential oils offer a promising adjunctive option to be used alongside conventional antibiotics.
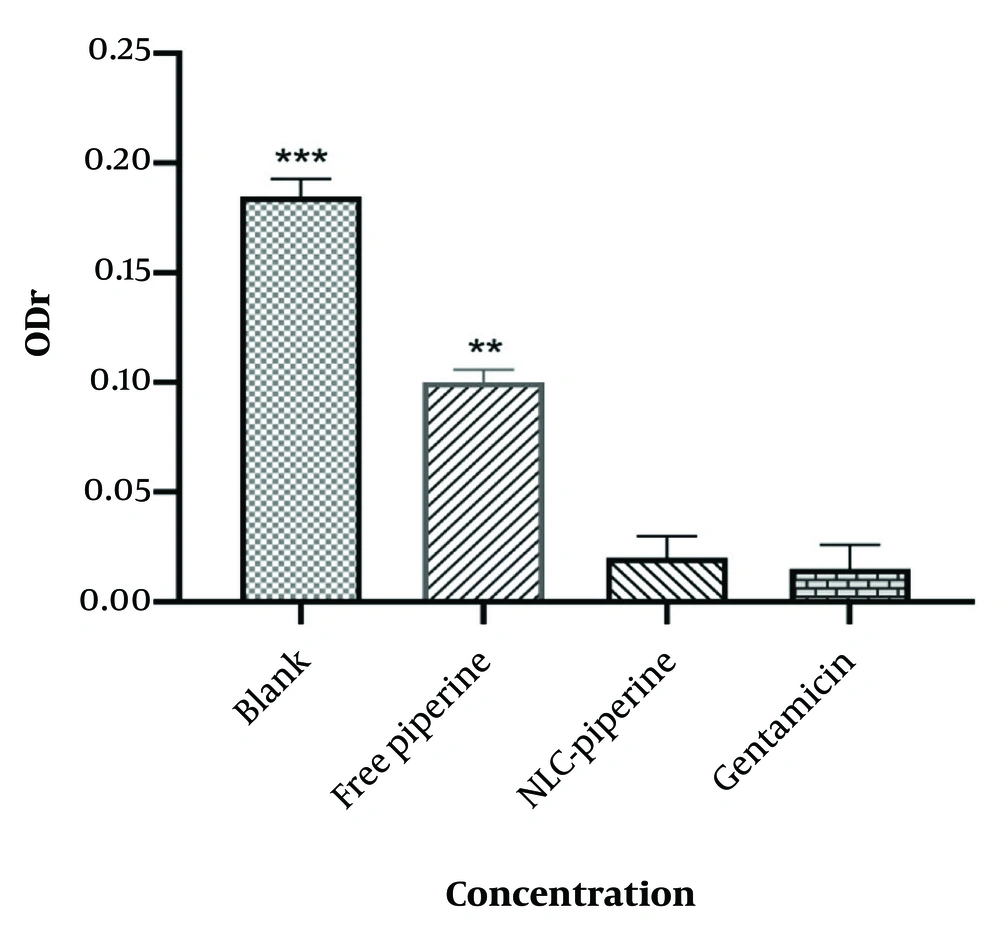
Figure 4 shows the magnitude of biofilm absorption for free piperine, NLC-Pip and blank NLCs.
According to Figure 4, a significant relationship was observed between the encapsulation of drug molecules via the NLCs formulation and a decrease in the optical density ratio (ODr). The spectrophotometric absorption data related to biofilm production indicated that in the NLC-Pip, the amount of biofilm formation had reached zero. As illustrated in Figure 4, in the NLC-Pip, the biofilm had almost disappeared. For gentamicin, the rate of biofilm formation was approximately zero, and there was no significant difference between NLC-Pip and gentamicin as a control drug. In contrast, free piperine and piperine-free NLCs exhibited the maximum ODr, indicating the highest biofilm formation (P < 0.001 and P < 0.01, respectively), compared to the synthesized formulation. Specifically, NLC-Pip demonstrated an enhancement in the antimicrobial efficacy of piperine by approximately five-fold compared to its free form (23). The most pronounced effect was detected in the NLCs-Pip formulation because the formulation can effectively bind to the bacterial surface and improve the release of piperine (32).
5. Discussion
Lipid nanoparticles are increasingly utilized in various fields of biology and medicine. Due to their small size, these nanoparticles can transport a wide range of compounds, including both hydrophilic and hydrophobic drugs (33). Piperine, known for its therapeutic benefits, is gaining significant attention from researchers. However, its clinical application is limited by poor solubility and bioavailability. To enhance these properties, SLNs and NLCs are being extensively studied (34).
