1. Background
Aging is a complex and inevitable process involving many factors (1). It is a complex biological phenomenon that gradually reduces the efficiency of many physiological functions of organs, the body's homeostatic capacity, and leads to the accumulation of harmful substances in cells, increasing vulnerability and impairing physical function. With age, there is a decrease in testosterone secretion, a decline in the body's antioxidant defense system, and an increase in apoptosis activity (2). Various factors, such as environmental conditions and nutrition, significantly impact the aging process. Approximately 100,000 people globally die every day due to age-related problems (3). Various theories have been proposed regarding aging. Some of the most popular include the programmed aging theory, free radical theory, and immunological theory. The programmed aging theory posits that aging is genetically programmed to occur over time, leading to eventual death (4). The free radical theory suggests that as the body ages, its capacity to counteract free radicals decreases, and aging is caused by accumulated damage to cells and tissues induced by free radicals (5). This hypothesis suggests that with aging, excessive production of reactive oxygen and nitrogen species can harm lipid membranes, DNA, and proteins, producing harmful byproducts such as malondialdehyde (MDA) and protein carbonyl (PC), ultimately leading to intracellular injury and cellular dysfunction (6, 7). Two key factors involved in oxidative stress in aging include decreased availability of antioxidant nutrients and abundant production of oxidative byproducts from biological compounds (8). Under normal circumstances, these reactive species are balanced by antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) (6, 9). With increasing age, antioxidant enzymes become less effective and are unable to counteract the excessive production of free radicals, resulting in cellular damage and the onset of DNA mutations (10).
Sirtuins are class III proteins of a family of histone deacetylase enzymes that catalyze the hydrolysis of histone acetylated ends, deform chromatin, produce heterochromatin, and thus inhibit transcription of mitochondrial pathway-dependent cell death genes such as P53. Among the SIRT, sirtuin 1 (SIRT1) in heart tissue, through mechanisms involving Peroxisome proliferator-activated receptor-gamma coactivator (PGC-1α) and inhibition of nuclear factor kappa B (NF-κB), increases mitochondrial biogenesis, resulting in NAD modulation and increased cellular respiration. As a result, oxidative stress decreases and telomere length increases (11-13). Sirtuins are NAD(+)-dependent deacetylases that are highly conserved between yeast and humans. The SIRT1 is the most extensively studied member of this family (14). The SIRT1 plays a positive role in autophagy and longevity and can activate AMPK/mTOR signaling in various pathologies (15).
Peroxisome proliferator-activated receptor γ coactivator-1α (PGC1-α) is a key transcriptional factor broadly involved in mitochondrial quality control of various physiological functions, including cardiac roles and skeletal muscle metabolism. Generally, proximal tubular cells (PTCs) containing PGC1-α meet their metabolic energy demands (16). Reports have shown the association between PGC1-α and mitochondrial quality control on dysfunctional mitochondria in PTCs through kidney aging (14). The D-Galactose (D-Gal) is a sugar naturally produced in the body, significantly impacting aging and the pathogenesis of some diseases. At normal concentrations, D-Galactokinase and galactose-1-phosphate uridyltransferase can effectively metabolize this sugar. However, at high concentrations, this sugar can undergo conversion into both aldose and hydroperoxide via galactose oxidase catalysis. This conversion leads to the creation of superoxide anions and oxygen-derived free radicals (17).
Several hypotheses propose that D-Gal may play a significant role in this process. These hypotheses suggest that D-Gal may contribute to the production of advanced glycation end products (AGEs) and the cessation of sugar metabolism. Furthermore, studies have shown that the use of D-Gal can lead to the generation of free radicals, which can cause oxidative damage over time. This damage can gradually lead to the development of age-related disorders (3). D-Galactose accelerates aging and is widely used in animal models to study the mechanisms of aging and the effect of drugs on these changes. Although D-Gal causes aging through various mechanisms, such as increased production of oxidants and subsequent oxidative damage, the molecular mechanisms of its effect in causing aging remain unknown (18).
Recently, there has been increased awareness of the harmful effects of artificial drugs. Additionally, it is believed that natural remedies, especially plant-based medicines, are safer and more sustainable than synthetic drugs, leading to an increasing trend toward their use in medicine (19). Polyphenols are chemicals that naturally occur in plants, fruits, and vegetables. Among these phenolic compounds, those found in olive fruit and leaves are particularly noteworthy due to their significant pharmacological properties. These compounds have been shown to possess a range of health benefits and are considered one of the most important factors contributing to the therapeutic potential of olive products (20).
