1. Background
Alzheimer’s disease (AD) and other forms of dementia rank as the sixth leading cause of death among older adults globally. The AD induces neurodegeneration, impairing various cognitive functions such as attention, language, orientation, and judgment. The primary symptoms include progressive memory loss and cognitive disorder, ultimately resulting in death (1). Key features of AD include disrupted synapses, accumulated amyloid beta (Aβ) plaques, neurofibrillary tangles, and neuronal loss (2). Neuronal reduction is influenced by degenerative processes like Aβ plaques, oxidative stress, and inflammation (3). The cortex and hippocampus are particularly vulnerable to these degenerations early in the disorder. The hippocampus, crucial for learning and memory, undergoes atrophy due to AD-related pathological mechanisms (4). Despite extensive research, many FDA-approved AD drugs are ineffective, unable to cure or slow AD progression, costly, and often cause adverse side effects (5). Consequently, researchers are exploring alternative products to mitigate AD pathogenesis, aiming for more accessible treatments with fewer side effects.
Recent studies highlight the significant role of oxidative and inflammatory processes in AD pathogenesis, contributing to senile plaques and neurofibrillary tangles. The abnormal accumulation of Aβ and tau proteins exacerbates redox imbalance, leading to oxidative stress associated with Aβ or tau-induced neurotoxicity. Evidence suggests oxidative stress enhances Aβ aggregation and facilitates tau protein polymerization and phosphorylation, initiating a negative cycle that contributes to AD onset and progression. Therefore, developing therapeutic strategies targeting neuroinflammation and oxidative stress is crucial in combating AD pathogenesis (6).
Natural products are vital sources for drug discovery, with compounds derived from them extensively evaluated for developing novel AD treatments (5). Pomegranate (Punica granatum L.), cultivated in West Asia, the Mediterranean, and other regions, contains compounds like hydrolysable and condensed tannins, flavanols, anthocyanins, phenolics, and natural acids, studied for their health benefits. Compared to other fruits and vegetables, pomegranates have higher concentrations of antioxidant polyphenolic compounds, providing neuroprotective benefits in various models (7). Pomegranate seed oil (PSO) is rich in linolenic, linoleic, punicic, stearic, palmitic, and oleic acids (8). Punicic acid exhibits antioxidant, anti-inflammatory, nephroprotective, hepatoprotective, neuroprotective, anti-cancer properties, improves carbohydrate metabolism, and reduces insulin resistance (9). The PSO improves sensorimotor function and memory performance dose-dependently (10). It acts as an antioxidant by scavenging reactive oxygen species (ROS) and restoring cell viability (11). Phenolic compounds in PSO show neuroprotective effects, reducing Aβ-induced toxicity, demonstrating antioxidant activity, and protecting against Aβ-induced cell death (12). Neuronal degeneration is controlled by inhibiting inflammation and amyloidogenesis in interleukin-1β treated SK-N-SH cells (13). Pomegranates reduce soluble Aβ levels and Aβ deposition as plaques, improving behavioral performance in APPsw/Tg2576 mice (14). Pomegranate seed extract ameliorates memory impairments following bilateral common carotid artery occlusion (15). Pomegranate extract exhibits significant bioactive properties, including anti-inflammatory and antioxidant effects (6). Thus, pathways regulating neuroinflammation and oxidative stress contribute to pomegranate's neuroprotective effects.
Scopolamine is widely used in neuroscience research to induce cognitive disorders in experimental models due to its ability to cross the blood-brain barrier. In AD, it is significant as it triggers cholinergic dysfunction and enhances Aβ deposition — key disease features (16). Scopolamine administration induces oxidative stress-mediated activation of the c-Jun N-terminal kinase (JNK) pathway, leading to synaptic dysfunction, neuroinflammation, and neurodegeneration. It induces lipid peroxidation (LPO) and ROS production while downregulating antioxidant proteins like Nrf2 and HO-1. Scopolamine promotes neuronal loss by upregulating pro-apoptotic markers such as Bax, Pro-Caspase-3, and Caspase-3 while decreasing the anti-apoptotic protein Bcl-2 (17). Despite numerous studies on pomegranate, further investigations are needed to understand pomegranate-related cellular and molecular mechanisms in animal models to confirm its efficacy as a therapeutic factor (18).
