1. Background
Programmed cell death, known as apoptosis, is commonly dysregulated in cancer, particularly in acute lymphoblastic leukemia (ALL). B-cell ALL, a hematological malignancy affecting the blood and bone marrow, poses significant challenges. The 5-year survival rate for ALL is approximately 70%, but it is markedly lower in adults over 65. While many B-acute lymphoblastic leukemia (B-ALL) patients achieve remission, 36% experience relapses that necessitate further treatment (1-3). In vitro studies using the chemotherapy-resistant CCRF-CEM ALL cell line have shown that high-dose prednisolone increases pro-apoptotic Bax expression and decreases anti-apoptotic Bcl-2 expression, factors associated with remission or failure in ALL. Research suggests that promoting apoptosis can effectively induce cell death in ALL cell lines and primary ALL cells. Ongoing studies are exploring novel strategies to modulate apoptosis by targeting multiple pro-apoptotic or anti-apoptotic factors (4, 5).
Evasion of apoptosis is another hallmark of tumor progression, believed to be regulated by microRNAs (miRNAs). MicroRNAs are small, single-stranded RNA molecules consisting of 19 - 25 nucleotides. They are often located in intron regions of genes and specifically bind to the 3' untranslated region (3'-UTR) of target mRNAs, leading to either target gene degradation or translation inhibition (6, 7). In recent years, miRNAs have gained increasing attention in the field of apoptosis. For example, miR-21, which is overexpressed in many types of cancer, has been shown to inhibit apoptosis by targeting multiple pro-apoptotic genes (8). Similarly, miR-148a and miR-24-2c directly suppress Bcl-2 expression and induce apoptosis (9). Conversely, miR-155 is overexpressed in various cancers and inhibits apoptosis by targeting multiple pro-apoptotic genes (4). Notably, miR-125b-5p, miR-181b-5p, and miR-17-5p play critical roles in regulating key signaling pathways, including NF-κB, p53, PI3K/Akt/mTOR, ErbB2, Wnt, PTEN, and others (10, 11). These miRNAs are involved in controlling essential cellular pathways, particularly apoptosis.
In vitro studies have demonstrated the significant anticancer properties of vitamin C against various human tumor cells, including those in leukemia. However, vitamin C has shown controversial roles in cancer treatment in some studies (12). Therefore, this study aimed to investigate the impact of combining vitamin C with vincristine, a chemotherapy drug, or parthenolide on the apoptosis process in the NALM6 cell line through alterations in the aforementioned miRNAs.
2. Objectives
The ability of vitamin C to induce apoptosis has made it an attractive candidate for new investigations in the development of potential cancer treatments. Given the conflicting data and the importance of miRNAs and apoptosis cross-talk in treating ALL, this study aims to explore the effect of vitamin C in combination with parthenolide and vincristine in the NALM6 cell line.
3. Methods
The Ethics Committee of Shiraz University of Medical Sciences approved this experimental study (IR.SUMS.REC.1401.173). The B-cell precursor leukemia (NALM6, CRL-3273) cell line was cultured following ATCC instructions. All cell culture materials and reagents were obtained from Gibco Life Technologies (Waltham, MA) and Sigma-Aldrich (Munich, Germany).
3.1. Cell Culture
The NALM-6 cell line (CRL-3273) was cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin-streptomycin, and 2 mM glutamine, following the ATCC protocol. Culturing was conducted at 37°C in a humidified incubator with 5% CO2. Cell viability was assessed using the Trypan blue exclusion method.
3.2. Propidium Iodide-Staining
To assess the inhibitory effects of vitamin C on cell growth, propidium iodide (PI) was used as a viability stain, purchased from Sigma-Aldrich. A total of 1 × 105 cells were suspended in 800 µL of fresh medium and plated in a 24-well microtiter plate, followed by overnight incubation. Subsequently, vitamin C was added at specified concentrations (0, 1, 0.5, 0.25, and 0.125 mM). After 48 hours, cells were harvested and washed twice with cold phosphate-buffered saline (PBS). A mixture of 100 µL containing 1 × 105 cells and 5 µL of PI was prepared according to the manufacturer's instructions and incubated at room temperature in the dark for 15 minutes. The binding of Annexin V-FITC and PI was analyzed quantitatively using a FACSCalibur flow cytometer.
3.3. Apoptosis Assay
For the apoptosis assay, 1 × 105 cells were suspended in 800 µL of fresh medium in each well of a 24-well microtiter plate and incubated overnight. Vitamin C and its combinations were then added at specified concentrations. After 48 hours, cells were harvested and washed twice with cold PBS. The washed cells were resuspended in 90 µL of 1x Annexin-V binding buffer at a concentration of 1 × 105 cells/mL. A mixture of 1 × 105 cells, 5 µL of Annexin V-FITC, and 5 µL of PI (from MabTag’s Annexin-V Apoptosis Detection Kit) was prepared according to the manufacturer's instructions and incubated at 25°C in the dark for 15 minutes. After adding 400 µL of 1x Annexin-V binding buffer, the samples were centrifuged at 400 × g for 5 minutes. Analysis was performed using a FACScalibur flow cytometer and FlowJo software.