Over the years, numerous studies have investigated the various physiological properties of oleuropein (OLE). The OLE is a polyphenolic component with a great antioxidant effect equivalent to a hydrosoluble analog of α-tocopherol or vitamin E. Many studies have revealed that OLE has numerous biological effects in both animals and humans, such as antioxidant, antidiabetic, anticancer, anti-inflammatory, hypolipidemic, and hypoglycemic effects (21). In vitro studies have demonstrated the ability of OLE to scavenge free radicals, protect against oxidative stress, and reduce inflammation (22). Oleuropein enhances cardiac dysfunction following ischemic reperfusion damage, as evidenced by reduced left ventricular end-diastolic pressure (LVEDP) and augmented maximum and minimum dp/dt, and left ventricular developed pressure (LVDP). These cardioprotective properties of OLE can be attributed to its antioxidant features (23).
2. Objectives
Considering that aging is increasing dramatically worldwide and it is predicted that in the coming decades, there will be more elderly people suffering from debilitating diseases, this study was conducted to examine the protective impact of OLE on D-Gal-induced aging in heart tissue.
3. Methods
3.1. Ethics Committee Approval
This study approved by ethics committee of Ahvaz Jundishapur University of Medical Sciences with code IR.AJUMS.ABHC.REC.1400.004.
3.2. Chemical Materials
Oleuropein and D-Gal were perchased from Sigma-Aldrich Company (St. Louis, MO, USA).
3.3. Animals
A total of 40 male Wistar rats were utilized in this study. The rats were obtained from the animal house of Jundishapur University of Medical Sciences in Ahvaz, Iran. They had an average weight of 220 ± 20 g and were between 8 and 10 months old. The rats were housed in polypropylene cages and provided with standard rat chow and water ad libitum. The living conditions were maintained at a constant temperature of 23 ± 2°C with a humidity level of 40% to 50% and a 12-hour light-dark cycle.
3.4. Study Design
One week before the experiments, the rats were brought to the laboratory to acclimate to the environment. They were divided into five groups. Group 1 received normal saline for 8 weeks as a control. Group 2 received D-Gal (100 mg/kg intraperitoneally) for the same period as a positive control group. Groups 3 to 5, designated as treatment groups, were administered a specific amount of OLE (doses of 20, 40, and 80 mg/kg, respectively) orally, in addition to receiving D-Gal (100 mg/kg intraperitoneally) for 8 consecutive weeks, as described by Khalili et al. (20).
3.5. Sample Collection
Twenty-four hours after the last administration, the animals were anesthetized using a combination of ketamine and xylazine (80/8 mg/kg, intraperitoneally). Blood samples were obtained from the rats by puncturing the left ventricle of the heart. Subsequently, the animals were sacrificed, and their chest cavities were opened to remove the heart, which was then weighed immediately. A portion of the heart tissue was allocated for histological studies, while the remainder was preserved at -80°C for various biochemical and gene expression analyses. Blood samples were subjected to centrifugation at 3000 rpm for 10 minutes to obtain serum. The serum specimens were stored at -20°C for subsequent tests.
3.6. Serum Analyses
Serum samples were used to measure biomarkers of cardiac dysfunction. The activity of creatine kinase-MB (CK-MB) was determined using Bishop's method (24). The level of cardiac troponin I (cTnI) was measured using the Rat ELISA Kit, following the manufacturer's instructions (MyBioSource, San Diego, CA, USA; Catalog number: MBS727624). Additionally, the Pars Azmoon kit was employed to measure lactate dehydrogenase (LDH) and aspartate aminotransferase (AST).
3.7. Examination of Tissue Biochemical Parameters
The heart tissue was thoroughly homogenized at a concentration of 10% w/v in ice-cold 50 mM phosphate buffer (pH = 7.4). The homogenate was then subjected to centrifugation at 3,000 g for 15 minutes at 4°C, and the resulting supernatant was used to measure oxidative stress biomarkers. The protein concentration was determined using the Bradford method (25).
3.8. Malondialdehyde Assay
The level of lipid peroxidation in the heart tissue was measured by assessing the concentration of MDA and evaluating the formation of thiobarbituric acid reactive substances (TBARS), as previously described (26).