2. Objectives
This study aimed to evaluate the effects of PSO on memory, hippocampal neuronal density, and amyloid plaques in a scopolamine-induced AD model in rats.
3. Methods
3.1. Animals
The experimental protocol adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). The study received approval from the Research Ethics Committee of Golestan University of Medical Sciences, Gorgan, Iran (Agreement License No. IR.GOUMS.REC.1397.260). In this study, 56 male Wistar rats, weighing between 180 and 220 g, were obtained from the animal facility of Golestan University of Medical Sciences in Gorgan, Iran. Optimal conditions were maintained at a temperature of 22 ± 3 °C with a 12-hour light/dark cycle, and the rats had free access to standard food and drinking water.
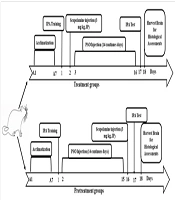
3.2. Experimental Design
The rats were randomly assigned to seven groups (n = 8 per group). All animals underwent behavioral testing and received daily treatments as follows:
- Control group: Received no injection.
- Sco + Saline group: Received a single intraperitoneal (i.p.) injection of scopolamine at 3 mg/kg (19), followed by injections of 0.9% saline (1 mL/kg, i.p.) for 14 days.
- Sco + PSO (0.32 or 0.64 mg/kg) groups: Received a single i.p. injection of scopolamine at 3 mg/kg (19), followed by continuous injections of pomegranate seed oil at either 0.32 or 0.64 mg/kg (20) for 14 days.
- Saline + Sco group: Received continuous i.p. injections of saline for 14 days, followed by a single i.p. injection of scopolamine.
- The PSO (0.32 or 0.64 mg/kg) + Sco groups: Received continuous i.p. injections of pomegranate seed oil at either 0.32 or 0.64 mg/kg for 14 days, followed by a single i.p. injection of scopolamine (Figure 1).
Scopolamine hydrobromide was dissolved in 0.9% saline immediately before injection. The pomegranate seed oil was procured from Golnar Golestan Company (Gonbadkavos, Iran).
3.3. Inhibitory Passive Avoidance Memory Test
All rats were trained in a passive avoidance apparatus 24 hours before drug injection, and the memory retention test was conducted 24 hours after the final injection (19). The test spanned two days:
- Training day: Each rat was placed in the light chamber, and the latency time to enter the dark chamber was recorded (without foot shock). Rats that did not enter within a maximum of 2 minutes were excluded from the study. Thirty minutes later, the test was repeated, during which the rat received a foot shock upon entering the dark chamber (50 Hz, 1 mA, 3 seconds). The rat was then promptly returned to its cage. After 2 minutes, the latency time was measured again (with a maximum of 120 seconds). Successful acquisition of inhibitory passive avoidance was determined for rats that did not enter the dark chamber within 2 minutes.
- Probe day: The step-through latency time was recorded for a maximum of 300 seconds.
3.4. Tissue Preparation
After anesthetization with ketamine (100 mg/mL) and xylazine (20 mg/mL), the rats were transcardially perfused with 4% paraformaldehyde (Scharlau, Spain). The brains were fixed for one week in 4% paraformaldehyde in PBS. Histological processing was performed using an automated tissue processor (Did Sabz, Iran), and the tissues were subsequently embedded in paraffin. The brains were sectioned in the coronal plane at 6 μm (21) using a rotary microtome (Pooyan MK 1110, Iran). The sections were divided into two sets: One set was used for Congo red staining to detect Aβ plaques, and the other set was used for cresyl violet staining to visualize neurons.
3.5. Congo Red Staining
The sections were initially deparaffinized in xylene and rehydrated through a descending alcohol series. The brain sections were then stained by immersion in Congo red (DDK, Italy) for one hour at room temperature, followed by washing in distilled water. Subsequently, the sections were quickly differentiated with an alcoholic solution and washed for 5 minutes. For counterstaining, hematoxylin solution was applied for 5 minutes, followed by a 10-minute wash. The slides were dehydrated using 95% and 100% alcohols and cleared in xylene. Finally, all slides were coverslipped with Entellan glue (Merck, Germany) (22).
