1. Background
Parkinson’s disease (PD), the second most common neurodegenerative disorder, impacts over 1% of the elderly population globally (1). Although numerous factors contribute to the pathology of PD, its primary etiology remains elusive. This syndrome is characterized by a progressive degeneration of dopaminergic neurons in the substantia nigra, resulting in a decreased dopaminergic neuron count in the striatum. This reduction in neuronal density results in significant motor impairments, including resting tremors, bradykinesia, muscle stiffness, and overall postural instability (2). Although PD is primarily known as a complex motor disorder, individuals with PD also experience a broad spectrum of non-motor symptoms, such as depression, anxiety, pain, disturbances in sleep, deficits in attention, learning challenges, memory problems, cognitive impairments, and autonomic dysfunctions like constipation (3).
Research has highlighted non-motor symptoms as critical components influencing the quality of life related to health and the progression of disability in those with PD. Moreover, these non-motor signs might present in the initial phases of PD, even before motor symptoms develop (4). Thus, it is clear that the neurodegenerative process associated with PD extends beyond the dopaminergic system in the substantia nigra; non-motor dysfunctions may arise from disruptions in other neurotransmitter systems, including serotonin, norepinephrine, and acetylcholine (ACh), affecting various brain regions, such as the hippocampus (5).
Many features of PD can be reproduced in animal models by administering substances like 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone, and paraquat. However, administering high doses abruptly often leads to damage in the substantia nigra, employing motor impairments that resemble those found in the disease's advanced stages. On the other hand, the rapid and severe damage caused by these methods does not help in tracking the progression of motor dysfunction, nor does it allow for the evaluation of non-motor symptoms in the initial phases (6). In contrast, repeated doses of reserpine (RES) enable the study of the gradual development of parkinsonism, including assessing non-motor symptoms in the early phases and exploring possible neuroprotective treatments (7). Reserpine is a potent inhibitor of vesicular monoamine transporter 2 (VMAT2), leading to tremors, muscle rigidity, and akinesia by depleting monoamine stores in the brain (7). Recent findings by Li et al. indicated that RES elicits both motor and non-motor symptoms akin to PD through mechanisms involving alpha-synuclein accumulation, impairment of autophagy, and decreased tyrosine hydroxylase in the substantia nigra (8). These findings suggest that the RES model is a valuable tool for studying PD.
Currently, no treatments have succeeded in stopping or decelerating the degeneration of neural tissue in PD, leaving only symptomatic therapies available for patients (9). The primary approach to managing the motor symptoms of PD is dopamine replacement through levodopa (L-DOPA). However, over time, the effectiveness of L-DOPA diminishes, and motor issues such as tremors and dyskinesia tend to reemerge with prolonged L-DOPA treatment (10). Furthermore, non-motor symptoms of PD generally do not respond favorably to dopaminergic therapies (11). Recent research has focused on discovering novel pharmacological agents that enhance both motor and non-motor symptoms, potentially modifying the therapeutic trajectory of PD.
Given the significant role of oxidative stress in the neurodegeneration of dopaminergic neurons in PD, identifying antioxidant compounds could provide therapeutic or preventive strategies for managing the disease (12). Several studies have demonstrated that a range of pure compounds extracted from herbal medicine can significantly reduce the motor complications of PD in both in vivo and in vitro models (13).
Hesperidin (3,5,7-trihydroxyflavanone 7-rhamnoglucoside; HES) is a bioflavonoid in citrus fruits that exhibits anti-inflammatory, anti-apoptotic, neuroprotective, and synaptic plasticity-enhancing properties (14). The antioxidant activity of HES is applied through direct inhibition of free radicals scavenging or indirect inhibition of prooxidative enzymes that participate in the production of these radicals and also as a chelator of metals that participate in reaction with reactive oxygen species (ROS) (15). The antioxidant properties of HES are shown by reducing the production of ROS and increasing the activity of antioxidant enzymes, such as catalase (CAT) and superoxide dismutase (SOD) (16).
This compound has been demonstrated to mitigate oxidative stress, enhance memory, and confer neuroprotection in a mouse model treated with methotrexate (MTX). Additionally, HES stimulates adenosine monophosphate-activated protein kinase (AMPK), a vital signaling pathway involved in neurogenesis (17).
2. Objectives
The investigation aims to determine the antioxidant, neuroprotective, and cholinergic function of HES in ameliorating motor and non-motor (cognitive) symptoms in a RES-elicited rat paradigm of PD.
3. Methods
3.1. Animals
This research involved 40 male Wistar rats, approximately 8 months old, with an average weight of 200 ± 20 g. The rats were kept in groups of five within transparent plastic cages (30 × 37 × 16 cm) under standard laboratory conditions, including a controlled temperature of 22 ± 1°C, 45 ± 5% humidity, and a 12-hour light/dark cycle starting at 6:00 a.m. The animals had unlimited access to water and standard lab food. To help them adjust to their environment and monitor food consumption per rat, they were kept for one week at the Islamic Azad University, Shiraz branch, without any experimental treatments. All procedures were approved by the Institutional Animal Care and Use Committee (IR.IAU.SHIRAZ.REC.1403.223). Every effort was made to reduce pain, suffering, and discomfort, and the experiment was conducted using the minimum number of animals necessary.
3.2. Drugs
Hesperidin (purity ≥ 80%) and RES were sourced from Sigma-Aldrich (St. Louis, MO, USA). Hesperidin was prepared by dissolving it in normal saline at 100 mg/kg based on a previous study (17). Reserpine was first dissolved in 0.3 mL of glacial acetic acid and then diluted with distilled water to achieve a 0.2 mg/mL concentration (17). Reserpine was initially dissolved in 0.3 mL glacial acetic acid and then diluted with distilled water to a 0.2 mg/mL concentration. For the RES vehicle group (VR), a solution of glacial acetic acid with distilled water was used (18). Hesperidin and RES were prepared daily and stored at 4°C among doses during treatment.
