1. Background
Type 2 diabetes mellitus (DM2) is a disease characterized by hyperglycemia. Untreated DM2 patients develop severe damage to organs and systems, resulting in complications such as cardiac failure, retinopathy, and neuropathy (1, 2). The primary mechanism of insulin in regulating glucose and lipid metabolism involves the phosphatidylinositol 3-kinase (PI3K) pathway, which is compromised in DM2 patients (3, 4). Under stress conditions (e.g., physical exercise and prolonged fasting), glucose assimilation is associated with the adenosine monophosphate-activated protein kinase (AMPK) pathway. Therefore, regulating this pathway is crucial for preventing and treating diabetes (4, 5). The etiology of diabetes has been linked to inflammation. Chronic high glucose concentration produces metabolites that increase the levels of proinflammatory cytokines, inducing low insulin sensitivity, hyperinsulinism, and diabetes. Consequently, modulating proinflammatory cytokine levels is a strategy for preventing and treating diabetes (4, 6). Leptin levels are also associated with obesity and diabetes, and this parameter is decreased in DM2 patients (7, 8). Current commercial treatments for diabetes have not shown sufficient efficacy, highlighting the need for new therapies. In this context, plant flavonoids have been shown to improve glucose assimilation and metabolism, including hesperetin, luteolin, catechin, genistein, quercetin, and kaempferol (6). For example, a Wisteria sinensis extract enriched in luteolin and apigenin derivatives improved glucose metabolism in diabetic rats (9). Our research group demonstrated that the methanol extract of Echeveria subrigida (B.L. Rob. & Seaton) rose leaves has high in vitro antioxidant activity, and the main phenolics present in the extract were isorhamnetin (ISO), quercetin, and kaempferol derivatives (10, 11). The α-glucosidase inhibitory activity of the same extract (IC50 25.21 - 50.57 μg/mL) was stronger than that of acarbose (IC50 3.59 mg/mL), suggesting its potential as an antidiabetic agent (12). Fractionation of the methanol extract of E. subrigida identified isorhamnetin-3-O-glucoside (I3G) (IC50 = 166.4 μg/mL), quercetin-3-O-glucoside (Q3G) (IC50 = 131.1 μg/mL), and tannins (IC50 = 9.6 μg/mL) as the primary compounds responsible for the α-glucosidase inhibition (13). A hydroalcoholic extract of E. subrigida leaves showed high adaptogenic and immunomodulatory activities, which could contribute to its antidiabetic effects (14). Based on these studies, an I3G-standardized hydroalcoholic extract of E. subrigida leaves was prepared, and an in vivo assay with normoglycemic mice showed significant hypoglycemic and antihyperglycemic effects (15). These results support the antidiabetic potential of the I3G-standardized hydroalcoholic extract of E. subrigida. These effects could be due to activation of the PI3K and AMPK pathways.
2. Objectives
The aim of this study was to investigate the antidiabetic mechanisms of ethanolic (EEEs) and methanolic (MEEs) extracts of E. subrigida, standardized for I3G, in streptozotocin (STZ)-induced diabetic rats.
3. Methods
3.1. Chemicals
The reagents were purchased from Sigma Aldrich (St. Louis, MO, USA), and HPLC solvents were obtained from TEDIA (Fairfield, OH, USA).
3.2. Plant Material and Preparation of the Ethanolic and Methanolic Extracts
Leaves of E. subrigida rose (B. L. Rob. & Seaton) were collected near the town of El Palmito, Concordia, Sinaloa (2,000 m above sea level; N 23° 34ʹ 06ʺ, W 105° 50ʹ 53ʺ; Vega-Aviña R.; 11742) in November 2019. They were transported to the Laboratory of Chemistry of Natural Products, School of Chemical and Biological Sciences, Autonomous University of Sinaloa. The E. subrigida leaves were freeze-dried (VirTis 25EL, VirTis Co., Gardiner, NY, USA), milled, and passed through a number 40 mesh. The powder (100 g) was mixed with methanol (1:10 w/v) to obtain the MEEs or with 80% ethanol (1:10 w/v) to obtain the EEEs. The extraction was carried out for three days with daily solvent exchange. The solvent was removed at 40°C using a rotary evaporator (BÜCHI Labortechnick AG, Switzerland), followed by the removal of any residual solvent from EEEs in a vacuum oven (Thermo Scientific™, Waltham, MA, USA) at 40°C. Finally, both MEEs and EEEs samples were freeze-dried. Both extracts were stored at -20°C in darkness until use.