3.6. Cresyl Violet Staining
Brain sections were dewaxed in xylene, rehydrated through an alcohol series, and washed with distilled water. The sections were then stained in 0.02% cresyl violet (Sigma, USA) for 5 minutes. Following dehydration with 96% and 100% alcohols and clearing in xylene, the brain sections were coverslipped with Entellan glue (23).
3.7. Estimating the Number of Neurons and Aβ Plaques
Images were captured using an Olympus BX53 microscope at 40x magnification, equipped with a digital camera (DP73, Olympus, Japan). The CA1, CA3, and dentate gyrus (DG) subfields of the hippocampus were evaluated. Quantitative analyses of the images were conducted using cellSens Standard 1.14 software (Olympus, Tokyo, Japan). The number of neurons and Aβ plaques in the hippocampus were randomly counted within a field of 12,000 μm2 (24, 25).
3.8. Statistical Analysis
The results were presented as mean ± SD. Since the data distribution was not normal, the Kruskal-Wallis’s test was conducted, followed by Dunn's multiple comparisons test. Statistical significance was set at P < 0.05.
4. Results
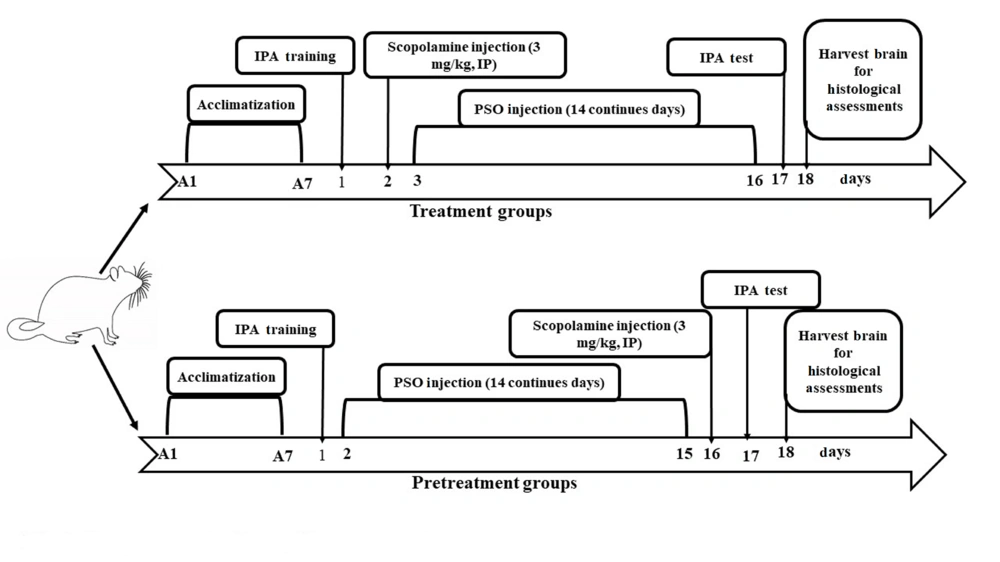
4.1. Pomegranate Seed Oil Effects Step-Through Latency Time
The results showed that the Sco + Saline group exhibited a significant reduction in step-through latency time to enter the dark chamber compared to the control group (Figure 2 P < 0.01). In the PSO (0.32 mg/kg) + Sco and Sco + PSO (0.32 mg/kg) groups, the step-through latency increased relative to the saline groups; however, these increases were not statistically significant (Figure 2A and B). The Sco + PSO (0.64 mg/kg) group showed an improvement in latency time compared to the Sco + Saline group. The PSO (0.64 mg/kg) + Sco group did not demonstrate effectiveness in enhancing memory performance compared to the Saline + Sco group. Conversely, it significantly decreased latency time compared to the control group (Figure 2 P < 0.01).
A and B, Passive avoidance test. Pomegranate seed oil improved the step-through latency time in treatment (B) group rats; however, it was not significant. B, the result showed that Sco injection in Sco + Saline could significantly decrease the memory, (P < 0.01). Data were expressed as the mean ± SD. Kruskal-Wallis’s test followed by Dunn's multiple comparisons test. ** P < 0.01. Abbreviations: PSO, pomegranate seed oil; Sco, scopolamine.