3.3. Group Allocation and Experimental Design
The animals were assigned randomly to five groups, each consisting of eight rats: (1) Control group; (2) vehicle group receiving HES solvent (normal saline; NS) and RES solvent (VR + NS); (3) RES and regular saline group (RES + NS); (4) HES and RES vehicle group (HES + VR); and (5) RES + HES group (RES + HES). Reserpine was delivered intraperitoneally at a dose of 0.2 mg/kg body weight for 13 days, with one injection every day. Upon observation of Parkinsonian motor symptoms, HES was administered at 100 mg/kg body weight orally via gavage for 21 days following RES administration.
3.4. Behavioral Tests
3.4.1. Catalepsy Test
The bar test evaluated catalepsy in all groups (n = 8). The apparatus used in this test was a wooden horizontal bar with a platform. The bar was positioned 9 cm above the platform, with a diameter of 0.9 cm. The time the animal's forepaws stayed fixed on the horizontal bar was recorded, with a maximum cutoff limit of 30 seconds. The test was terminated when the animal removed one of its forepaws from the bar or moved its head inquisitively. This procedure was repeated three times at 3-minute intervals (19).
3.4.2. Passive Avoidance Memory
Passive avoidance memory was evaluated in all groups (n = 8) employing a shuttle box apparatus. The shuttle box, made of plexiglass, is divided into two compartments—one brightly lit and the other dark—separated by a guillotine door. The base of the apparatus consists of steel rods linked to an electrical circuit, enabling the passage of an electric current with a defined duration, intensity, and frequency when the circuit is activated. This test is based on rodents' instinctive escape from light and the presence of an aversive factor (electric shock) in the dark environment. The procedure involves three main phases. The initial step is the Adaptation phase, in which the animals are placed in a brightly lit compartment for one minute to become familiar with their surroundings. After 30 seconds, the guillotine door is opened, allowing them to move voluntarily into the dark compartment. The next stage, the Acquisition phase, is conducted 24 hours later. During this phase, the animals are again positioned in the illuminated compartment for 30 seconds before the guillotine door is raised, permitting entry into the dark compartment. Upon entry, the door closes, and a 2 mA electric shock is applied through the floor for 2 seconds at 50 Hz. The animals are removed from the apparatus 20 seconds post-shock and returned to their cages. Finally, the Retention phase is performed 24 and 48 hours after the Acquisition phase.
At this point, the animal is once more positioned in the lit compartment for 30 seconds, after which the guillotine door is opened. The latency to move into the dark compartment (LDR) and the total duration spent in the dark compartment (TDR) are recorded (19). During the retention phase, no electric shocks were provided. The maximum test time was 300 seconds. A more significant initial delay in entering the dark container during the test phase suggests memory retention.
3.4.3. Spatial-Working Memory
The Y-maze test assessed Working memory in all groups (n = 8). The Y-maze consists of three identical arms, shaped like the letter "Y," each measuring 15 × 30 × 40 cm and connected by a central area. At first, the animal was gently put in one of the three arms without stress, and its motions were recorded for five minutes. The number of entries into each arm was noted. An entrance was made by placing the animal's hind paws inside an arm. Alternation behavior was defined as consecutive and sequential entry into all three arms of overlapping triplet sets. Working memory performance was evaluated by calculating the percentage of alternation behavior. This was determined by dividing the number of successful alternations by the total number of arm entries minus two, then multiplying the result by 100. Successful entries refer to consecutive and serial entries into all three arms (20).
3.5. Oxidative Stress Markers
At the end of the treatment duration, a subset of animals from each group (n = 4) was deeply anesthetized with chloroform inhalation, and the head was immediately decapitated employing a rodent guillotine. The complete brain was extracted from the skull and immediately placed on ice for preservation. Employing a stereomicroscope (Olympus, Japan), the hippocampus was meticulously isolated from the remaining brain tissue. Following dissection, the tissue was rinsed with saline containing Tris buffer (Sigma, Germany). The tissue was then homogenized using a homogenizer (IKA, Germany) at 5000 rpm for five minutes. The homogenate was subsequently centrifuged in a refrigerated centrifuge (Hermle, Germany), with 0.5 mM phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich, Germany) employed as a protease inhibitor (21). After centrifugation, the supernatant was collected to evaluate oxidative stress markers. The concentrations of CAT, SOD, and glutathione peroxidase (GPx) within the hippocampus were quantified employing the ELISA technique with an ELISA reader (Stat Fax, USA) and kits sourced from Arman Biotech. Additionally, the tissue levels of MDA were evaluated through its reaction with thiobarbituric acid (Merck, Germany), employing spectrophotometry to measure absorbance at 535 nm and comparing the results against a standard curve derived from tissue samples.
3.6. Cholinergic Function
Acetylcholinesterase (AChE) function in hippocampal tissue was measured employing the Ellman method, which involves the hydrolysis of ACh (22). A small aliquot of supernatant (0.4 mL) from the hippocampal tissue homogenate was combined with 2.6 mL of a phosphate buffer solution (0.1 M, pH 7.4). Then, 0.1 mL of 5,5'-dithio-bis (2-nitrobenzoic acid) (DTNB) was added to the mixture. Then, 0.1 mL of ACh iodide was incorporated into the reaction blend. The optical density (OD) was subsequently measured at a wavelength of 412 nm employing a spectrophotometer, and the change in OD over two minutes was recorded. Acetylcholinesterase activity was evaluated based on the colorimetric change resulting from the reaction between thiocholine and DTNB. The OD rate per minute change was determined, with AChE enzymatic activity expressed in μmol/min/mg protein.