3.9. Protein Carbonyl Assays
To measure PC, the homogenized tissue was mixed with 0.5 mL of 1% 2,4-dinitrophenylhydrazine (DNPH) in 2 N hydrochloric acid (HCl) and incubated at room temperature for 1 hour. Sedimentation and pellet formation were achieved by adding 1 mL of 20% trichloroacetic acid (TCA). Protein separation was performed by centrifugation at 10,000 rpm for 1 minute, and the supernatant was removed. The remaining unreacted DNPH was treated with a 0.5 mL solution of ethanol and ethyl acetate in a 1:1 ratio, in triplicate. The recovered cellular protein was dried using nitrogen gas and then suspended in 1 mL of Tris-buffered 8 M guanidine – HCl, pH 7.2. The solubilized hydrazones were quantified by measuring the absorbance at 370 nm. Tetraethoxypropane was used as the standard for calibration (27).
3.10. Glutathione Assay
Glutathione was measured using the Ellman method (28). For this purpose, 200 μL of tissue homogenate was mixed with 200 μL of phosphate buffer, followed by the addition of 40 μL of 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB) reagent (10 mM). An ELISA reader was used to measure the absorbance of the yellow color of the complex formed at a wavelength of 412 nm. The concentration of glutathione (GSH) in the sample was determined using a calibration curve.
3.11. Measurement of Superoxide Dismutase, Catalase and Glutathione Peroxidase Activities
The activity of SOD, CAT and GPx was evaluated using colorimetric enzymatic assay kits from ZellBio (GmbH, Ulm, Germany).
3.12. Measurement of Sirtuin 1 and PGC1 Genes Expression
To quantify the expression of SIRT1, PGC1, and GAPDH genes, real-time polymerase chain reaction (PCR) was employed following specific steps.
3.12.1.RNA Extraction
Total RNA was extracted from heart tissues using RNX Plus™ solution (Cinnagen, Iran), following the manufacturer's instructions.
3.12.2.Complementary DNA Synthesis
The RNA was transcribed into complementary DNA (cDNA) using a reverse transcriptase enzyme and oligo-dT primers, as described by Bart et al. (29).
3.12.3.Real-time Polymerase Chain Reaction
The cDNA was amplified using specific primers for the SIRT1, PGC1, and GAPDH genes and a fluorescent dye-based detection system (Table 1). The PCR reaction was performed in a real-time PCR machine (Rotor-Gene 6000 system, Corbett, Concorde, NSW, Australia) using 5.5 μL of Fermentas SYBR Green master mix (Fermentas Life Sciences, St Leon-Rot, Germany), which allows for simultaneous amplification and detection of the PCR product. Melting curve analysis was performed for the SIRT1, PGC1 genes, and GAPDH (as the internal control gene). A single peak was observed at the melting temperature (Tm) point for all these genes, indicating a specific PCR reaction. This specificity was further confirmed by electrophoresis of the PCR products.
| Primer Sets and Probs | Product Size (bp) |
|---|---|
| GAPDH-rat | 162 |
| F: ATGCTGGTGCTGAGTATGTC | |
| R: AGTTGTCATATTTCTCGTGG | |
| PGC-1α | 133 |
| F: GCAACATGCTCAAGCCAAAC | |
| R: TGCAGTTCCAGAGAGTTCCA | |
| SIRT1 | 147 |
| F: TACCTTGGAGCAGGTTGCAG | |
| R: GACACCGAGGAACTACCTGATT |
Abbreviation: SIRT1, sirtuin 1; PGC-1α, proliferator-activated receptor-gamma coactivator.
3.13. Data Analysis
The real-time PCR data were analyzed using the following formula to determine the relative expression levels of the SIRT1 and PGC1 genes in heart tissues. This was done by comparing the cycle threshold (Ct) values of the SIRT1 and PGC1 genes to the Ct values of the GAPDH gene.
3.14. Histopathological Assessments
To prepare the heart tissues for histological examination, they were first fixed in 10% formalin to preserve their structure. The tissues were then dehydrated using a series of ethanol solutions. The dehydrated tissue was embedded in paraffin wax and cut into thin sections of 5 µm thickness using a microtome. The sections were mounted onto glass slides and stained with hematoxylin and eosin (H&E) dye. The stained sections were then examined under a microscope to evaluate the tissue structure and identify any pathological changes. Based on inflammatory cell infiltration, congested vessels, and myofibrillar loss, the heart tissue was scored as typical (-), mild (+), moderate (++), or severe (+++) (30).