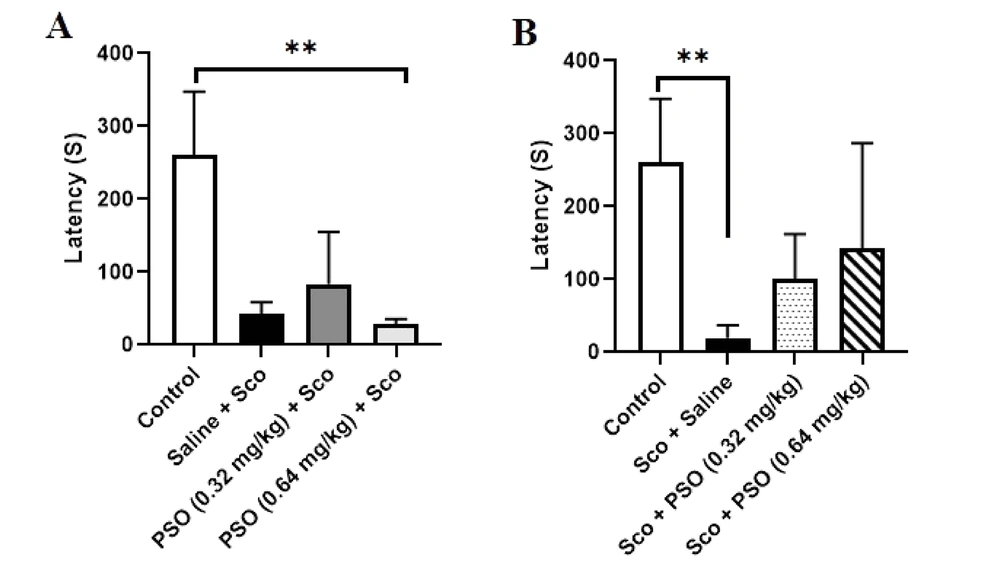
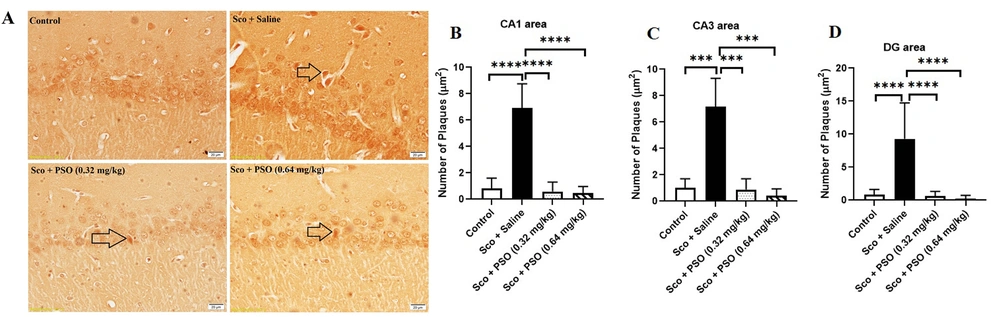
4.2. Pomegranate Seed Oil Declined the Density of Aβ Plaques
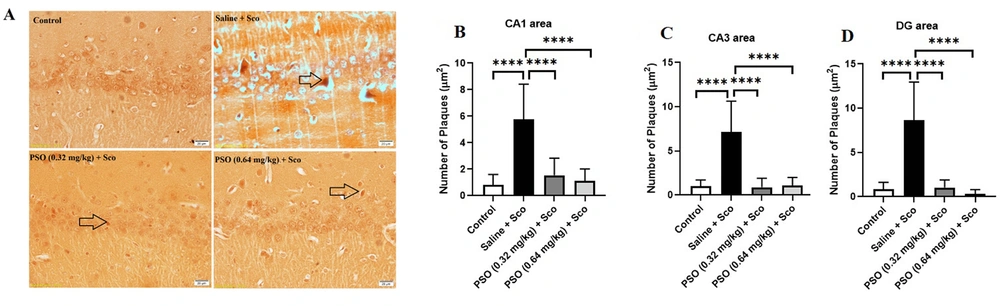
Figure 3A and 4A demonstrated a notable increase in the hippocampal density of Aβ plaques in the Saline + Sco and Sco + Saline rats compared to the control group (Figure 3 - C and 4B - C, P < 0.0001). Treatment with PSO, both before and after the injection of scopolamine, significantly reduced the density of Aβ plaques across all areas of the hippocampus relative to the Sco + Saline and Saline + Sco groups (Figures 3 and 4, P < 0.0001). However, no significant differences were observed in the density of hippocampal Aβ plaques among the experimental groups receiving PSO.