3.3. Determination of Isorhamnetin-3-O-Glucoside by HPLC
The concentrations of I3G, Q3G, and tannins in the extracts were quantified by HPLC analysis using an ACCELA HPLC-DAD (Thermo Scientific, USA) equipped with an ACE EXCEL C18 – Amide column (150 × 30 mm × 3 μm) (Advanced Chromatography Technologies, UK) as described by Heredia-Mercado et al. (15). The mobile phase consisted of 1% formic acid (A) and acetonitrile (B): 0.5% B, linear gradient to 16% B in 4 minutes, linear gradient to 60% B in 17 minutes, and isocratic for 5 minutes. The separation conditions were as follows: Operating time of 35 minutes, flow rate of 0.3 mL/min, injection volume of 15 µL, and detection at 280, 320, and 350 nm. For the HPLC analysis, 100 mg of extract was dissolved in 10 mL of methanol; a 1:1 dilution was prepared, passed through a PVDF filter (17 mm, 0.45 μm, TITAN, USA), and injected. Flavonoids were measured using calibration curves of I3G and Q3G, and tannins were quantified using a calibration curve of catechin; values were reported as milligrams of compound per gram of extract. It was previously reported that the standardized extracts of E. subrigida leaves contain at least 4.82 mg of I3G or 3.01 mg of Q3G per gram (16).
3.4. Animals
Six-week-old male Wistar rats were used. The animals were kept in the FCQB-UAS vivarium at 24°C, 50% humidity, and 12-hour light-dark cycles. They were fed a standard diet containing 3% fat, 49% carbohydrates, 23% protein, 6% fiber, 7% ash, and 12% moisture (Nutricubos, Nutrimentos Purina S.A. de C.V., Mexico). The research was conducted in accordance with the Official Mexican Standard NOM-062-ZOO-1999.
3.5. Animal Experimental Design
The rats underwent a 7-day adaptation period with a regular diet. After this period, the animals were provided with a 10% sucrose solution instead of drinking water for 15 days. They then fasted for seven hours (7:00 a.m. to 2:00 p.m.) with free access to water, and diabetes was induced by intraperitoneal (i.p.) administration of STZ [50 mg/kg body weight (b.w.)]. The STZ was resuspended in cold citrate buffer (0.1 M citric acid, pH 4.5) prepared at the time of application, and the animals were injected within the following 5 minutes. Thirty minutes later, rats were provided with a 5% sucrose solution instead of drinking water for 48 hours to prevent hypoglycemia and death. Control rats received an equivalent i.p. dose of the citrate buffer used as a vehicle (pH 4.5, 10 mL/kg b.w.). One week after injection, glucose levels were measured in blood sampled from the tail using an Accu-Chek® instant glucometer. Rats with blood glucose levels higher than 200 mg/dL were considered to have DM2 (17) and were used for experimentation. Six groups of animals (n = 6) were formed: Healthy control (HC); diabetic control (DC); MET: Diabetic + metformin treatment (500 mg/kg b.w.); ISO: Diabetic + isorhamnetin (1.9 mg/kg b.w.); EEEs: Diabetic + E. subrigida ethanolic extract (400 mg/kg b.w.); and MEEs: Diabetic + E. subrigida methanolic extract (284.7 mg/kg b.w.). The employed doses of ISO, EEEs, and MEEs were adjusted to provide the same quantity of ISO. Oral treatments were administered once a day (17:30 h) for 30 days. Body weight and blood glucose levels were measured at the start of the experiment and weekly.
At the end of the experiment, rats were euthanized following the Mexican Official Norm NOM-062-ZOO-1999. The animals were anesthetized with ether, and blood was collected by cardiac puncture in polystyrene tubes without anticoagulant (BD Vacutainer®). Subsequently, manual cervical dislocation was performed. Serum was immediately recovered by centrifugation (PowerSpin™ MX Centrifuge, UNICO, Chicago, IL, USA) at 2500 rpm at room temperature for 10 minutes. Animals were dissected to obtain the liver and pancreas. Portions of the liver and pancreas were sliced, fixed, and stained for histological analysis. The remaining liver tissue was frozen with liquid nitrogen until processing.