3.7. Histopathology
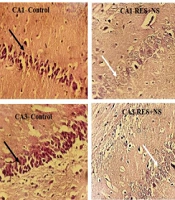
To evaluate hippocampal tissue and assess neuronal density in the CA1 and CA3 regions at the end of the HES treatment duration, animals (n = 4 per group) were deeply anesthetized employing chloroform in a desiccator, followed by cardiac perfusion with 10% formalin after flushing the blood from the body with sodium chloride. The brain was carefully extracted from the skull and processed employing an autotechnicon. Paraffin blocks were prepared, and sections from the hippocampal region were sourced with guidance from the Paxinos and Watson atlas. Slides were stained with hematoxylin-eosin (H-E), and microscopic imaging was done with an Olympus-BH2 light microscope linked to a computer system and processed with StereoLite software.
Neuronal density in the CA1 and CA3 regions was measured employing random sampling and the dissector method. Briefly, cell counting was conducted within a reference frame. Cells in both frames were not tallied, but cells in the reference frame, not in the next frame (second portion), were counted. The formula used to determine neuronal density is:
Where NA is neuronal density, ΣQ is the total number of cells counted in a sample, ΣP is the number of sampling instances in a sample, A is the area of the sampling frame, and H is the distance among two successive sections or the thickness of each section (23). A minimum of 10 slides from each hippocampal sample (50 slides per group) were counted to evaluate neuronal density.
3.8. Statistical Analysis
Statistical analyses across the various groups were conducted using SPSS software (version 22). The Kolmogorov-Smirnov test was utilized to assess the normality of data distribution. Once normality was confirmed, one-way ANOVA followed by Tukey’s post hoc test was employed to identify significant differences between the groups. A P-value of less than 0.05 was deemed statistically significant.
4. Results
4.1. Cataleptic Behavior
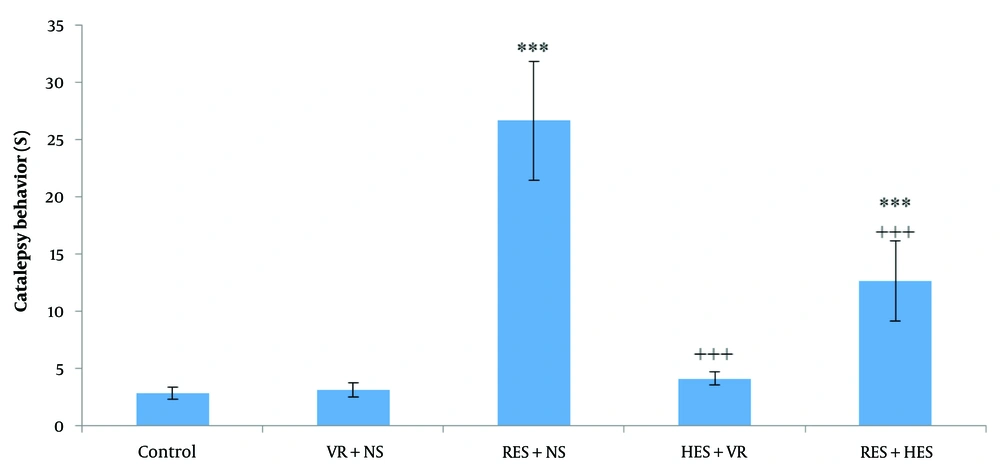
Analysis via one-way ANOVA and Tukey's post hoc test demonstrated significant differences between the experimental groups (P < 0.001, F = 29.7, df = 4,35). Notably, the duration of the bar test (indicating cataleptic behavior) was significantly elevated in the RES + NS and RES + HES groups in relation to the control group (P < 0.001). In contrast, a significant decrease in bar test duration was observed in the HES + VR and RES + HES groups in relation to the RES + NS group (P < 0.001). Nonetheless, no statistically significant difference was observed between the HES + VR and control groups (P > 0.05, Figure 1).
Contrast of mean (M) ± standard deviation (SD) of bar test duration among the investigation groups. The results indicate a noteworthy difference among the control and reserpine (RES) + NS and RES + hesperidin (HES) groups (*** P < 0.001). A notable reduction was noted in the HES + VR and RES + HES groups in relative assessment with the RES + NS group (+++ P < 0.001).