A - D, The density of hippocampal Aβ plaques in pretreatment groups. A, Congo red stained Aβ plaques were indicated by arrows (×40); B, pretreatment with pomegranate seed oil reduced the number of Aβ plaques in the rat hippocampal CA1; C, CA3; and D, DG areas. Data were expressed as the mean ± SD. Kruskal-Wallis’s test followed by Dunn's multiple comparisons test. **** P < 0.0001. The scale bar shows 20 μm. Abbreviations: PSO, pomegranate seed oil; Sco, scopolamine.
A - D, Hippocampal Aβ plaques density in treatment groups. A, congo red stained Aβ plaques were indicated by arrows (×40); B, treatment with pomegranate seed oil reduced the number of Aβ plaques in the rat hippocampal CA1; C, CA3; and D, DG areas. Data were expressed as the mean ± SD. Kruskal-Wallis’s test followed by Dunn's multiple comparisons test. **** P < 0.0001, *** P < 0.001. The scale bar shows 20 μm. Abbreviations: PSO, pomegranate seed oil; Sco, scopolamine.
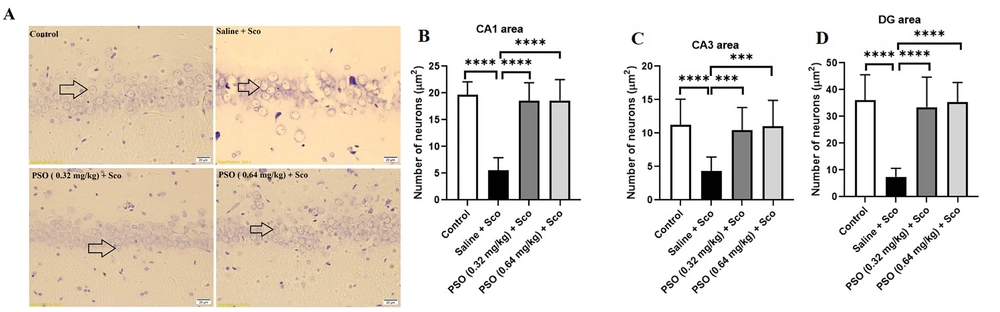
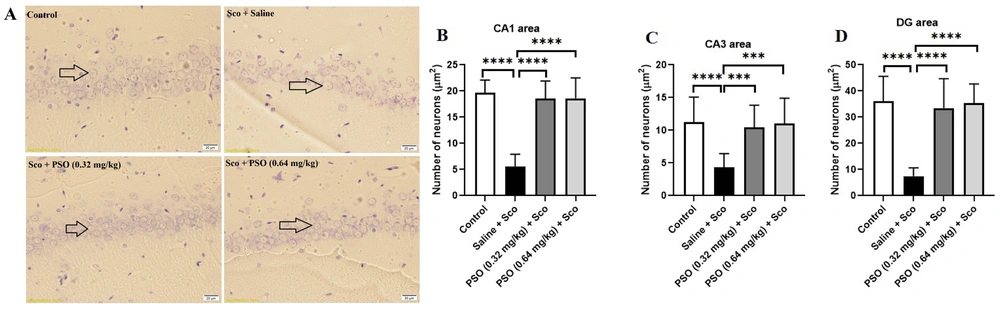
4.3. Pomegranate Seed Oil Enhanced the Density of Neurons
Figures 5A and 6A illustrate the effect of pomegranate seed oil on the number of cresyl violet-stained neurons before and after scopolamine administration. As shown in Figures 5B - D and Figure 6B – D, exposure to scopolamine significantly decreased the number of neurons (P < 0.0001) compared to the control group. Injection of pomegranate seed oil at doses of 0.32 and 0.64 mg/kg significantly increased the number of neurons (P < 0.0001) compared to the Sco + Saline and Saline + Sco groups.