3.15. Statistical Analysis
Data analysis was performed using GraphPad Prism software, version 5. The results were reported as mean ± SEM, and a one-way ANOVA test was used for statistical comparisons. Further analyses of the data were conducted using Tukey's post hoc test.
4. Results
4.1. The Effect of Oleuropein on the Serum Biomarkers of Cardiac Function in Rats Exposed to D-Galactose
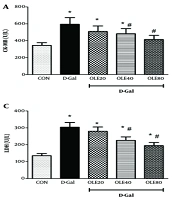
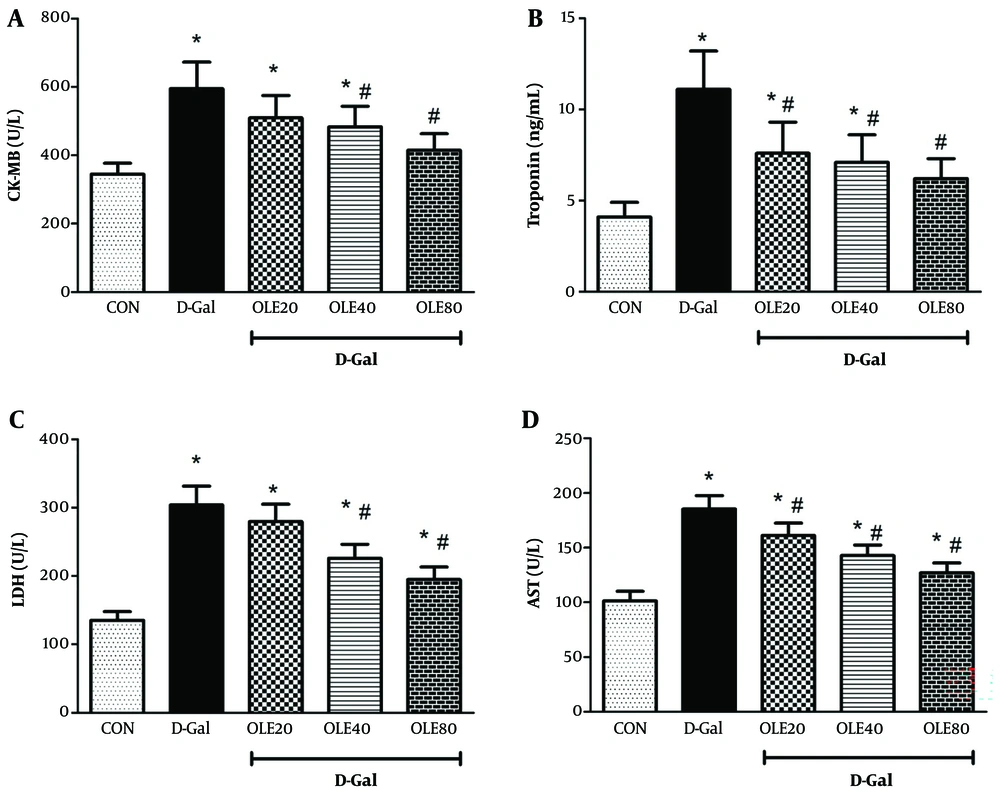
In our study, the adverse effects of D-Gal were evaluated in several aspects. Measurement of serum markers of cardiac function showed that the levels of LDH, AST, CK-MB, and cTnI were significantly increased in the D-Gal group compared to the control group (all P < 0.05) (Figure 1). Administration of OLE reduced these markers (LDH, AST, CK-MB, and cTnI) in a dose-dependent manner, reflecting the cardioprotective effect of OLE on heart tissue in D-Gal-treated rats (Figure 1).
Treatment effects of oleuropein (OLE) on the markers of cardiac dysfunction A, creatine kinase-MB (CK-MB);B, cardiac troponin I (cTnI); C, lactate dehydrogenase (LDH); D, aspartate aminotransferase (AST), and 24 hours after the last intervention. Group I received normal saline for 8 weeks; group II (induction control) was given D-Galactose (D-Gal) (100 mg/kg/ip) for 8 weeks. Group III – VI, was given oral deses of 20, 40 and 80 mg/kg of OLE, respectively for 8 weeks, simultaneously with D-Gal administration (100 mg/kg/ip). Each value represents means ± SEM of 8 mice per group.*: Significantly different from the control group (*: P < 0.05); #: Significantly different from the D-Gal group (#: P < 0.05).