4.2. Passive Avoidance Memory
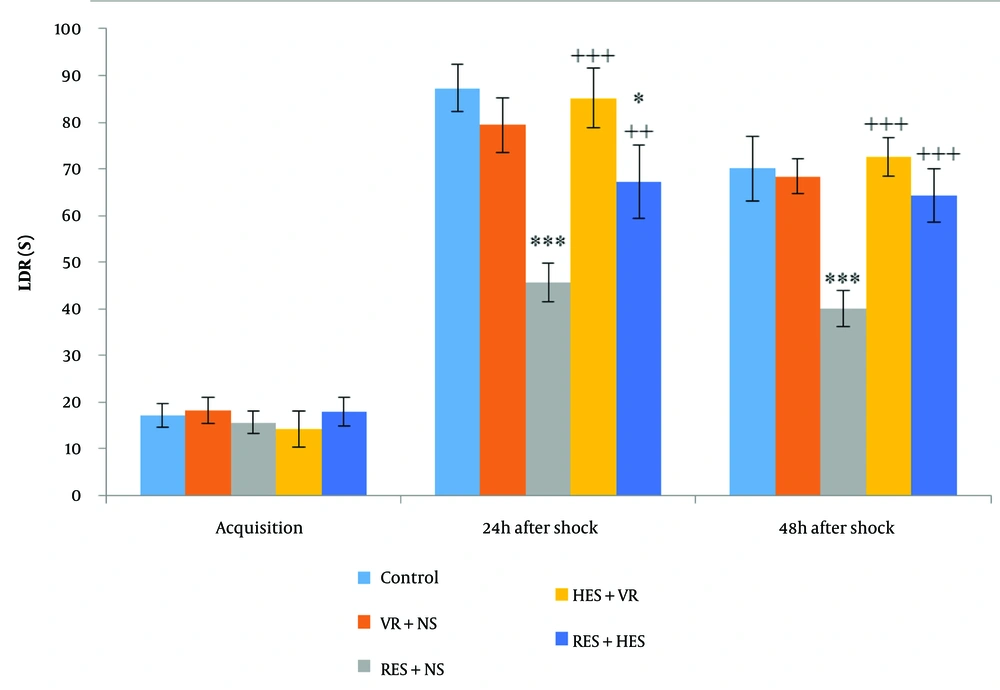
ANOVA demonstrated no noteworthy disparities in the LDR during the acquisition phase among the investigation groups (Figure 2). However, LDR measured 24 hours after the shock induction demonstrated noteworthy disparities among the groups (P < 0.001, F = 45.73, df = 4, 35). Tukey's post hoc test demonstrated a remarkable decrease in LDR for the RES + NS and RES + HES groups in contrast to the control group (P < 0.001 and P < 0.05, in that order). In contrast, in relative assessment with the RES + NS group, the HES + VR and RES + HES groups demonstrated a notable enhancement in LDR (P < 0.001 and P < 0.01, in that order). Forty-eight hours after shock induction, group disparities remained substantial (P < 0.001, F = 37.45, df = 4, 35). Concerning the control group, the RES + NS group noteworthyly reduced LDR (P < 0.001). Conversely, the HES + VR and RES + HES groups demonstrated notable enhancements in LDR about the RES + NS group (P < 0.001).
Contrast of mean (M) ± SD of LDR: No noteworthy disparities were noted among groups before shock induction (acquisition phase). Noteworthy disparities were found 24 hours after shock induction among the control group and the reserpine (RES) + NS and RES + hesperidin (HES) groups 48 hours after shock among the control group and the RES + NS group (*** P < 0.001, * P < 0.05). In relation to the RES + NS group, the HES + VR and RES + HES groups demonstrated notable enhancements in LDR at 24- and 48-hours post-shock (+++ P < 0.001, ++ P < 0.01).
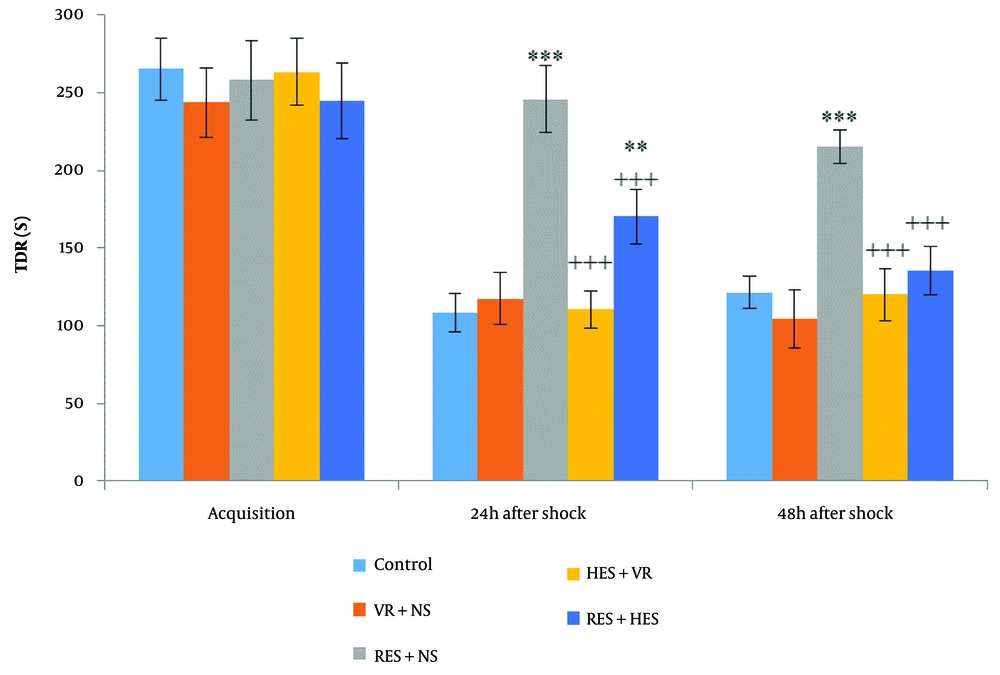
Figure 3, one-way ANOVA, demonstrated no noteworthy disparities in the meantime spent by animals in the dark compartment (TDR) among the investigation groups during the acquisition phase. However, 24 hours (P < 0.001, F = 25.13, df = 4, 35) and 48 hours (P < 0.001, F = 31.05, df = 4, 35) after shock induction, TDR was noteworthyly different among the groups. Tukey's post hoc test demonstrated that 24 hours after the shock, TDR increased dramatically in the RES + NS and RES + HES groups in relative assessment with the control group (P < 0.001 and P < 0.01, in that order). Forty-eight hours after the shock, TDR also noteworthyly increased in the RES + NS group concerning the control group (P < 0.001). Furthermore, in relative assessment with the RES + NS group, TDR was noteworthyly reduced in the HES + VR and RES + HES groups at 24- and 48-hours post-shock (P < 0.001).
Contrast of mean (M) ± SD of TDR: No noteworthy disparities were noted among groups before shock induction (acquisition phase). Noteworthy disparities were found 24 and 48 hours after shock induction among the control and reserpine (RES) + NS groups (** P < 0.01, *** P < 0.001). The hesperidin (HES) + VR and RES + HES groups demonstrated noteworthy disparities from the RES + NS group at both time points post-shock (+++ P < 0.001).