A - D, Hippocampal neuronal density in pretreatment groups. A, cresyl violet stained neurons were indicated by arrows (×40); B, pretreatment with pomegranate seed oil boosted the neuronal density in the rat hippocampal CA1; C, CA3; and D, DG areas. Data were expressed as the mean ± SD. Kruskal-Wallis’s test followed by Dunn's multiple comparisons test. **** P < 0.0001, *** P < 0.001. The scale bar shows 20 μm. Abbreviations: PSO, pomegranate seed oil; Sco, scopolamine.
A - D, Hippocampal neuronal density in treatment groups. A, cresyl violet stained neurons were indicated by arrows (×40); B, treatment with pomegranate seed oil enhanced the neuronal density in the rat hippocampal CA1; C, CA3; and D, DG areas. Data were expressed as the mean ± SD. Kruskal-Wallis’s test followed by Dunn's multiple comparisons test. **** P < 0.0001, *** P < 0.001. The scale bar shows 20 μm. Abbreviations: PSO, pomegranate seed oil; Sco, scopolamine.
5. Discussion
In agreement with other studies that used scopolamine to model AD-like conditions (26, 27), our findings confirmed impaired memory in the scopolamine-injected groups compared to the control group. We found that PSO re-established scopolamine-induced impairment in passive avoidance memory. Furthermore, we observed a notable reduction in the density of Aβ plaques and an increase in the number of neurons across various subfields of the hippocampus, the main structure involved in memory formation and stabilization.
Pomegranate is recognized as a medicinal plant that offers effective neuroprotection against several neurodegenerative disorders (28-30). Sarkaki (15), in 2013, reported that continuous treatment with pomegranate seed extract for 14 days could ameliorate both passive and active memory impairments in cerebral ischemia model rats, due to the phytoestrogen and antioxidative effects of pomegranate seed extract (10). Another study revealed that pomegranate juice at a 20% concentration for 29 days improved learning and memory while protecting brain cells from degeneration induced by aluminum chloride (31). Moreover, pomegranate juice has been found to enhance spatial learning performance by reducing amyloid formation in AD model transgenic mice (7).
Dietary supplementation with pomegranate has shown promising effects on cognitive functions, particularly in APPsw/Tg2576 mice, where a 4% pomegranate diet improved memory, learning, locomotor functions, and reduced anxiety (14). The results of the current study showed that treatment with 0.32 mg/kg and 0.64 mg/kg of pomegranate seed oil could improve passive avoidance memory in scopolamine-treated rats; however, these improvements were not significant. Remarkably, pretreatment with 0.64 mg/kg of PSO resulted in decreased memory performance.
The dysfunction of the cholinergic system results in the deterioration of learning and memory processing in AD. Scopolamine blocks acetylcholine muscarinic receptors, leading to increased acetylcholinesterase activity in the hippocampus and cortex (32). This enhancement is associated with memory deficits and oxidative stress in the brain. Notably, PSO has been shown to possess neuroprotective properties by inhibiting acetylcholinesterase activity and enhancing antioxidant capacity (33).
Synaptic plasticity is essential for the maintenance of learning and memory, which is impaired in AD pathology. Administration of 4% pomegranate over 15 months ameliorated the loss of key synaptic proteins, including PSD-95, Munc18-1, SNAP25, and synaptophysin (34). These proteins are vital for synaptic function and plasticity, indicating that pomegranate may play a significant role in enhancing cognitive functions through its protective effects on synaptic integrity.
The protective effects of PSO and pomegranate extract are thought to stem from their ability to neutralize reactive oxygen species (ROS), upregulate the expression of antioxidant genes, and exert anti-inflammatory, anti-apoptotic, and ATP-replenishing effects (11, 35). Studies have demonstrated that PSO protects against toxins such as mercuric chloride, hexachlorobutadiene, diazinon, cisplatin, gentamicin, and H2O2 (36, 37). In addition, pretreatment with pomegranate extract before ischemia/reperfusion in rats resulted in protection against brain injury and DNA damage (35).
Amyloid aggregation is a crucial pathological feature of AD (38). Deposition of Aβ peptides, especially Aβ1-42, increases oxidative stress, neuroinflammation, and neuronal death (39). The administration of scopolamine increased Aβ production and oxidative stress while reducing neuronal density in rodent brains (19, 38). These findings align with the current study, which demonstrated that a single injection of scopolamine (3 mg/kg) elevated the density of Aβ plaques and reduced neuronal density in the hippocampus of rats.