4.2. The Effect of Oleuropein on the Oxidative Stress Biomarkers in Heart Tissue of Rats Exposed to D-Galactose
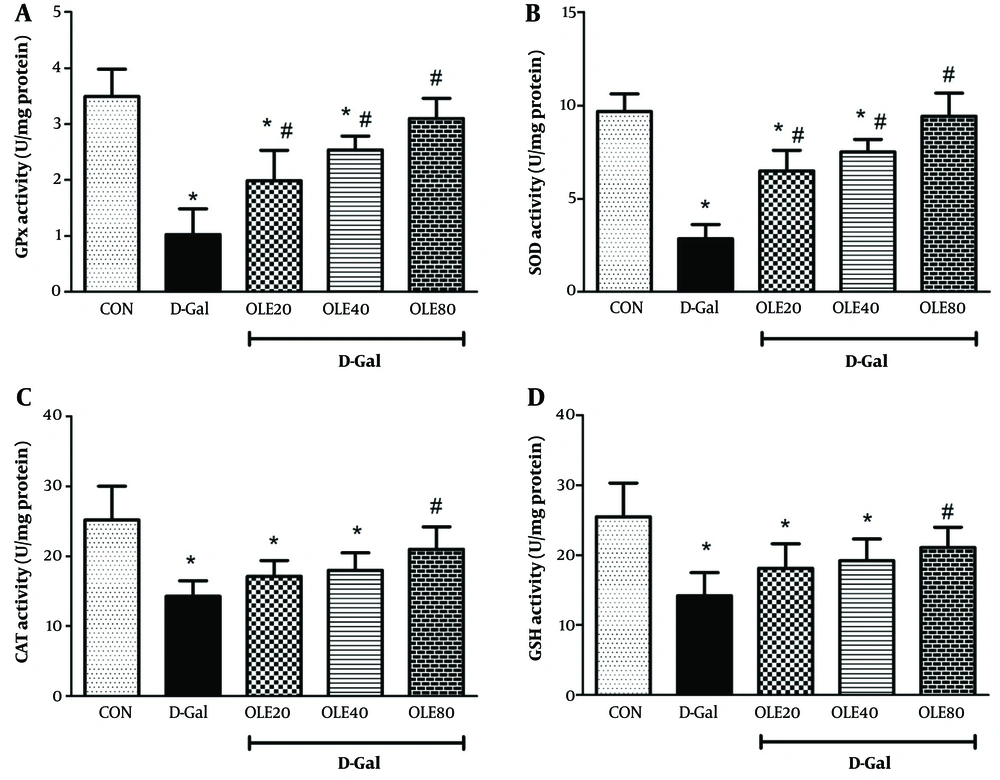
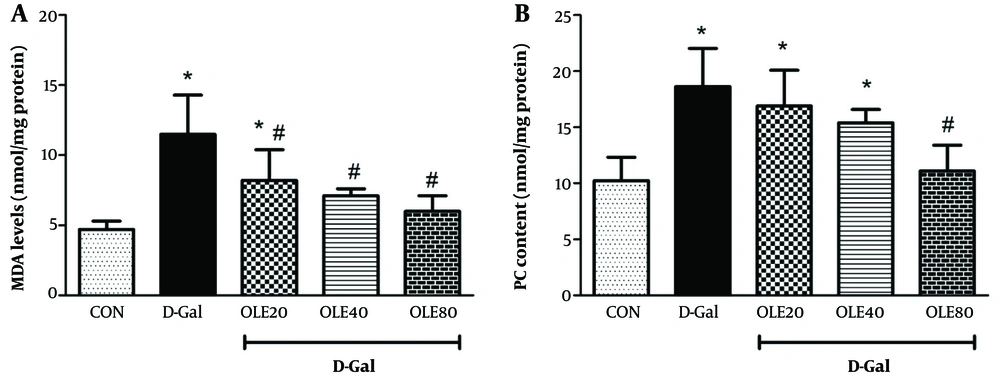
The results of oxidative stress factors (MDA, GSH, PC, and the activities of SOD, CAT, and GPx) for evaluating the effects of OLE in rat heart tissue are shown in Figures 2 and 3. Examination of MDA and PC in different groups showed a significant increase in MDA and PC levels in the D-Gal group compared to the control group by the end of the 8 weeks (Figure 2). Administration of OLE decreased MDA and PC levels in a dose-dependent manner compared to the D-Gal group. Regarding MDA, this reduction was significant at all doses of OLE, but for PC, the reduction was significantly different from the D-Gal group only in group 5 (P < 0.05).