4.3. Spatial-Working Memory
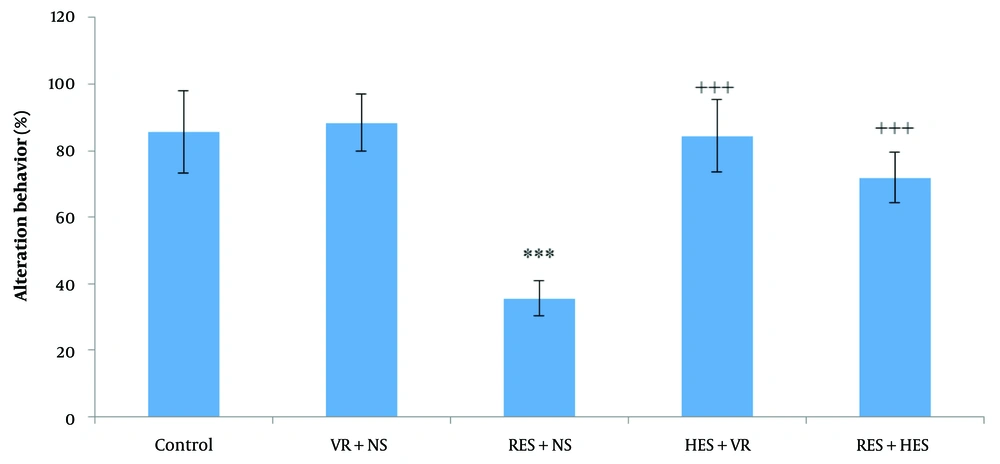
Statistical analysis of spontaneous alternation behavior percentage employing one-way ANOVA and Tukey's post hoc test demonstrated noteworthy disparities among the investigation groups (Figure 4 P < 0.001, F = 36.5, df = 4, 35). Specifically, the percentage of alternation behavior (working memory) was noteworthyly decreased in the RES + NS group in relative assessment with the control group (P < 0.001). Conversely, a notable enhancement in alternation behavior was noted in the HES + VR and RES + HES groups in relative assessment with the RES + NS group (P < 0.001).
The contrast of mean (M) ± SD percentage of spontaneous alternation behavior among the investigation groups. The results indicate a noteworthy difference among the control and RES + NS groups (*** P < 0.001). In relation to the reserpine (RES) + NS group, the hesperidin (HES) + VR and RES + HES groups have demonstrated notable enhancements (+++ P < 0.001).
4.4. Cholinergic Function
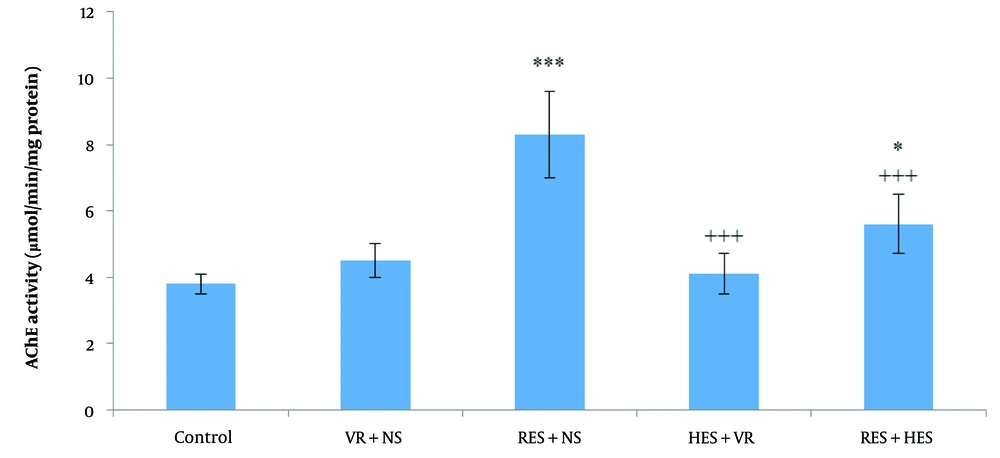
The results from one-way ANOVA and Tukey's post hoc test demonstrated noteworthy disparities in AChE function levels in the hippocampus among the investigation groups (P < 0.001, F = 7.102, df = 4, 11). Specifically, there was a notable enhancement in the AChE function in the RES + NS and RES + HES groups in relative assessment with the control group (P < 0.001 and P < 0.05, in that order). Additionally, noteworthy decreases in hippocampal AChE function were noted in the HES + VR and RES + HES groups in contrast to the RES + NS group (Figure 5 P < 0.001).
Contrast of mean (M) ± SD of hippocampal acetylcholinesterase (AChE) function among the investigation groups. The results indicate noteworthy disparities among the control and reserpine (RES) + NS and RES + hesperidin (HES) groups (* P < 0.05 and *** P < 0.001). AChE function in the HES + VR and RES + HES groups was noteworthyly reduced in relative assessment with the RES + NS group (+++ P < 0.001).
4.5. Oxidative Stress Factors in the Hippocampus
One-way ANOVA and Tukey's post hoc test were employed to assess oxidative stress factors among the different groups. The function levels of CAT in the hippocampus demonstrated noteworthy disparities among the investigation groups (P < 0.001, F = 9.215, df = 4, 11). Specifically, the RES + NS and RES + HES groups had noteworthyly decreased CAT function in relative assessment with the control group (P < 0.001 and P < 0.05, in that order). Additionally, the HES + VR and RES + HES groups demonstrated notable enhancements in hippocampal CAT function about the RES + NS group (Table 1 P < 0.001).