3.4. Real-time Polymerase Chain Reaction
Total RNA was extracted following mono or combination therapy of all reagents using TRIzol reagent. RNA quality was assessed by 260/280 and 260/230 ratios on a NanoDrop spectrophotometer. Complementary DNA (cDNA) synthesis was performed with 2 µg of total RNA using a reverse transcriptase microRNA PCR kit, following the manufacturer's protocol (Pars Genome, Iran). Samples were incubated at 45°C for 60 minutes and then heated to 85°C for 5 minutes to inactivate the reverse transcriptase enzyme. Quantitative real-time polymerase chain reaction (qRT-PCR) assays for miR-17-5p, miR-125b-5p, and miR-181b-5p were conducted using an ABI 7500 RT-PCR system, SYBR Green master mix, and specific primers. 5SrRNA served as the housekeeping gene for normalization (positive control). Cycling conditions were: 95°C for 15 minutes, followed by 40 cycles of 95°C for 30 seconds, 62°C for 30 seconds, and 72°C for 30 seconds. Relative quantification was calculated using the 2-ΔΔCt method.
3.5. Molecular Docking
Molecular docking was performed to assess the binding affinity and interactions between miRNA and ligands. The 2D structures of the ligands were downloaded as SDF files from the PubChem database and optimized through energy minimization and partial molecular dynamics using Chem3D 17.1, with the final structures saved as PDB files. MiRNA sequences were obtained from the RNAcentral database, and their structures were generated with Avogadro software v1.2.0, also saved as PDB files. Molecular docking was conducted using AutoDock Vina. Polar hydrogen atoms and Gasteiger charges were added to the target and ligand molecules, which were then converted to PDBQT files. The grid box size was determined to encompass the entire miRNA structure. Docking poses were selected based on binding affinity, with interactions visualized using Biovia Discovery Studio v.2021 and UCSF Chimera v1.16. To evaluate the binding affinity of ligands in combination, complexes of all ligands with miRNAs were created in Biovia Discovery Studio and subjected to molecular docking with AutoDock Vina again.
3.6. Statistical Analysis
The statistical analysis for this experimental study was conducted using GraphPad Prism (version 8.4.3, GraphPad Software, Inc., USA). Data were analyzed using one-way ANOVA followed by Tukey's test for multiple comparisons, with statistical significance set at P < 0.05. Additionally, correlation analysis was performed using Spearman's rho test with SPSS software version 22.
4. Results
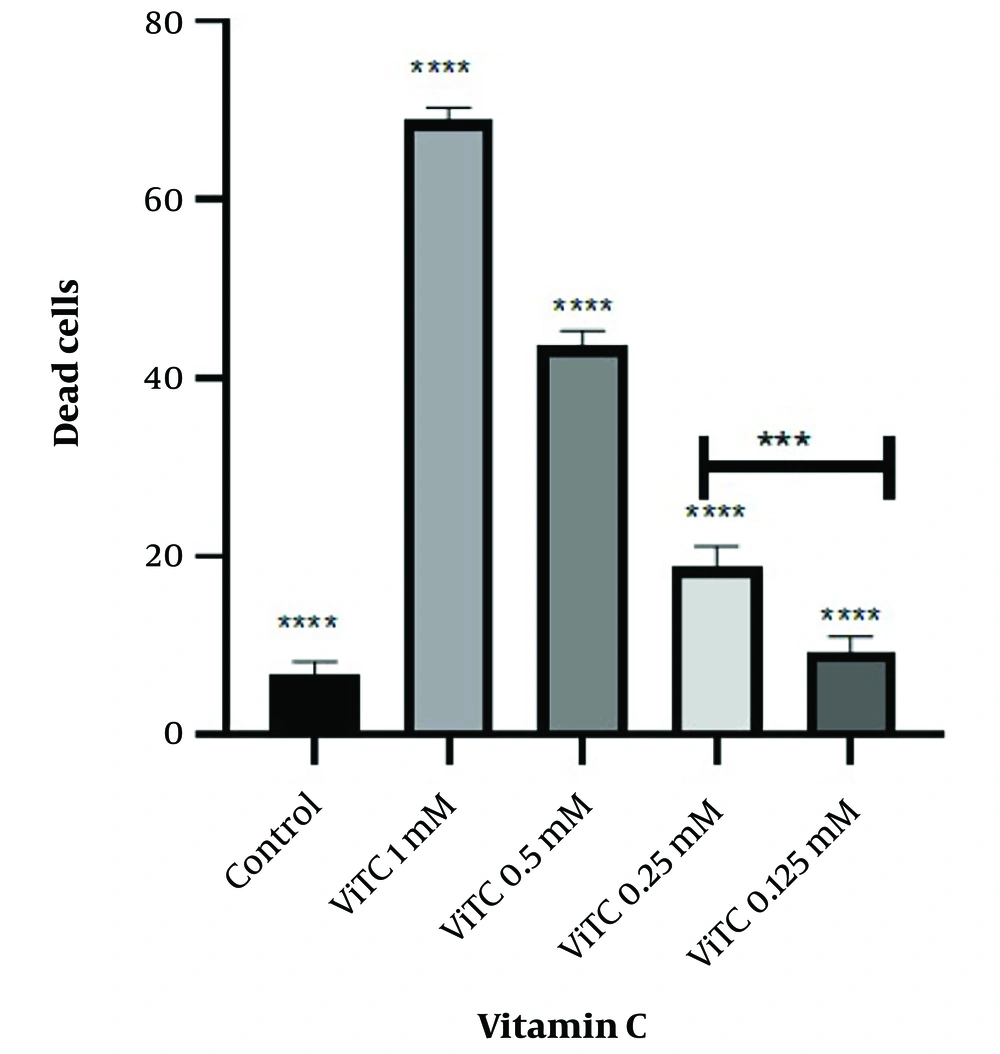
4.1. Vitamin C Reduces NALM6 Cell Viability