Treatment effects of oleuropein (OLE) on the antioxidant biomarkers A, glutathione peroxidase (GPx); B, superoxide dismutase (SOD) activity; C, catalase (CAT); and D, glutathione (GSH), in heart tissue, 24 hours after the last intervention. Group I received normal saline for 8 weeks; group II (induction control) was given D-Galactose (D-Gal) (100 mg/kg/ip) for 8 weeks. Group III –VI was given oral deses of 20, 40 and 80 mg/kg of OLE, respectively for 8 weeks, simultaneously with D-Gal administration (100 mg/kg/ip). Each value represents means ± SEM of 8 mice per group.*: Significantly different from the control group (*: P < 0.05); #: Significantly different from the D-Gal group (#: P < 0.05).
Treatment effects of oleuropein (OLE) on the oxidative stress biomarkers A, malondialdehyde (MDA); and B, carbonyl protein (PC), in heart tissue, 24 hours after the last intervention. Group I received normal saline for 8 weeks; group II (induction control) was given D-Galactose (D-Gal) (100 mg/kg/ip) for 8 weeks. Group III – VI was given oral deses of 20, 40 and 80 mg/kg of OLE, respectively for 8 weeks, simultaneously with D-Gal administration (100 mg/kg/ip). Each value represents means ± SEM of 8 mice per group.*: Significantly different from the control group (*: P < 0.05); #: Significantly different from the D-Gal group (#: P < 0.05).
Glutathione levels and the activities of CAT, SOD, and GPx were also studied to evaluate oxidative stress. The findings showed that the GSH level and the activities of CAT, SOD, and GPx in the D-Gal group decreased significantly compared to the control group (P < 0.05). In the treatment groups, which received different doses of OLE, GSH levels and the activities of CAT, SOD, and GPx increased compared to the D-Gal group. This elevation was dose-dependent, with the highest increase in these parameters observed in group 5 (P < 0.01).
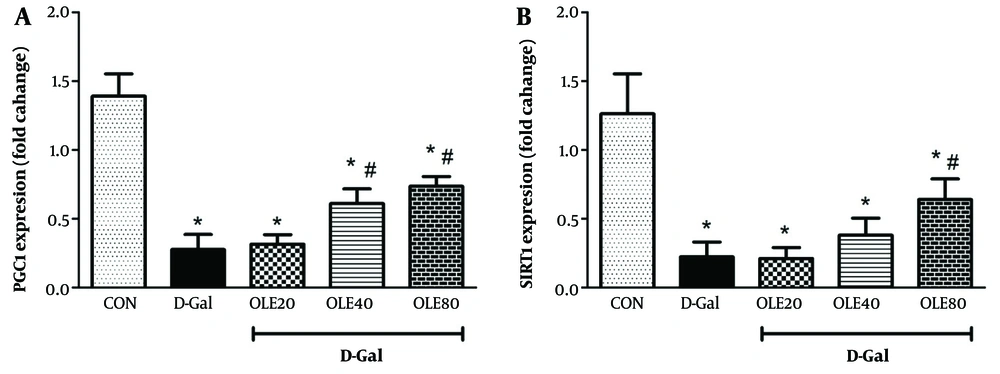
4.3. The Effect of Oleuropein on the Sirtuin 1 and PGC1 Genes Expression in Heart Tissue of Rats Exposed to D-Galactose
The expression of SIRT1 and PGC1 genes was also studied to evaluate the aging process in heart tissue (Figure 3). The results showed that the expression of SIRT1 and PGC1 genes in the D-Gal group significantly decreased compared to the control group (P < 0.001). In the treatment groups, which received different doses of OLE, the expression of SIRT1 and PGC1 genes increased compared to the D-Gal group. The highest increase in the expression of these genes was observed in group 5, which was significantly different from the D-Gal group (P < 0.01).