3.6. Oral Glucose Tolerance Test
On day twenty-one of treatment, an oral glucose tolerance test was performed after a 6-hour daytime fast. Blood was drawn to measure basal glucose, and then treatments were administered. Thirty minutes later, an oral sucrose load of 3.0 g/kg body weight (b.w.) was administered. Blood was obtained by tail puncture after 30, 60, and 120 minutes to measure glucose using an Accu-Chek® instant glucometer.
3.7. MCP-1 and Leptin Analysis
Serum levels of monocyte chemoattractant protein-1 (MCP-1) (PicoKine™ ELISA) and leptin (no. RAB0335, Sigma-Aldrich, USA) were analyzed using ELISA kits according to the manufacturer's protocols. Three biological replicates were measured per duplicate: Rats in each experimental group were randomly allocated into groups of two, and their sera were mixed to measure the MCP-1 and leptin levels.
3.8. Cytokine Analysis
The levels of IL-4, IL-6, IL-10, IL-17A, and IFN-γ were analyzed using a flow cytometer (BD Accuri C6, USA) with the commercial Th1/Th2/Th17 kit (catalog number 560485; BD Bioscience-Pharmingen) according to the manufacturer's instructions. The analysis was carried out in triplicate, as indicated in section 3.7. The level of each cytokine was measured using the corresponding standard curve.
3.9. Liver and Pancreas Histology
Liver and pancreas slices were fixed in 4% formaldehyde, embedded in paraffin, and stained with hematoxylin-eosin (HE) (TissuePro Technology, Gainesville, FL, USA). The stained tissues were observed with an optical microscope (Nikon ECLIPSE E200, Nikon Instruments Inc., Melville, NY, USA) at 40× magnification.
3.10. Analysis of Proteins from the PI3K and AMPK Pathways
The levels of expression and phosphorylation of proteins from the PI3K and AMPK pathways were analyzed by Western blot. The analysis was performed with pooled livers from each group; three extractions were carried out and analyzed in duplicate. Proteins from the liver samples were extracted by sonication for 30 minutes in an ice bath using the RIPA kit (Thermo Scientific™ Buffer RIPA Pierce™) containing the protease and phosphatase inhibitors cocktail (Halt™, 100X). The samples were centrifuged (Eppendorf 5417R, Fairfield, OH, USA) at 12,000 × g for 15 minutes at 4°C, and the supernatant was recovered (9). Protein concentration was determined with the BCA kit (Pierce™ BCA Protein Assay Kit) and adjusted to 60 μg/μL. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane (Hybond™-ECL™ Amersham Biosciences). The membrane was blocked with 5% non-fat milk proteins (Svelty, Nestle®) and incubated for 2 hours with a primary monoclonal antibody (Cell Technology Inc., Williamsburg, VA, USA): The p-Akt (1:1000), Akt (1:1000), p-AMPKα (1:1000), AMPKα (1:1000), p-AS160 (1:1000), or AS160 (1:1000). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:1000) was used as an expression control. Membranes were then incubated for 90 minutes with the secondary antibody conjugated with horseradish peroxidase (1:1000). The membranes were treated with 3,3’-diaminobenzidine (Research Organics), and protein band images were analyzed with a ChemicDoc XRS photodocumentation system (Bio-Rad, Hercules, CA, USA) (18).
3.11. Transcriptional Expression of PPARα and SREBP-1c
The mRNA expression was measured with the pooled livers of each group; three extractions were carried out and analyzed in duplicate. Liver RNA was obtained using the Trizol method, and its quantity and quality were assessed using a spectrophotometer (Nanodrop™ Lite Thermo Scientific™). RNA was converted to cDNA using the iScript IV First-Strand cDNA Synthesis kit (ABP Biosciences, Rockville, MD, USA). The cDNA was used with the One Taq RT-PCR kit (NEW ENGLAND BioLabs® Inc., Ipswich, MA, USA) to determine the expression level of the genes for peroxisome proliferator-activated receptor alpha (PPARα) and sterol regulatory element binding protein (SREBP-1c). Glyceraldehyde 3-phosphate dehydrogenase was used as a housekeeping gene to normalize the mRNA levels of target gene expression. Primers were designed using the UGENE and NCBI Primer-BLAST programs (Table 1). A 2720 thermal cycler (Applied Biosystems™, Foster City, CA, USA) was used, and the running conditions included initial denaturation at 95°C for 30 seconds, followed by 35 cycles at 94°C for 15 seconds, 60°C for 35 seconds, and 72°C for 40 seconds.