Firstly, flow cytometry was used to confirm the identification of the NALM6 cell line based on CD19 and CD3 markers. The results showed that the cells were positive for CD19 and negative for CD3 (Appendix 1 in Supplementary File). To investigate the effect of vitamin C on the growth of the NALM6 cell line, the cells were exposed to different concentrations of vitamin C (0, 1, 0.5, 0.25, and 0.125 mM) for 48 hours. After treatment with vitamin C, the viability of the NALM6 cell line significantly decreased in a dose-dependent manner. Specifically, the cell death rates after 48 hours of vitamin C treatment were 6.767% ± 1.4%, 68.87% ± 1.4%, 43.55% ± 1.7%, 18.80% ± 2.35%, and 9.380% ± 1.6% (Figure 1).
4.2. Vitamin C Combined with Vincristine or Parthenolide Enhances Apoptosis in NALM6 Cells
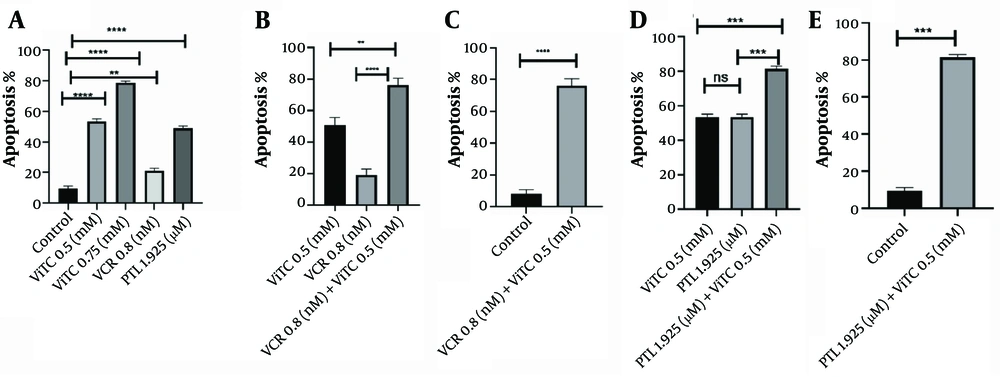
Vitamin C has been found to induce apoptosis in the NALM6 cell line. It was observed that the control group had 10.73% apoptotic cells, while the cells treated with the optimal combination of vitamin C (0.5 mM) and vincristine (0.8 nM) for 48 hours showed a significant increase to 79.22% apoptotic cells. Meanwhile, when treated individually for 48 hours, vitamin C or vincristine alone resulted in only 54.68% and 22.28% apoptotic NALM6 cells, respectively. Furthermore, the distribution of early apoptotic cells was 7.38%, 13.1%, and 8.32% in the vitamin C, vincristine, and vitamin C/vincristine groups, respectively. The combination treatment using 0.5 mM vitamin C with 0.8 nM vincristine exhibited the strongest synergistic inhibitory effect, leaving only 10.3% cell survival after 48 hours of treatment. In the next step, we found that combination therapy with vitamin C and vincristine enhances apoptosis 1.44- and 3.55-fold higher than when the NALM6 cell line was treated with individual vitamin C and vincristine. Our results also demonstrated that vitamin C triggers apoptosis significantly more than vincristine, with a 2.45-fold higher effect. In addition, an increase in the number of early apoptotic and dead (late-apoptotic) cells was observed after the co-incubation of parthenolide with vitamin C at concentrations of 1.925 µM and 0.5 mM, resulting in 82.52% ± 1.520% apoptosis (early and late apoptotic cells), which is about 1.64 times and 1.50 times when NALM6 cells are exposed to parthenolide or vitamin C alone. Also, only 6.50% ± 1.697% of cells survived. Moreover, we observed that the rate of early apoptotic cells tended to be reduced when combination therapy was applied (only 3.92%) (Appendix 2 in Supplementary File and Figure 2).