| Parameter | CAT (mIU/mL) | SOD (pg/mL) | GPx (pg/mL) | MDA (ng/mL) |
|---|---|---|---|---|
| Control | 121.92 ± 7.12 | 71.23 ± 6.20 | 97.38 ± 5.11 | 34.12 ± 3.25 |
| VR + NS | 118.43 ± 6.45 | 68.11 ± 5.34 | 106.54 ± 6.54 | 33.8 ± 3.57 |
| RES + NS | 47.06 ± 4.91 ** | 27.86 ± 4.91 ** | 42.31 ± 3.90 ** | 55.81 ± 2.72 ** |
| HES + VR | 130.27 ± 6.27 b | 73.55 ± 5.76 b | 108.15 ± 5.41 b | 31.67 ± 3.18 b |
| RES + HES | 102.15 ± 5.46 *,b | 52.39 ± 3.72 **,b | 89.5 ± 4.76 b | 41.29 ± 3.89 *,b |
Mean Function Levels of Catalase, Superoxide Dismutase, Glutathione Peroxidase, and Malondialdehyde Content in the Hippocampus a
Noteworthy disparities in hippocampal SOD function were also noted among the investigation groups (P < 0.001, F = 14.231, df = 4, 11). A noteworthy reduction in hippocampal SOD function was found in the RES + NS and RES + HES groups in relative assessment with the control group (P < 0.001). Conversely, the HES + VR and RES + HES groups demonstrated notable enhancements in hippocampal SOD function in relative assessment with the RES + NS group (Table 1 P < 0.001).
The results of GPx enzyme function in hippocampal tissue demonstrated noteworthy disparities among the different investigation groups (P < 0.001, F = 10.35, df = 4, 11). A notable decrease was noted in the RES + NS group in relation to the control group (P < 0.001). Notable enhancements were pointed out in the HES + VR and RES + HES groups in relative assessment with the RES + NS group (Table 1 P < 0.001).
The measurement of hippocampal malondialdehyde (MDA) levels demonstrated noteworthy disparities among the groups (P < 0.001, F = 7.30, df = 4, 11). There was a notable enhancement in MDA levels in the RES + NS and RES + HES groups in relative assessment with the control group (P < 0.001 and P < 0.05, in that order). Contrasts of the groups receiving HES with the RES + NS group demonstrated that the HES + VR and RES + HES groups had noteworthyly reduced MDA levels (Table 1 P < 0.001).
4.6. Neuronal Density in the Hippocampus
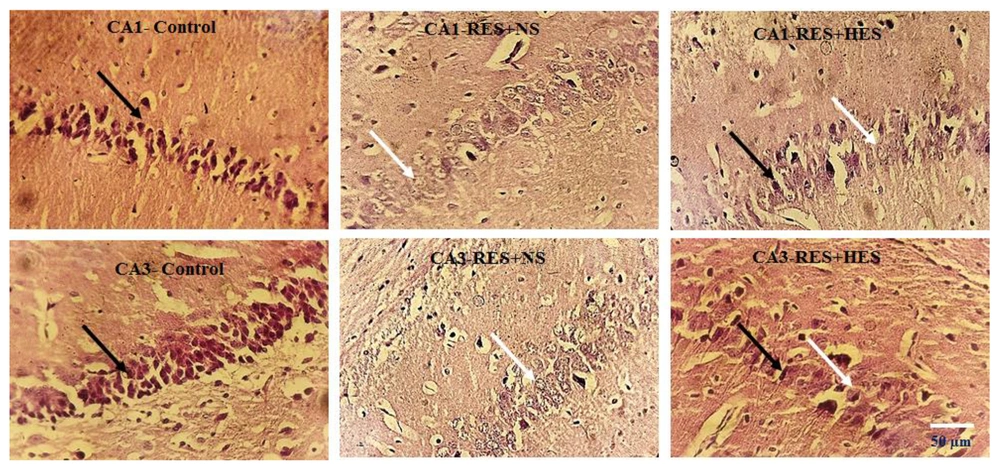
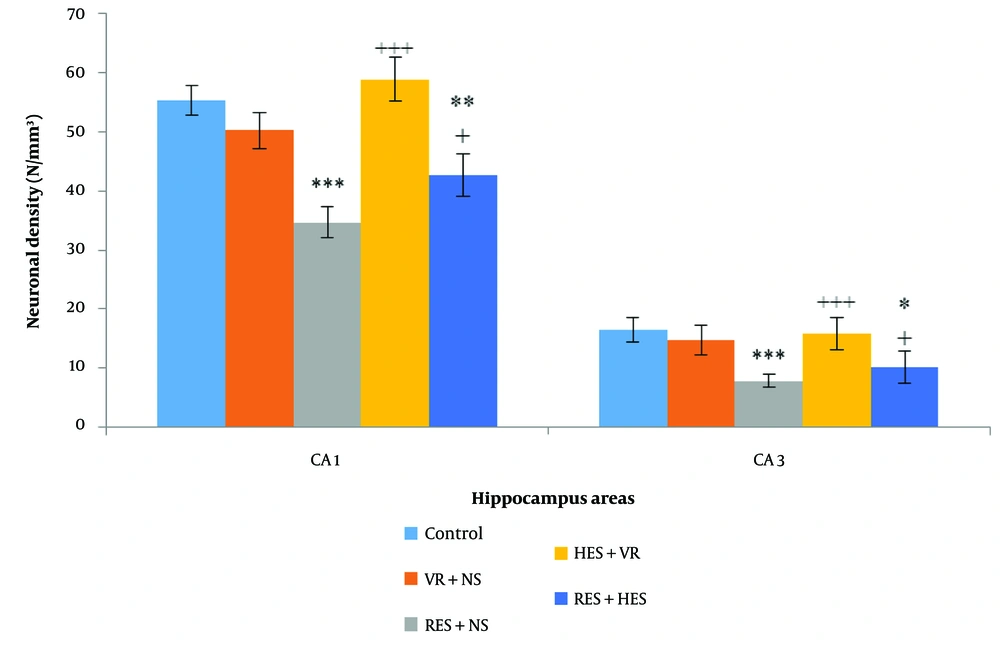
In relative assessment, a reduction in neuronal density was noted in the RES + NS group, with the control group in multiple hippocampus subareas employing H-E staining (Figure 6). One-way ANOVA results demonstrated noteworthy disparities among the investigation groups in the CA1 (P < 0.001, F = 6.237, df = 4, 11) and CA3 regions (P < 0.001, F = 8.109, df = 4, 11). Tukey's post hoc test demonstrated that the reduction in neuronal density in the CA1 and CA3 regions of the RES + NS group was noteworthy in the control group (P < 0.001). A notable decrease in neuronal density was also noted in the CA1 and CA3 regions of the RES + HES group in relative assessment with the control group (P < 0.01 and P < 0.05, in that order). Conversely, in relative assessment with the RES + NS group, the HES + VR and RES + HES groups demonstrated a notable enhancement in neuronal density in the CA1 (P < 0.001) and CA3 regions (P < 0.05, Figure 7).