4.4. The Effect of Oleuropein on the Histopathology of Heart Tissue of Rats Exposed to D-Galactose
The results of the histopathological examination showed that in the control group, the tissue structure was completely normal, and no lesions were observed. In the group receiving D-Gal, the tissue damage was severe, including congested vessels, myocardial irregularity, myofibrillar loss, and infiltration of inflammatory cells. In the group receiving D-Gal and 20 mg/kg OLE, the amount of lesions was almost similar to the positive control group. In the group receiving D-Gal and 40 mg/kg OLE, the rate of congested vessels, myofibrillar loss, and infiltration of inflammatory cells was moderate. In the group receiving D-Gal and 80 mg/kg OLE, the rate of congested vessels, myocardial irregularity, myofibrillar loss, and inflammatory cell infiltration was similar to the negative control group (Figure 4).
Treatment effects of oleuropein (OLE) on the A, PGC1 genes; B, expression rate of sirtuin 1 (SIRT1), in heart tissue, 24 hours after the last intervention each value represents means ± SEM of 8 mice per group.*: Significantly different from the control group (*: P < 0.05); #: Significantly different from the D-Galactose (D-Gal) group (#: P < 0.05)
5. Discussion
When there is damage to cardiac tissue, enzymes such as CK-MB, AST, and LDH, along with proteins, are released into the bloodstream, resulting in an increase in their serum levels. Therefore, measuring the levels of these enzymes and proteins in the blood can be used as a diagnostic tool for detecting heart dysfunction (31). Administration of D-Gal increases these cardiac markers in serum, while co-administration of OLE with D-Gal reduces these disturbances. It is worth mentioning that normalization of serum levels of these markers via OLE indicates protection of cardiac cells (32).
The role of oxidative stress in causing heart damage has been well demonstrated (33). If the body is unable to neutralize oxidative stress, an imbalance is created, leading to cardiovascular diseases. Oxidative stress can also promote inflammation and contribute to the development of hypertension, heart failure, and other cardiovascular disorders. Additionally, oxidative stress can cause damage to cardiac cells, leading to cell death and the release of enzymes and proteins into the bloodstream, which can be used as markers of heart dysfunction. Therefore, reducing oxidative stress has become a vital target for the prevention and treatment of these diseases (33, 34).
Mammalian bodies have multiple antioxidant defense mechanisms (such as SOD, GPx, and CAT) that help manage free radicals, maintaining equilibrium between oxidants and antioxidants, and preventing oxidative stress (35, 36). Current studies have provided insight into the disease-protective mechanism of OLE and elucidated many of its effects through oxidative stress modulation and antioxidant action (37). Oleuropein has demonstrated the capability to inhibit the production of reactive oxygen and nitrogen species (ROS and NOS, respectively) in vitro biochemical tests, as well as in human cells, where it prevented excessive ROS generation. It was also shown to reduce oxidative stress by modulating the ERK/Nrf2 pathway-mediated signaling, with downstream effects on a variety of cellular processes (38).
Increasing biomarkers such as MDA and PC, and decreasing antioxidant enzymes such as SOD, GPx, and CAT, in some ways indicate oxidative damage (39). Our findings showed that aging in the D-Gal group was associated with an elevation in both MDA and PC content and a reduction in GSH, CAT, SOD, and GPx activities. These findings indicated that intraperitoneal administration of D-Gal for 8 weeks increased oxidative stress in the heart tissue of rats. These findings are consistent with other studies. In a similar study, Yang et al. (40) showed that the administration of 150 mg/kg of D-Gal for 8 weeks increased oxidative stress, evidenced by decreased GSH and other enzymatic antioxidants such as SOD. Studies conducted by Fu et al. in 2018 (41), Yang et al. in 2016 (40), and Coban et al. in 2015 (42) also showed that subcutaneous administration of D-Gal for 8 weeks increased oxidative stress in different tissues. Another study conducted by Bo-Htay et al. revealed that D-Gal administration could cause cardiac damage through upregulation of the expression of senescence markers. They suggested that increased oxidative stress, decreased antioxidant levels, and heightened apoptosis are some of the mechanisms involved in promoting aging induced by D-Gal (43).