The results of apoptosis following different treatments in NALM6 cell line after 48 hours. A, the statistical analysis of the data after treatment with vincristine and vitamin C, separately (**** P < 0.0001, ** P: 0.0025); B, the analysis of the combined data compared to separate treatments (** P: 0.0024, **** P < 0.0001); C, vitamin C combining with vincristine compared to control (**** P < 0.0001); D, combination therapy with vitamin C and parthenolide (ns: Non-significant, *** P = 0.0008); E, vitamin C combining with parthenolide compared to control (*** P = 0.0005). VCR, vincristine; ViTC, vitamin C; PTL, parthenolide.
4.3. Vitamin C with Vincristine or Parthenolide Reduces miR-17/125b/181b-5p Expression in NALM6 Cells
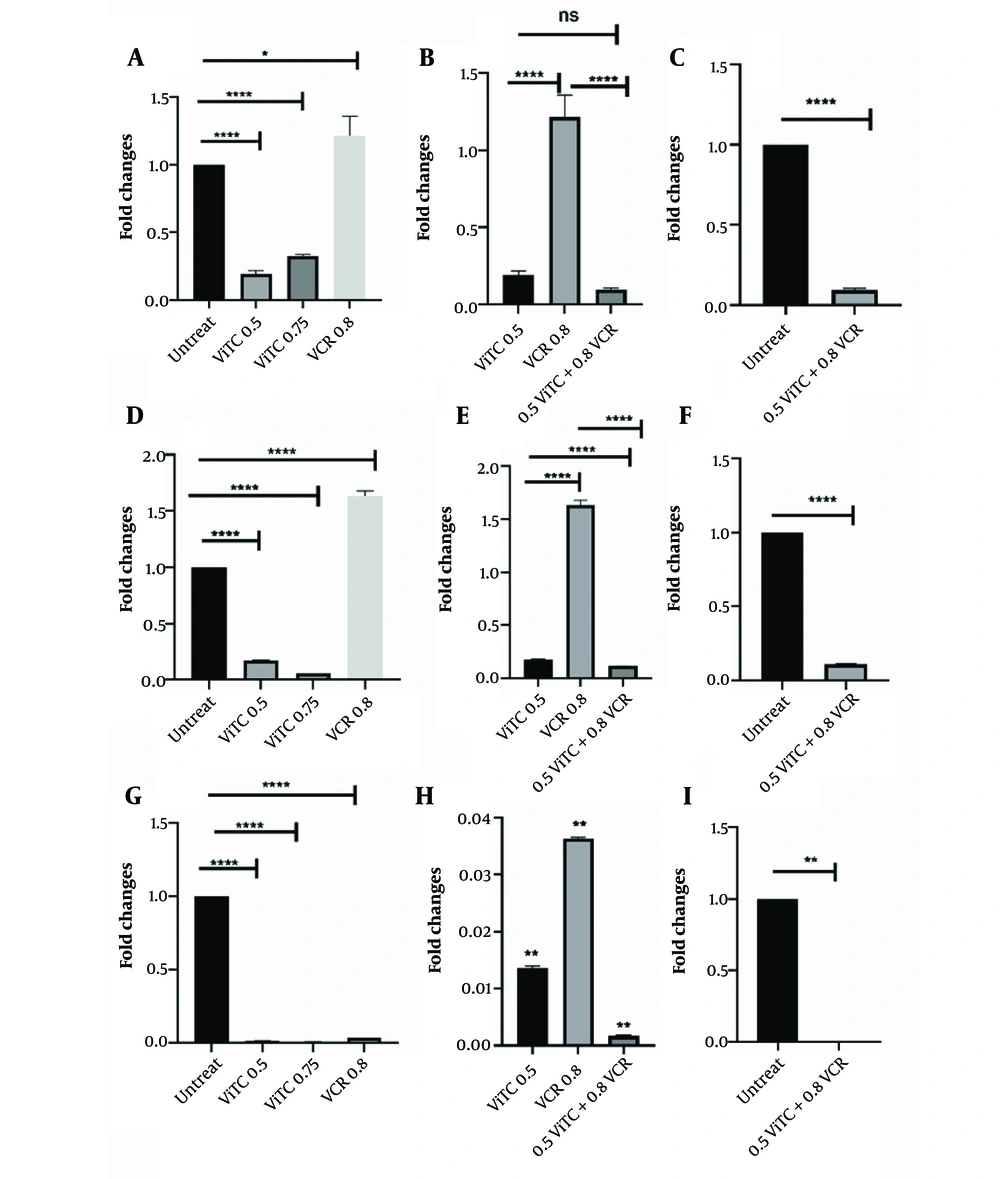
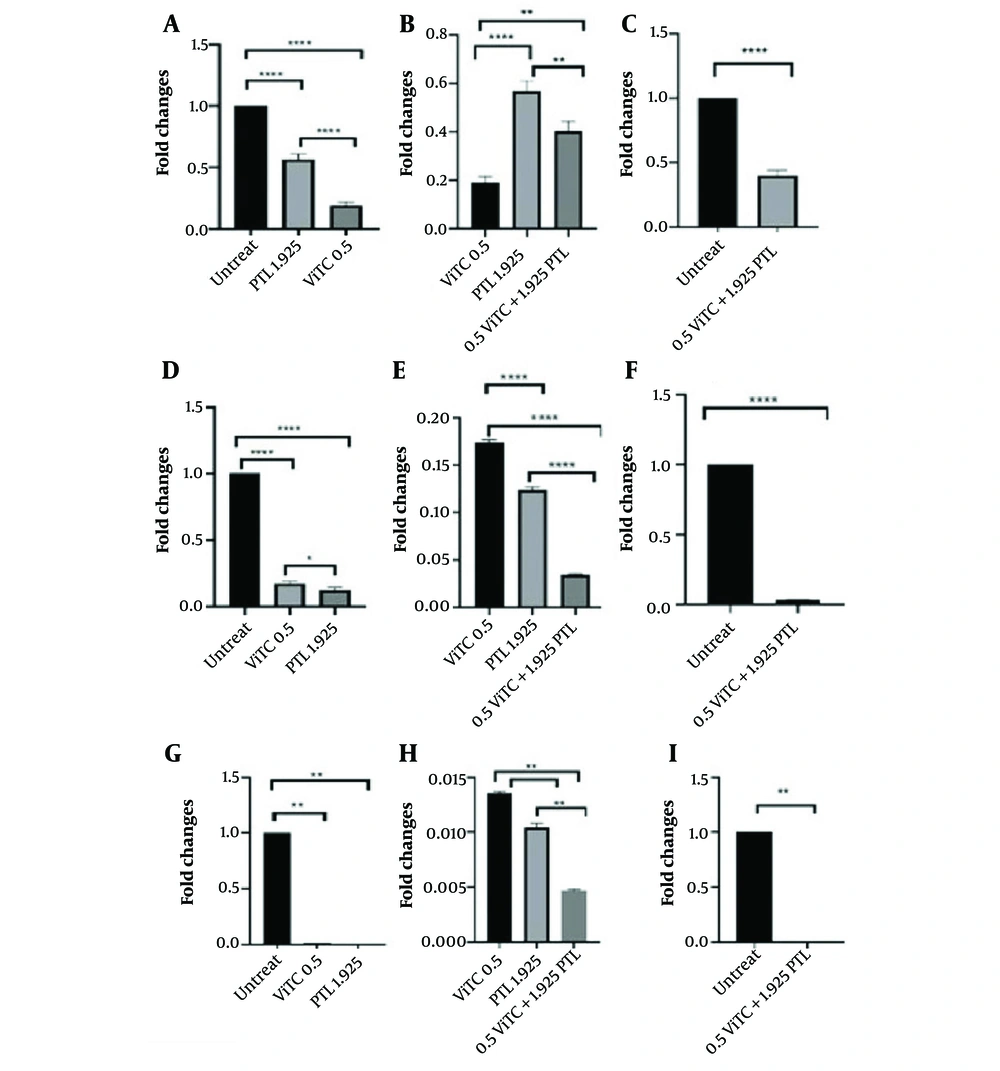
Statistical analysis shows that vitamin C resulted in decreased miR-17-5p expression. Similarly, combining vincristine and vitamin C significantly decreased miR-17-5p expression compared to untreated cells and individual treatments. Additionally, the RT-PCR results indicated that vitamin C reduced miR-125b-5p expression. The concentration of 0.8 mM of vitamin C and 0.8 nM of vincristine significantly reduced miR-125b-5p expression compared to untreated cells and individual treatments. miR-181b-5p exhibited a significant decrease following treatment with vitamin C and vincristine. Likewise, its expression was noticeably reduced following combination therapy (Figure 3). Moreover, vitamin C combined with parthenolide led to a drop in the expression of the three miRNAs as well. For miR-17-5p, vitamin C caused