Photomicrographs of the CA1 and CA3 hippocampus subareas in the investigation groups. In relative assessment with the control group, a reduction in neuronal density is noted in the reserpine (RES) + NS group. In contrast, the RES + hesperidin (HES) group shows a notable increase in neuronal density. H-E staining. White arrows indicate damaged neurons, and black arrows indicate normal neurons. Images are at 40X magnification.
Contrast of mean (M) ± SD of neuronal density in different hippocampus regions among the investigation groups. The results show noteworthy disparities in both regions examined among the control group and the reserpine (RES) + NS and RES + hesperidin (HES) groups (* P < 0.05, ** P < 0.01, *** P < 0.001). Additionally, the HES + VR and RES + HES groups noted a notable enhancement in neuronal density in relation to the RES + NS group (+++ P < 0.001).
5. Discussion
This investigation examined the neuroprotective impacts of HES in a rat PD paradigm following repeated RES administration. The results demonstrated that HES prevents RES-elicited cataleptic behavior, and improves passive avoidance and working memory. In fact, the amelioration of RES-induced cognitive and motor deficits occurs through the modulation of the hippocampal activity of CAT, SOD and GPx enzymes, the reduction of lipid peroxidation and AChE activity in the hippocampus. In addition, HES prevents the reduction of neuronal density in CA1 and CA3 areas of hippocampus by modulating the antioxidant capacity and cholinergic activity of hippocampus.
Reserpine inhibits vesicular monoamine transporter 1 (VMAT-1) and vesicular monoamine transporter 2 (VMAT-2), leading to a reduction in vesicular monoamine storage and a noteworthy decrease in monoamine concentrations within the brain (24). This effect results in both motor and non-motor symptoms, neurochemical alterations, and heightened oxidative stress in the brains of humans and animals, resembling the changes noted in individuals with PD (24).
Reserpine induces cognitive impairments in laboratory animals, such as attention deficits, learning disorders, and episodic and executive memory dysfunctions (25).
The current investigation demonstrated a notable enhancement in cataleptic behavior in the RES + NS group, consistent with prior research (26). Conversely, treatment with HES prevented RES-elicited cataleptic behavior (Figure 1). Cataleptic behavior is characterized by an animal's inability to adjust to an externally imposed posture, analogous to the challenges individuals with PD face when initiating movements (27). This behavioral change is associated with dysfunction in specific brain regions, including the striatum and globus pallidus, and alterations in the nigrostriatal system's dopamine levels (27). Consequently, the catalepsy test is a valuable tool for assessing motor impairments related to PD and can be elicited by dopamine receptor antagonists (28). It is proposed that dopamine receptor agonists may mitigate this behavior. Therefore, HES might activate dopaminergic receptors, providing protective effects during the catalepsy test (29).
Reserpine causes disturbances in the storage of synaptic vesicular monoamines. The depletion of monoamines at nerve terminals and the increase in intracellular monoamine levels result in the auto-oxidation of dopamine and its oxidative metabolism by monoamine oxidase (MAO), leading to oxidative stress (30). Since PD motor impairments are associated with oxidative stress, they are mitigated by antioxidant compounds. As demonstrated in Table 1, HES likely reduced RES-elicited cataleptic behavior by enhancing the antioxidant defense system.
Regarding non-motor changes, cognitive deficits are widespread in PD, often preceding motor symptoms. Progressive parkinsonism elicited by repeated doses of RES is a valuable tool for evaluating non-motor disorders, including cognitive deficits in rodents (18). This investigation noted cognitive impairment in the passive avoidance and working memory tests. As mentioned, pathways other than the nigrostriatal pathway play an essential role in manifesting PD's non-motor symptoms (27). The current investigation demonstrated impaired passive avoidance memory by employing the shuttle box test 24 hours after the final dose of RES.
Additionally, the percentage of alternation behavior in the Y-maze, indicative of spatial working memory or short-term memory performance, was noteworthyly reduced in rats receiving RES. Despite this, HES noteworthyly improved spatial working memory by increasing the percentage of alternation behavior. Cognition-related brain areas, such as the hippocampus and medial frontal cortex, are innervated by serotonergic and dopaminergic projections, which stem from the raphe complex and the mesocorticolimbic pathway, respectively (31).
The memory impairment elicited by RES in this investigation was likely due to neurochemical changes in the hippocampus, which damaged pyramidal neurons in the CA1 and CA3 regions.