| Genes | Forward Sequence 5'→3' | Reverse Sequence 5'→3' |
|---|---|---|
| PPARα | CCATCTGTCCTCTCTCCCCA | TGATGACAGAGCCCTCCGAG |
| SREBP-1c | AAGATGTACCCGTCCGTGCC | CAGGCTTGAGTACCCCAGCA |
| GAPDH | GCTGAGAATGGGAAGCTGGT | GGCGGAGATGATGACCCTTT |
Abbreviations: PPARα, peroxisome proliferator-activated receptor alpha; SREBP-1c, sterol regulatory element binding protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
3.12. Statistical Analysis
Evaluations were carried out at least in triplicate, and the data are reported as mean ± SD. The normality of data distribution was assessed by the Shapiro-Wilk Test. A one-way ANOVA was used, and the means were compared by Fisher’s Test (P ≤ 0.05). All analyses were carried out using StatGraphics Centurion XVI.I (Statistical Graphics Corporation™, USA).
4. Results
4.1. Flavonoid and Tannins Composition of the Echeveria subrigida Extracts
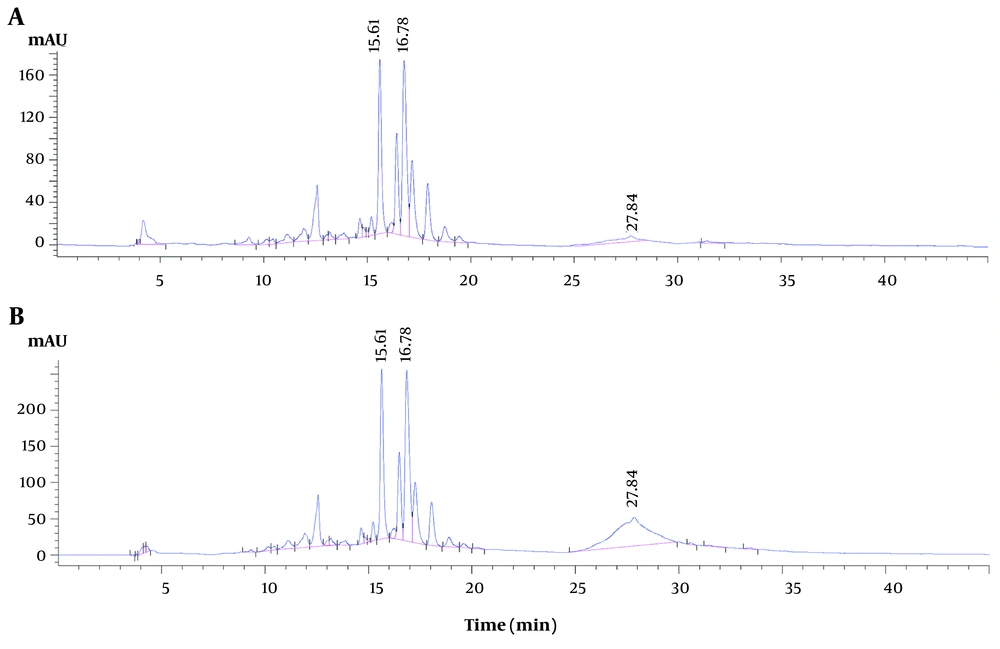
The contents of Q3G, I3G, and tannins (as equivalents of catechin) (mg per gram of dry extract) in the E. subrigida extracts were as follows: The EEEs, 3.30 ± 0.073, 4.76 ± 0.201, 2.70 ± 0.150; and MEEs, 5.01 ± 0.187, 6.67 ± 0.201, 46.36 ± 2.116. The peak purities of Q3G (999.36) and I3G (998.69) indicated good separation (> 990), while the peak of tannins resulted in a mixture of compounds with a value of 977.08 (Figure 1).
HPLC chromatogram of the ethanolic (A), and methanolic (B) extracts (EEEs and MEEs) of Echeveria subrigida. The compounds associated with the hypoglycemic effect (retention time and peak purity) are quercetin-3-O-glucoside (Q3G) (15.61 min, 999.36), isorhamnetin-3-O-glucoside (I3G) (16.78 min, 998.69), and tannins (27.84 min, 977.08).