Previous studies have shown that pomegranate juice and extracts exert neuroprotective effects against AD pathogenesis in several transgenic animal models, though the exact bioactive compounds have not yet been fully identified. Urolithins are suggested to mediate the neuroprotective effects of pomegranate against AD, as they inhibit Aβ fibrillation in vitro (40). It has been reported that pomegranate prevents amyloidogenesis in SK-N-SH cells stimulated with interleukin-1β. It is assumed that pomegranate is a potent nutritional strategy to slow neurodegeneration in AD (41). Pomegranate peel extract (800 mg/kg/day) reduced the density of Aβ plaques in Aβ peptide-treated mice (28). In transgenic mice, pomegranate juice hindered the accumulation of soluble Aβ1-42 and amyloid deposition in the hippocampus (7).
Moreover, the phenolic compounds in PSO have demonstrated significant neuroprotective effects. Shrivas et al. (2023) developed a stable microemulsion incorporating PSO as an adjuvant for galantamine hydrobromide (GHBr). The optimized ratio of GHBr to PSO showed promising outcomes, including reduced toxicity, enhanced antioxidant activity, and protection against Aβ-induced cell death. The study highlights PSO as a potential treatment to enhance the efficacy of anti-Alzheimer’s therapies (12). Additionally, PSO can inhibit key enzymes, reduce ROS, prevent microglial activation, inhibit tau protein hyperphosphorylation, maintain synaptic plasticity, exhibit anti-inflammatory activity, and suppress beta-secretase-1 (BACE-1) (42).
These benefits are largely attributed to punicic acid, the primary bioactive compound in PSO, which is an omega-5 isomer of conjugated α-linoleic acid. Punicic acid possesses potent antioxidant and anti-inflammatory properties, reducing oxidative damage and inflammation by upregulating peroxisome proliferator-activated receptors. It further mitigates AD pathology by reducing beta-amyloid deposition and tau hyperphosphorylation through mechanisms such as enhanced GLUT4 protein expression and inhibition of calpain activation (43).
Pomegranate peel extract has been shown to decrease apoptosis and chromatolysis in the DG and CA3 areas of the hippocampus, reduce neurofibrillary tangles and senile plaques, and restore Nissl granules (44, 45). Consistent with these findings, the current study discovered that pretreatment and treatment with pomegranate seed oil (0.32 and 0.64 mg/kg) can reduce the density of Aβ plaques in scopolamine-injected rats. Additionally, it increased the density of neurons in the rat hippocampus. These results confirm the neuroprotective properties of pomegranate seed oil against the adverse effects of AD pathogenesis. Essa et al. (46) observed that long-term administration of pomegranate (for 15 months) decreased the levels of Aβ1–40 and Aβ1–42 in the brains of transgenic AD mice. In the current study, the protective effects of PSO were observed during a two-week treatment, which reduced the density of Aβ plaques in the hippocampus of scopolamine-injected rats. It has also been reported that pomegranate extract is more protective when administered as a pretreatment rather than as a therapeutic, as it reduces the histopathological features of AD (44). However, this study found that both pretreatment and treatment with pomegranate seed oil can be protective against the extension of Aβ plaques and the loss of neuronal density.
5.1. Conclusions
This study highlights the neuroprotective effects of PSO in a scopolamine-induced Alzheimer's disease rat model. The PSO reduced amyloid-β plaques, preserved neuronal density in the hippocampus, and restored memory function. While further research is needed to elucidate detailed mechanisms and validate these findings in humans, this study suggests that PSO is a promising agent for AD treatment.
5.2. Limitation
This study had several limitations. First, while the neuroprotective effects of PSO were demonstrated, the specific bioactive compounds responsible and their mechanisms of action were not fully clarified. Additionally, the molecular pathways through which PSO reduces amyloid-β plaques and oxidative stress remain unclear. These aspects should be further explored in future studies. Moreover, it is recommended to focus on clinical trials to confirm the neuroprotective effects of PSO and evaluate its potential as a therapeutic agent for Alzheimer’s disease.