a larger decrease in expression than parthenolide. The combination therapy reduced miR-17-5p expression more than parthenolide alone but less than vitamin C alone (Figure 4A, B, and C). A different pattern was seen for miR-125b-5p. Parthenolide lowered its expression more than vitamin C, while the combination had a powerful effect (Figure 4D, E, and F). A similar pattern was seen for miR-181b-5p, with this miRNA decreased more than the other two, and the combination therapy reduced this miRNA nearly to zero (Figure 4G, H, and I). Overall, parthenolide demonstrated the strongest inhibitory effects on the expression of miR-125b-5p and miR-181b-5p individually. The combination of parthenolide and vitamin C had the greatest suppressive effect on miR-181b-5p levels.
RT-PCR analysis of miRNAs following different treatment after 48 hours. A, miR-17-5p expression changes treated with vincristine and vitamin C, separately (**** P < 0.0001, * P: 0.0298); B, comparison of combination therapy with separated treatment (**** P < 0.0001); C, before and after 236 combination therapy (**** P < 0.0001); D, shows miR-125b-5p expression changes after treatment with vincristine and vitamin C, separately (**** P < 0.0001); E, comparison between combination therapy and separated ones (**** P < 0.0001); F, combination therapy (**** P < 0.0001); G, miR-181b-5p expression changes treated with vincristine and vitamin C, separately (**** P < 0.0001); H, comparison of combination with separated treatment (** P < 0.01); I, combination therapy (** P < 0.01). RT-PCR, real-time polymerase chain reaction; VCR, vincristine; ViTC, vitamin C.