4.2. Body Weight
The body weight gain of the diabetic rat groups (DC, MET, EEEs, MEEs, and ISO) over four weeks was lower than that of the HC group (Table 2). At the end of the experiment, the gain percentage of the diabetic groups (18% - 39%) was lower (P < 0.05) than that of the HC group (59%).
| Groups | Weeks of Treatment | ||||
|---|---|---|---|---|---|
| Initial | 1st | 2nd | 3rd | 4th | |
| HC | 244.63 ± 15.66 aD | 329.50 ± 17.18 aC | 357.00 ± 24.18 aB | 377.67 ± 23.77 aAB | 385.67 ± 28.38 aA |
| DC | 245.73 ± 14.43 aC | 288.83 ± 24.30 cdB | 288.00 ± 11.73 dB | 308.17 ± 15.56 cdA | 308.83 ± 11.65 cdA |
| MET | 243.00 ± 14.99 aC | 308.33 ± 19.04 bB | 324.00 ± 27.09 bAB | 339.83 ± 26.59 bA | 336.67 ± 31.21 bAB |
| ISO | 244.88 ± 4.97 aC | 274.00 ± 9.17 dB | 293.83 ± 22.12 cdA | 302.50 ± 12.55 cdA | 290.50 ± 16.40 dAB |
| EEEs | 244.50 ± 14.15 aD | 297.67 ± 5.57 bcC | 313.17 ± 7.39 bcB | 318.50 ± 6.25 bcAB | 325.17 ± 7.28 bcA |
| MEEs | 252.00 ± 12.39 aB | 285.50 ± 13.53 cdA | 296.83 ± 15.28 cdA | 296.33 ± 19.76 dA | 301.67 ± 22.03 cdA |
Abbreviations: HC, healthy control; DC, diabetic control; SD, standard deviation ; MET, metformin; ISO, isorhamnetin; EEEs, ethanol extract of Echeveria subrigida; MEEs, methanol extract of E. subrigida.
a Values are expressed as mean ± SD (n = 6).
b Different lowercase letters in the same column and uppercase letters in the same row represent significant differences (Fisher, P < 0.05).
c MET: Diabetic + MET (500 mg/kg b.w.); ISO: Diabetic + ISO (1.9 mg/kg b.w.); EEEs: Diabetic + EEEs (400 mg/kg b.w.); MEEs: Diabetic + MEEs (284.7 mg/kg b.w.).
4.3. Glucose Levels
The rats in the HC group maintained normal glucose levels (96.25 - 109.25 mg/dL) throughout the experiment. At the start of the experiment, DM2 rats had fasting glucose levels higher than 200 mg/dL (Table 3) and exhibited signs of polydipsia, polyphagia, and polyuria, meeting the criteria for inclusion in the study. All treatments, except for the DC and ISO groups, decreased glucose levels in the DM2 rats, with values for the MET and MEEs groups similar to those of the HC group during the experiment, whereas the EEEs group reached similar values by the fourth week (Table 3).
| Groups | Weeks of Treatment | ||||
|---|---|---|---|---|---|
| Initial | 1st | 2nd | 3rd | 4th | |
| HC | 102.63 ± 5.55 bB | 108.00 ± 5.73 dAB | 96.25 ± 7.23 cC | 105.63 ± 4.17 cAB | 109.25 ± 6.11 bA |
| DC | 275.25 ± 63.30 aBC | 254.13 ± 60.99 abC | 334.00 ± 69.90 aAB | 354.88 ± 44.60 aA | 315.53 ± 65.18 aAB |
| MET | 312.00 ± 50.25 aA | 161.75 ± 59.74 cdB | 163.38 ± 72.85 bcB | 147.50 ± 57.36 bcB | 183.75 ± 61.89 bB |
| ISO | 306.63 ± 53.54 aA | 314.88 ± 124.86 aA | 354.50 ± 121.90 aA | 313.63 ± 139.19 aA | 313.63 ± 139.19 aA |
| EEEs | 269.75 ± 24.01 aA | 218.25 ± 79.12 bcAB | 202.00 ± 39.84 bBC | 181.25 ± 59.50 bBC | 150.13 ± 39.04 bC |
| MEEs | 310.60 ± 46.10 aA | 174.20 ± 71.57 bcdB | 153.20 ± 31.55 bcB | 146.80 ± 44.91 bcB | 168.00 ± 48.10 bB |
Abbreviations: HC, healthy control; DC, diabetic control; SD, standard deviation ; MET, metformin; ISO, isorhamnetin; EEEs, ethanol extract of Echeveria subrigida; MEEs, methanol extract of E. subrigida.
a Values are expressed as mean ± SD (n = 6).
b Different lowercase letters in the same column and uppercase letters in the same row represent significant differences (Fisher, P < 0.05).
c MET: Diabetic + MET (500 mg/kg b.w.); ISO: Diabetic + ISO (1.9 mg/kg b.w.); EEEs: Diabetic + EEEs (400 mg/kg b.w.); MEEs: Diabetic + MEEs (284.7 mg/kg b.w.).