Simultaneous administration of OLE by gavage for 8 weeks led to remarkable alterations in the tissue antioxidant status of the heart, resulting in increased GSH, CAT, SOD, and GPx activities and reduced MDA and PC levels in treated groups compared to the group that received D-Gal alone. These findings suggest that the reduction in oxidative stress is likely due to an increase in antioxidant defense mechanisms. These effects obtained from the present study are comparable to another study conducted by Coban et al. in 2014 (44). They evaluated the positive effect of olive leaf extract on oxidative stress in vital tissues of elderly rats. The findings showed that the consumption of this extract can decrease oxidative stress in organs such as the liver, heart, and brain of rats (44).
Some studies have shown that SIRT1 has a protective role against apoptosis and plays a role in the survival of cardiac myocytes and neurons under stress in vitro. Overexpression of SIRT1 (2.5-fold to moderate 7.5-fold) in the hearts of transgenic mice reduces age-related effects, including cardiac hypertrophy, apoptosis, fibrosis, cardiac dysfunction, and expression of aging-related markers (45, 46). Our findings showed that D-Gal administration resulted in downregulation of SIRT1 gene expression in cardiac tissue. Oleuropein administration resulted in significant expression of the SIRT1 gene in cardiac tissue, as shown by the current study. Our findings are consistent with those of a study conducted by Alcendor et al. They reported that a moderate increase in SIRT1 expression can provide protection for heart tissue against oxidative stress while also promoting the expression of antioxidant enzymes (47).
A limitation of D-Gal is that, as a sugar, it can be consumed by cells, potentially reducing its inducing effect. The difference between D-Gal in inducing aging and the natural aging process is that natural aging requires time, whereas D-Gal can induce aging in a short period (48). The PGC-1α is highly expressed in tissues with high energy demands, such as the heart, skeletal muscle, and liver. It is activated by various stimuli, including exercise, cold exposure, and fasting, and can induce the expression of genes involved in energy metabolism. The PGC-1α has been shown to play a role in the pathogenesis of metabolic disorders such as obesity, type 2 diabetes, and cardiovascular disease. Therefore, it has become an attractive target for the development of therapeutic interventions (49-51).
The results of the present study indicated that administration of D-Gal caused downregulation of PGC-1α in heart tissue. This reduction was significant compared to the control (healthy) group. Co-administration of OLE with D-Gal for 8 weeks by gavage increased PGC-1α gene expression, showing a reduction in the effect of D-Gal. Notably, this effect was more prominent and higher at a dose of 80 mg/kg OLE. Histopathological results of heart tissue also confirmed the findings of antioxidant tests and gene expression, showing that D-Gal consumption led to severe damage to heart tissue, while OLE administration reduced the severity of heart tissue damage. In histopathological studies of heart tissue, performed using conventional H&E staining, signs of D-Gal toxicity in the form of hemorrhage, myocardial irregularity, myofibrillar loss, and inflammatory cell infiltration were clearly evident. The histopathological results of heart tissue in the present study align with studies conducted by Wang et al. in 2012 and He et al. in 2009 (52, 53).
Practically, the effect of OLE has been shown to prevent heart aging. Thus, including olives (the source of OLE) in the human diet could be beneficial. Overall, the results of the present study and other reports indicate the role of OLE in improving cardiac function and reducing oxidative stress. Therefore, this issue should be confirmed with further studies and its use in humans, which requires additional investigation.
The limitations of this study include the use of D-Gal to induce aging instead of naturally aged rats, which were not available. Additionally, given the complexity of the antioxidant-oxidative stress system, the lack of measurement of DNA damage indices and total antioxidant capacity is another limitation. Considering the wide range of mechanisms for improving telomere function in heart tissue, the lack of measurement of key proteins in nuclear transcription, such as NRFs and PPARs, is also a limitation.
5.1. Conclusions
Our findings showed that OLE dose-dependently reduced heart lesions caused by D-Gal. The findings suggest that the protective effect of OLE is through the reduction of oxidative damage. The expression of genes involved in the aging process increased, and oxidative stress factors, such as MDA, were effectively suppressed. In fact, the oxidative phase decreased while the antioxidant phase increased, with an increase in PGC1. This indicates that PGC1 functions to increase the number of heart mitochondria, thereby improving heart function. Based on the results obtained in this study, we can explore the effect of this substance on other factors affecting aging. Considering our study, the findings showed that OLE administration increased the levels of SOD and GPx enzymes, as well as PGC1-α and SIRT1 gene expression, while decreasing MDA and PC levels in the heart tissue of rats. It is suggested that future studies evaluate the effect of this substance on mitochondrial biogenesis and the improvement of telomere and telomerase enzyme function.