Reserpine, as an inducer of oxidative stress, can reduce CAT, GPx, SOD, reduced glutathione, and adenosine triphosphate (ATP) enzymes (32). Increased lipid peroxidation (MDA) and NO have also been noted in multiple brain regions, including the midbrain and striatum, in a rat paradigm of PD. Increased lipid peroxidation results from free radical attacks on the cell membrane, leading to phospholipid oxidation and cell death (32). However, some research has reported contradictory results, possibly due to different RES doses, administration methods, and brain regions studied. For example, repeated administration of low doses of RES (0.1 mg/kg) has cumulative impacts on lipid peroxidation in the striatum. Simultaneously, these alterations have not been observed in rats' hippocampus. Catalase function is generally reduced in all brain regions except the striatum (7). However, in the current investigation, employing a dose of 0.2 mg/kg body weight RES, the enzyme levels of CAT, SOD, and GPx in the hippocampus of the RES + NS group demonstrated a noteworthy decrease in relative assessment with the control group at the end of the treatment duration. It seems that oxidative stress in the hippocampus following RES administration is sufficient for neuronal degeneration and reduced neuronal density in the hippocampus. Oxidative stress can weaken the brain's antioxidant system by producing free radicals, leading to irreversible brain damage (33). Oxidative stress and chronic inflammation caused by PD result in neuronal cell damage by altering the levels of miRNAs and their target proteins. However, according to prior research, antioxidants can protect neuronal cells by modulating the levels of miRNAs and their intended proteins (34).
Flavonoids, widely used in multiple fruits and vegetables, have numerous biological and pharmacological impacts. Flavonoid-rich diets are effective in regulating brain function. Hesperidin has potential antioxidant impacts in vivo and may act as a neuroprotective agent in the brain. This compound stabilizes biological membranes as an antioxidant, thus preventing cell membrane damage in neurological diseases such as Alzheimer's, Parkinson's, and Huntington's (35). In this investigation, HES increased the function levels of the enzymes CAT, SOD, and GPx and decreased MDA levels, consistent with prior research (14). Hesperidin increases the activity of antioxidant enzymes through upregulation of SIRT1 gene and inhibition of NADPH oxidase 4 (NOX4) enzyme (36). Also, studies have shown that HES decreases interleukin 1 beta (IL-1β), tumor necrosis factor (TNF-α) and MDA and increases hippocampal brain derived neurotrophic factor (BDNF) levels in the depression model. In addition, oxidative stress parameters, including SOD, CAT, nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1), were significantly increased following HES treatment in this model (37). By activating the Nrf2-antioxidant response element (Nrf2/ARE) pathway, HES activates the main antioxidant systems, and by stimulating the BDNF/protein kinase A (PKA)/ cAMP-response element binding protein (CREB) signaling pathway in the amygdala and hippocampus, it shows anxiolytic effects (38).
Although decreased AChE function in the midbrain of the RES paradigm of PD has been noted, which may be due to damage to cholinergic neurons (39), and noteworthy inhibition of AChE function has been recorded in the thalamus of PD patients (40), in the current investigation, AChE function in the hippocampus of rats in the PD paradigm of increased following RES treatment, which could explain cognitive impairment and memory and learning deficits in this paradigm of. Prior research has demonstrated that AChE function levels occur before neurodegeneration in Alzheimer's disease. Acetylcholinesterase hydrolyzes acetylcholine in the synaptic cleft, and changes in AChE function are considered an early indicator of cognitive disorders (41). In this investigation, HES improved learning and memory deficits caused by RES in the PD paradigm of rats by reducing AChE expression. Additionally, the noteworthy reduction in hippocampal AChE function after HES treatment may be due to its AChE-inhibiting impact.
Prior studies have demonstrated that HES can inhibit apoptosis and cell cycle arrest, thereby preventing cell death in various brain regions (38). In this investigation, the administration of HES led to an increase in neuronal density in the CA1 and CA3 regions of the hippocampus. This increase was associated with enhanced working memory, passive avoidance, and a reduction in cataleptic behavior in the RES + HES group in contrast to the vehicle-treated group (VR + NS). Hesperidin reduces oxidative stress in PD by regulating Nrf2 nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and reducing apoptotic cells (42). Chronic administration of HSP (HES precursor for five weeks) exhibited neuroprotective impacts against oxidative stress in the brains of mice. These impacts were associated with decreased lipid peroxidation levels and activation of endogenous antioxidant defense mechanisms, including CAT, SOD, and glutathione (GSH)-related enzymes (43). By scavenging high ROS levels and boosting antioxidant defense mechanisms—primarily by upregulating the expression of Nrf2 and HO-1—HES has demonstrated impressive antioxidant effects. By suppressing apoptotic cell death, lowering ROS and MDA generation, raising SOD and GSH expression, and triggering Kelch-like ECH-associated protein 1 (KEAP-1) and Nrf2/HO-1 signaling, HES considerably guards against severe oxidative stress (44).
5.1. Conclusions
The current investigation demonstrated that HES exhibits neuroprotective impacts, improves both passive avoidance and working memory, enhances cholinergic function, and prevents the reduction of neuronal density in multiple subareas of the hippocampus in the RES paradigm of PD. However, the limitations of this investigation should be considered, as molecular methods for evaluating the mechanisms underlying the protective impacts of HES were not conducted. Some of the limitations of the study can be mentioned: (1) The study lacks molecular insights or signaling pathway into HES's protective mechanisms; (2) findings are based solely on the RES model, which may not fully represent human PD; (3) the focus was on motor and cognitive functions, with limited exploration of other behavioral domains; (4) the sample size or possibility of potential biases. Further research is essential to enhance the understanding of PD prevention and therapy. This research should investigate the impact of HES on motor and non-motor modifications within the RES paradigm of PD. At the end of this research and for future studies, we recommended the investigation of molecular mechanisms underlying HES's neuroprotective effects, exploration of the long-term effects of HES on motor and non-motor symptoms, evaluation of the potential synergistic effects of combining HES with other treatments, additional behavioral assessments for non-motor symptoms such as anxiety and depression in the RES model of PD and conduction of the clinical trials to evaluate the efficacy and safety of HES for PD treatment.