4.4. Glucose Tolerance Test
Rats in the HC, EEEs, and MEEs groups showed a hyperglycemic peak 30 minutes after the sucrose load (Table 4), and their glucose levels decreased, reaching statistically similar values after 120 minutes. At the same time, the MET group also showed a decrease in glucose levels but did not reach the levels of the HC group.
| Groups | Minutes | |||
|---|---|---|---|---|
| Initial | 30 | 60 | 120 | |
| HC | 101.75 ± 4.68 cBC | 116.75 ± 12.15 cA | 107.75 ± 10.11 cB | 97.75 ± 5.26 cC |
| DC | 331.43 ± 84.51 aAB | 352.44 ± 92.50 aA | 368.90 ± 127.45 aA | 227.22 ± 123.52 abB |
| MET | 201.67 ± 87.65 bAB | 230.83 ± 92.63 bAB | 308.22 ± 128.60 aA | 205.00 ± 82.96 abB |
| ISO | 315.00 ± 116.17 aA | 373.00 ± 152.47 aA | 376.75 ± 144.68 aA | 286.75 ± 162.18 aA |
| EEEs | 169.17 ± 70.62 bcAB | 226.92 ± 75.87 bA | 209.46 ± 83.63 bA | 148.50 ± 61.12 bcB |
| MEEs | 159.20 ± 25.39 bcA | 223.17 ± 59.70 bA | 191.33 ± 51.43 bcA | 181.67 ± 62.76 bcA |
Abbreviations: HC, healthy control; DC, diabetic control; SD, standard deviation ; MET, metformin; ISO, isorhamnetin; EEEs, ethanol extract of Echeveria subrigida; MEEs, methanol extract of E. subrigida.
a Values are expressed as mean ± SD (n = 6).
b Different lowercase letters in the same column and uppercase letters in the same row represent significant differences (Fisher, P < 0.05).
c MET: Diabetic + MET (500 mg/kg b.w.); ISO: Diabetic + ISO (1.9 mg/kg b.w.); EEEs: Diabetic + EEEs (400 mg/kg b.w.); MEEs: Diabetic + MEEs (284.7 mg/kg b.w.).
4.5. Leptin in Serum
At the end of the experiment, the serum leptin levels of the DM2 groups, except for MEEs (0.77 ng/mL), were lower than those of the HC group (0.71 ng/mL) (P < 0.05). The leptin levels of the EEEs group (0.650 ng/mL) were closest to that of the HC group (0.71 ng/mL). Remarkably, the Echeveria extracts (MEEs and EEEs) were more effective than MET in improving leptin levels (Table 5).
| Groups | Leptin (ng/mL) |
|---|---|
| HC | 0.71 ± 0.19 A |
| DC | 0.17 ± 0.07 D |
| MET | 0.32 ± 0.05 C |
| ISO | 0.38 ± 0.05 C |
| EEEs | 0.65 ± 0.06 B |
| MEEs | 0.77 ± 0.09 A |
Abbreviations: HC, healthy control; DC, diabetic control; SD, standard deviation ; MET, metformin; ISO, isorhamnetin; EEEs, ethanol extract of Echeveria subrigida; MEEs, methanol extract of E. subrigida.
a Values are expressed as mean ± SD (n = 6).
b Uppercase letters represent significant differences (Fisher, P < 0.05).
c MET: Diabetic + MET (500 mg/kg b.w.); ISO: Diabetic + ISO (1.9 mg/kg b.w.); EEEs: Diabetic + EEEs (400 mg/kg b.w.); MEEs: Diabetic + MEEs (284.7 mg/kg b.w.).