RT-PCR analysis of miRNAs following different treatment after 48 hours. A, miR-17-5p expression level before and after treatment with ViTC and PTL (**** P < 0.0001); B, comparison of expression level of combination treatment with individual treatments (**** P < 0.0001, ** P < 0.01); C, comparison of miRNA expression level before and after 48h combination treatment (**** P < 0.0001); D, levels of miR-125b-5p expression before and after treatment with ViTC and parthenolide (**** P < 0.0001, * P: 0.0239); E, comparison of expression levels between combination treatment and individual treatments (**** P < 0.0001); F, comparison of miRNA expression levels before and after 48 hours of combination treatment (**** P < 0.0001); G, levels of miR-181b-5p expression before and after treatment with ViTC and parthenolide (** P < 0.01) ; H, comparison of expression levels between combination treatment and individual treatments (* P < 0.05); I, comparison of miRNA expression levels before and after 48 hours of combination treatment (** P < 0.01). RT-PCR, real-time polymerase chain reaction; PTL, parthenolide; ViTC, vitamin C
4.4. Molecular Docking with Auto Dock Vina
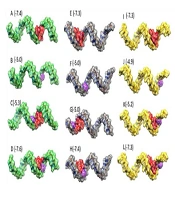
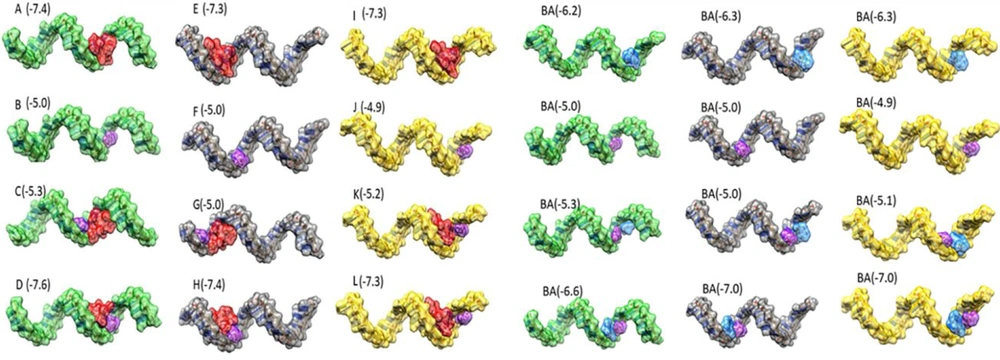
The process of molecular docking using AutoDock Vina involves several steps. Molecular docking was carried out using AutoDock Vina to analyze the binding potential and nucleotide region involved in the binding conformation (13). First, ligand molecules and miRNAs were converted to PDBQT files separately by AutoDock Vina. The grid box size was selected to encompass the entire molecule, taking into account the size of the miRNAs. The docking output poses were then chosen based on their binding affinity, ensuring the selection of the most favorable interactions. Biovia Discovery Studio v2021 was used to display the bases involved in ligand binding, and the relative positions of miRNAs and ligands were checked using UCSF Chimera v.1.16. (14). Complexes of miRNAs with all ligands were made using Biovia Discovery Studio v.2021. Docking was performed again on the constructed complexes to evaluate the binding of the ligand in the presence of other ligands. The results of the docking showed a good scoring value and better interactive behavior based on binding affinity (Figure 5).
Molecular docking analysis. The predicted 3D structures of mature miRNAs with vitamin C, vincristine and pathenolide. The green, gray, yellow, red, blue and purple color indicated as miR-17-5p, miR-125b-5p, miR-181b-5p, vincristine, parthenolide and vitamin C, respectively. The numbers in picture represent binding affinity of each interaction.