In a study, the effects of the natural compounds embelin and piperine on the formation of Streptococcus mutans biofilm were evaluated using the microtiter plate method (35). Both compounds were found to significantly inhibit biofilm formation, demonstrating notable anti-biofilm properties. Electron microscopy results indicated that lipid nanocarriers enriched with piperine have a smooth, uniform surface and spherical-shaped particles. Compared to other nanoparticle forms, the spherical shape minimizes contact with the aqueous environment of the dispersed phase, facilitating controlled release and drug protection.
According to the FTIR spectra, the major peaks in piperine, which indicate the main functional groups in the final formulation of NLC-Pip, have significantly diminished. This suggests that a substantial percentage of piperine has been incorporated into the nanoparticles, indicating successful encapsulation and loading of piperine. Similarly, the spectra for NLC-Pip and the blank formulation demonstrate effective entrapment of piperine by the NLCs. Observations related to the presence of functional groups may be attributed to the existence of free drugs at the nanoparticle level. The PDI measures particle non-uniformity, with a standard value range between zero and one (values closer to zero indicate greater uniformity and homogeneity). In this study, the PDI values of the formulations ranged from 0.241 to 0.933 (27).
The zeta potential is influenced by the concentration of electrolytes or ionic surfactants in the carrier. In our investigation, the zeta potential of the particles ranged from -21.2 to -23.4 (27). These results confirm that the incorporation of piperine into NLC particles is well-established, making NLCs suitable carriers for piperine due to enhanced drug loading and delivery capabilities. Additionally, the inclusion of lavender oil in the formulation increases the efficiency of NLC vehicles. In a research study, polysorbate-phospholipid micelles encapsulating piperine and azithromycin were synthesized to evaluate the oral absorption of piperine and its antibacterial effect. Optimization results indicated that 5.5% w/v of polysorbate achieved the highest solubilization of piperine. The study showed that the largest and smallest zones of inhibition were observed at concentrations of 100 mg/mL and 50 mg/mL, respectively (36). Another study reported the preparation of piperine and gentamicin liposomes using the dehydration-rehydration method. The antibacterial activity of these structures was tested against methicillin-resistant Staphylococcus aureus. A reduction in the MIC and MBC values of gentamicin and piperine was noted when gentamicin was incorporated into liposomes and liposomal combinations. Furthermore, piperine liposomes demonstrated efflux pump inhibition (37).
According to a report, the combination of piperine and pipelongomine with rifampicin and tetracycline offers a method to reduce the side effects associated with common antibiotics. The results indicated that piperine and pipelongomine can enhance the effectiveness of rifampicin and tetracycline (38). In this study, although the outcomes related to the diameter of the non-growth zone and the MIC in NLC-Pip were lower than those of free piperine at the same concentrations—due to the slow release of piperine from the nanoparticles—the assessment of the absorption of various nanoparticle concentrations demonstrated that NLC-Pip is more effective in biofilm removal than free piperine. The findings of this research suggest that lipid nanoparticles significantly enhance the efficacy of piperine. The use of nanometer-sized colloidal carriers is justified by their improved interaction with bacteria (39). The nanoparticles utilized in this study were within the size range of 100 - 200 nm.
Piperine likely mitigates biofilm formation by suppressing the genes involved in the initiation and expansion phases of biofilm development. It is established that bacterial biofilms have three-dimensional structures where cells are embedded in a substantial amount of extracellular polymeric substance (EPS). Piperine alone may not effectively inhibit biofilm formation due to its high density and limited permeability within the biofilm. In this context, utilizing nanocarriers could be an effective strategy to deliver piperine into the EPS of the biofilm. The continuous release of piperine from nanocarriers can have promising effects over time. Moreover, enhancing piperine release in acidic pH, characteristic of biofilm environments, may improve its biofilm inhibitory activity. Microbial tests indicate that NLC-Pip has a greater effect on Staphylococcus aureus biofilm compared to free piperine and nanoparticles without piperine.
5.1. Conclusions
The anti-biofilm bioavailability of piperine was significantly enhanced by the formulation of piperine-NLCs. The formulation was prepared using Tween and Span as stabilizers, stearic acid as the solid lipid, and lavender oil as the liquid lipid, resulting in a spherical nano-sized formulation with high entrapment efficiency. Studies and results concerning morphology, size, zeta potential, and infrared (IR) spectroscopy confirm that the NLC-Pip formulation with lavender oil is a suitable solution for targeted drug delivery, improving the effectiveness of piperine and reducing its side effects. Furthermore, the antimicrobial activity of NLC-Pip supports the potential effectiveness of these systems against bacterial biofilm, as piperine-NLCs exhibited a five-fold higher anti-biofilm activity compared to free piperine. In line with these results, NLCs may deliver piperine with sustained release, especially when formulated with lavender essential oil, which enhances piperine's anti-biofilm efficiency due to its intrinsic therapeutic properties.