4.6. Pro- and Anti-inflammatory Molecules
Compared with the cytokine levels (IL-10, IL-17A, IFN-γ, IL-6, IL-4, and MCP-1) in the HC group, the levels of these molecules in the DC group increased significantly, except for IL-17A levels, which did not change (Table 6). In contrast, the cytokine levels in the Echeveria-treated groups (EEEs and MEEs) decreased significantly, except for MCP-1, whose level was similar to that of the DC group. The cytokine levels in the ISO group were significantly higher than those in the HC group, but MCP-1 levels were similar (Table 6). On the other hand, the cytokine levels of the HC and MET groups were statistically similar, except for those of MCP-1, which were lower in the MET group (Table 6).
| Groups | Cytokines (pg/mL) | |||||
|---|---|---|---|---|---|---|
| IL-10 | IL-17A | IFNƴ | IL-6 | IL-4 | MCP-1 | |
| HC | 47.77 ± 12.15 B | 4.60 ± 0.14 C | 2.72 ± 0.55 C | 18.00 ± 3.46 C | 9.74 ± 0.58 B | 82.01 ± 15.89 BC |
| DC | 72.26 ± 12.91 A | 4.50 ± 0.55 C | 4.56 ± 0.63 B | 36.72 ± 2.91 A | 16.34 ± 2.06 A | 131.66 ± 34.24 A |
| MET | 40.87 ± 10.80 BC | 6.51 ± 1.03 B | 2.59 ± 0.13 C | 16.29 ± 2.66 C | 9.25 ± 1.20 B | 32.96 ± 21.38 D |
| ISO | 72.21 ± 7.15 A | 14.27 ± 1.22 A | 6.18 ± 0.41 A | 28.12 ± 1.64 B | 15.70 ± 0.63 A | 70.44 ± 26.32 C |
| EEEs | 29.64 ± 2.86 C | 2.69 ± 0.82 D | 1.11 ± 0.24 D | 9.85 ± 2.92 D | 6.71 ± 1.15 C | 116.51.0 ± 31.18 AB |
| MEEs | 26.65 ± 5.31 C | 1.20 ± 0.60 E | 1.25 ± 0.28 D | 7.30 ± 1.89 D | 5.55 ± 0.43 C | 143.90 ± 33.85 A |
Abbreviations: HC, healthy control; DC, diabetic control; SD, standard deviation ; MET, metformin; ISO, isorhamnetin; EEEs, ethanol extract of Echeveria subrigida; MEEs, methanol extract of E. subrigida.
a Values are expressed as mean ± SD (n = 6).
b Different lowercase letters in the same column and uppercase letters in the same row represent significant differences (Fisher, P < 0.05).
c MET: Diabetic + MET (500 mg/kg b.w.); ISO: Diabetic + ISO (1.9 mg/kg b.w.); EEEs: Diabetic + EEEs (400 mg/kg b.w.); MEEs: Diabetic + MEEs (284.7 mg/kg b.w.).
4.7. Histology Analysis
4.7.1. Pancreas Tissue
Streptozotocin treatment damages pancreatic cells and induces diabetes. The pancreas slices of the HC and DM2 (DC, MET, ISO, EEEs, and MEEs) groups showed normal acinar cells. However, the Langerhans islets of the DM2 groups showed hypertrophy, characterized by irregular islet contours and smaller sizes than those of the HC group (Figure 2).
Histological analysis of pancreas from healthy and diabetic rats. Optical micrographs of pancreas sections from the different study groups stained with hematoxylin and eosin (40X). Diabetic + metformin (MET) [500 mg/kg b.w.]; diabetic + isorhamnetin (ISO) [1.9 mg/kg b.w.]; diabetic + ethanol extract of Echeveria subrigida (EEEs) [400 mg/kg b.w.]; diabetic + methanol extract of E. subrigida (MEEs) [284.7 mg/kg b.w.]. Islets of langerhans (arrows); acinar cells (delimited by the circle). HC, healthy control; DC, diabetic control.
4.7.2. Liver Tissue
The hepatic parenchyma of all groups showed normal structure and absence of steatosis; the size and appearance of the central veins and hepatic sinusoids were similar among groups. However, the sinusoids in the ISO group were dilated (Figure 3).
Histological analysis of liver from healthy and diabetic rats. Optical micrographs of liver sections from the different study groups stained with hematoxylin and eosin (40X). Diabetic + metformin (MET) [500 mg/kg b.w.]; diabetic + isorhamnetin (ISO) [1.9 mg/kg b.w.]; diabetic + ethanol extract of Echeveria subrigida (EEEs) [400 mg/kg b.w.]; diabetic + methanol extract of E. subrigida (MEEs) [284.7 mg/kg b.w.]. Sinusoids (arrows); liver parenchyma (delimited by the circle). HC, healthy control; DC, diabetic control; CV, central vein.