4.5. Negative Correlation Between miRNAs Level and Apoptosis
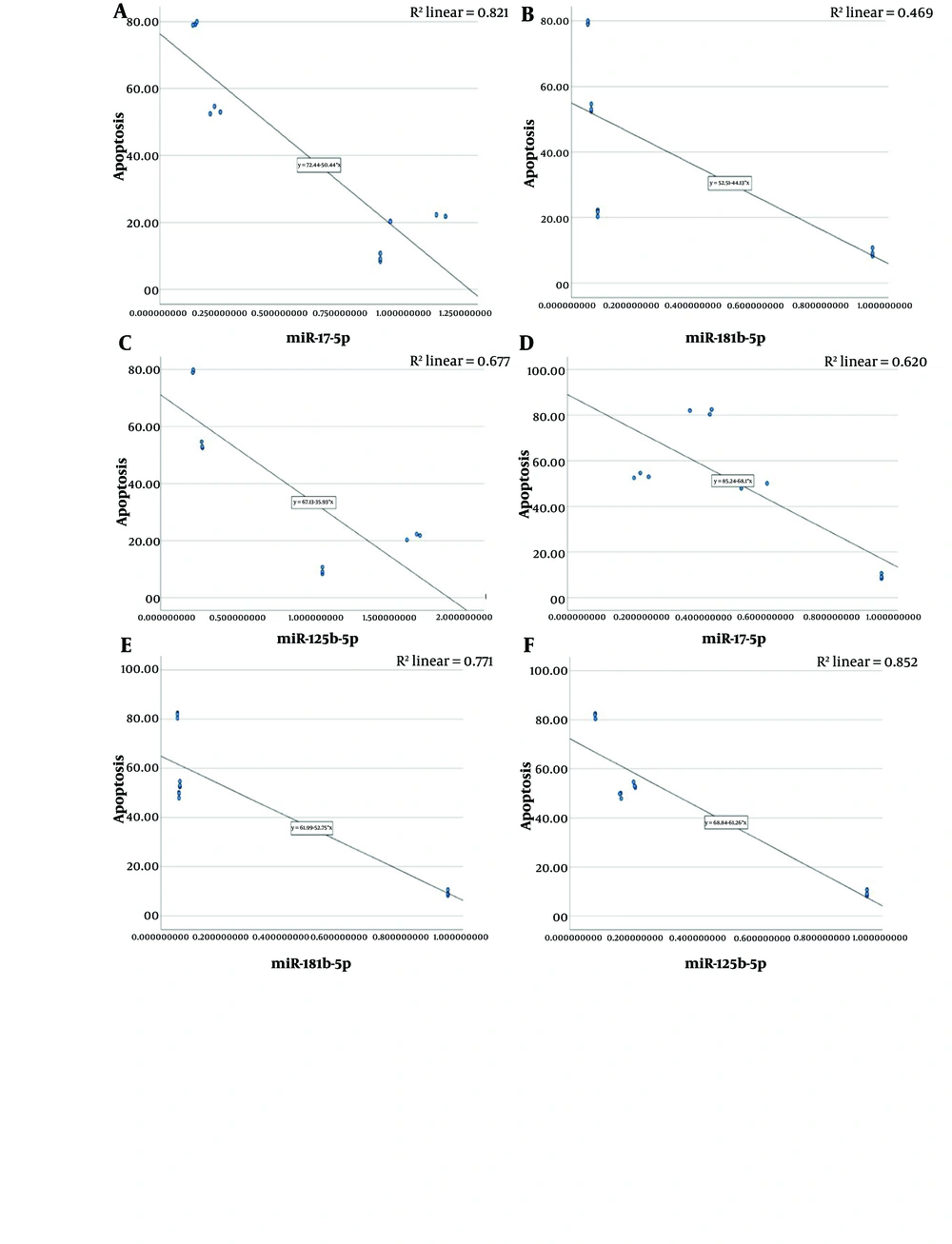
The correlation analysis revealed a significant negative slope between the percentage of apoptosis and the decrease in miR-181b-5p, miR-17-5p, and miR-125b-5p levels (Figure 6).
Correlation analysis between fold change of miRNAs obtained from real-time polymerase chain reaction (RT-PCR) and percentage of apoptosis; A, miR-17-5p (R: -0.732, P: 0.007); B, miR-181b-5p (R: -0.957, P < 0.001); C, miR-125b-5p (R: -0.758, P: 0.004) ; D, miR-17-5p (R: -0.732, P: 0.007); E, miR-181b-5p (R: -0.784, P: 0.003); F, miR-125b-5p (R: -0.789, P: 0.002). A, B, and C showed the negative correlation between the decreased miRNAs following treatment with vitamin C combining vincristine; D, E, and F showed the negative correlation between the decreased miRNAs following treatment with vitamin C combining part henolide; data analyzed with SPSS software version 22 (Spearman's rho test).
5. Discussion
Vitamin C exhibits both antioxidant and prooxidant effects in cancer. At dietary levels, it acts as an antioxidant, scavenging reactive oxygen species and preventing DNA damage. However, at high pharmacological doses, vitamin C may induce prooxidant effects that can be detrimental to cancer cells (15). The dual nature of vitamin C in cancer underscores the importance of considering its concentration and source in developing combination therapies. This study aimed to investigate the anti-apoptotic impact of high-dose vitamin C combined with vincristine (a chemotherapy drug) or parthenolide (a plant-derived compound) and its modulation of miR-125b-5p, miR-181b-5p, and miR-17-5p in the NALM6 cell line.
We observed that vitamin C exhibited synergistic anti-tumor activity, reducing cell viability in a dose-dependent manner and inducing apoptosis in the NALM6 cell line, either as monotherapy or in combination therapy. These effects were likely mediated by a decrease in the expression of the studied miRNAs, which are involved in regulating apoptosis signaling. Vitamin C, an essential nutrient, has shown potential for cancer treatment due to its high cytotoxicity against NALM6 cells and its ability to enhance apoptosis when combined with vincristine or parthenolide (Figures 1 and 2). This aligns with previous studies by Su et al. (16), El Banna et al. (17), Sant et al. (18), Zhao et al. (19), and Gong et al. (20), which demonstrated that vitamin C inhibited tumor growth, induced apoptosis, and enhanced the efficacy of standard cancer therapies in preclinical studies.
Vitamin C has been shown to selectively destroy cancer cells without harming normal cells due to several biological mechanisms. High levels of vitamin C generate hydrogen peroxide, a reactive oxygen species that damages cancer cells, which are less efficient at removing hydrogen peroxide compared to normal cells. Normal cells, with higher catalase content, can efficiently neutralize hydrogen peroxide, maintaining low levels and preventing damage (21, 22). Future studies should further explore this selective property of vitamin C, particularly its interaction with enzymatic antioxidant defense systems.
Parthenolide, an NF-κB signaling inhibitor, combined with high-dose vitamin C, also targeted the NF-κB transcriptional network. This network is linked to cell survival, metastasis, and apoptosis resistance in cancer. Vitamin C was shown to regulate NF-κB activation by inhibiting specific NF-κB-dependent genes and restricting the phosphorylation of IκBα and IKK, thereby reducing NF-κB activity in various cancers, including breast cancer (23).
Moreover, the use of vitamin C as an epigenetic modulator is of particular significance. MicroRNAs are small molecules that play a major role in gene regulation and have been demonstrated to regulate acute lymphoblastic leukemia (ALL) pathogenesis and apoptosis evasion. MiR-181b-5p, miR-17-5p, and miR-125b-5p are hypothesized to be involved in the pathological process of ALL due to their role as resistance factors in the apoptosis pathway(24, 25). Treatment with vitamin C, either alone or in combination with vincristine or parthenolide, led to a more consistent decrease in the levels of miR-17-5p, miR-181b-5p, and miR-125b-5p compared to monotherapy (Figures 3 and 4).
The fundamental hypothesis of this project centers on the role of miR-181b-5p, miR-125b-5p, and miR-17-5p as negative regulators of the apoptosis signal. Targeting these microRNAs with natural compounds to reduce their levels was expected to enhance apoptosis. Previous research has established that these microRNAs play a regulatory role in the apoptotic pathway (25, 26). Our recent research has revealed that these microRNAs primarily target the apoptosis pathway through BCL family genes. Furthermore, enrichment analysis of biological pathways has indicated that the TGF-β and PI3K pathways are key targets of these microRNAs (27). The PI3K/AKT signaling pathway is known to promote cell survival and has been reported to participate in apoptosis (28). In previous studies, it has been demonstrated that inhibiting the PI3K pathway not only inhibits the NF-κB pathway but also triggers the apoptosis pathway (23). Appendix 3 shows the genes regulated by miR-181b-5p and miR-17-5p in the PI3K pathways and others targeted by miR-181b-5p and miR-125b-5p in the TGF-β pathway.
Moreover, correlation analysis revealed a negative slope between apoptosis percentage and the decrease in miR-181b-5p, miR-17-5p, and miR-125b-5p levels. Hence, these results suggest that therapy with vitamin C in combination with vincristine or parthenolide may lead to a decrease in the expression of these miRNAs, which regulate apoptosis. This decrease in miRNA levels likely promotes apoptosis by targeting BcL-2 family genes and inhibiting the PI3K pathway, leading to suppression of the NF-κB pathway. Therefore, we suggest further exploration of PI3K, NF-κB signaling, and Bcl-2 family genes in future studies.
5.1. Limitations
Despite its merits, this study has some limitations. First, Compusyn software was not available to determine the Combination Index (CI). Second, we were not able to experimentally study the signaling proposed in the KEGG pathway. Third, other cell models of B-ALL were not accessible to examine the results of this study on them.
5.2. Future Study
Exploring the molecular mechanisms underlying the modulation of miR-17-5p, miR-125b-5p, and miR-181b-5p by these treatments could provide valuable insights into their specific gene targets and pathways involved in apoptosis and cell viability, particularly PI3K and NF-κB signaling. Additionally, evaluating the efficacy of vitamin C in combination with other chemotherapeutic agents or targeted therapies may further enhance the apoptotic effects. Extending the findings to in vivo models will help assess the therapeutic potential and safety of these combinations in a more complex biological environment. Conducting broader miRNA profiling to identify other miRNAs influenced by these treatments and their roles in mediating apoptosis and treatment response would be beneficial.
5.3. Conclusions
It can be inferred that therapy combining vitamin C with vincristine or parthenolide led to a reduction in the levels of miR-181b-5p, miR-17-5p, and miR-125b-5p in the NALM6 cell line, potentially supporting a negative regulatory role of these specific miRNAs. Additionally, correlation analysis confirmed the negative association of these miRNAs with apoptosis. Moreover, this study suggests that miR-181b-5p, miR-17-5p, and miR-125b-5p may target the TGF-β and PI3K pathways, which can lead to inhibition of NF-κB signaling. This study highlights the potential therapeutic significance of combining vitamin C with vincristine or parthenolide for treating B-ALL patients. However, further in vivo investigations are warranted to validate and expand upon these initial observations. These results emphasize the need for continued research to elucidate the precise mechanisms, including the PI3K pathway, and the clinical implications of this treatment approach.