4.8. Effect of the Echeveria Extracts on the PI3K Pathway in the Liver
The Western blot of hepatic protein extracts from all groups detected Akt, whereas the activated phosphorylated form (p-Akt) was present only in the ISO, EEEs, and MEEs groups. In the signaling cascade of Akt, p-Akt inactivates AS160 by phosphorylation (p-AS160). All groups expressed AS160 and p-AS160, but bands were better observed in the DC, MET, and ISO groups (Figure 4).
Western blot analysis of protein phosphorylation levels of the PI3K and AMPK signaling pathway in livers from the different study groups. Diabetic + metformin (MET) [500 mg/kg b.w.]; diabetic + isorhamnetin (ISO) [1.9 mg/kg b.w.]; diabetic + ethanol extract of Echeveria subrigida (EEEs) [400 mg/kg b.w.]; diabetic + methanol extract of E. subrigida (MEEs) [284.7 mg/kg b.w.]. HC, healthy control; DC, diabetic control.
4.9. Effect of the Echeveria Extracts on the AMPK Pathway Activation in the Liver
All groups of rats expressed AMPK, with higher levels observed in the DC, MET, and ISO groups. The activated phosphorylated form (p-AMPK) was detected in the HC, DC, MET, and ISO groups but not in those treated with Echeveria extracts (EEEs and MEEs) (Figure 4).
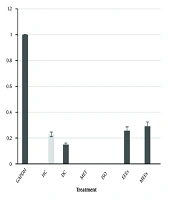
4.10. Effect of the Echeveria Extracts on the Transcriptional Expression of SREBP-1c and PPARα in the Liver
The transcription factor SREBP-1c activates the expression of glycolytic and lipogenic enzymes. The liver samples of the DC, EEEs, and MEEs groups expressed the SREBP-1c transcript (Figure 5). On the other hand, the transcription factor PPARα regulates peroxisomal fatty acid β-oxidation, glucose metabolism, and insulin sensitivity. PPARα was only detected in the HC group (Figure 5).
A, gene expression analysis of SREBP-1c and PPARα by RT-PCR; B, relative expression of SREBP-1c and PPARα. Diabetic + metformin (MET) [500 mg/kg b.w.]; diabetic + isorhamnetin (ISO) [1.9 mg/kg b.w.]; diabetic + ethanol extract of Echeveria subrigida (EEEs) [400 mg/kg b.w.]; diabetic + methanol extract of E. subrigida (MEEs) [284.7 mg/kg b.w.]. M, marker; NC, negative control; HC, healthy control; DC, diabetic control.
5. Discussion
5.1. Body Weight
Obesity is a risk factor for developing DM2, but patients often lose weight after the disease is established (19). Therefore, body weight control is crucial in the management of DM2. Metformin is a first-line treatment for DM2; it increases insulin sensitivity in peripheral tissues and decreases hyperinsulinism, hyperglycemia, and adipose tissue exposure to insulin. Consequently, MET contributes to body weight control in DM2 patients (20). These effects were observed in the present study. The body weight of the DM2 groups (DC, MET, ISO, EEEs, and MEEs) was significantly lower than that of the HC group. The mean value of the MET group was statistically higher than those of other DM2 groups but was similar to that of the EEEs group (Table 2). These results agree with the adaptogenic activity reported for the hydroalcoholic extract of E. subrigida in normoglycemic mice; the body weight of treated animals was lower than that of the stressed ones without changing the glucose levels (14).
5.2. Glucose Levels
The rats in the MET and MEEs groups significantly decreased their glucose levels within the first week of treatment (Table 3); these treatments showed the lowest values throughout the study. However, the glucose values for the MEEs and EEEs groups were statistically similar throughout the experiment. The glycemic control observed in the MET group aligns with previous reports. Mice treated with 400 mg/kg body weight (b.w.) of MET showed decreased hyperglycemia in the glucose tolerance test, an effect associated with decreased intestinal absorption (21). Additionally, mice treated with pharmacologic concentrations of MET (75 μM) showed reduced gluconeogenesis and increased ATP production (22). Echeveriasubrigida contains compounds with α-glucosidase inhibitory activity, such as I3G, Q3G, and proanthocyanidins, which could decrease intestinal glucose absorption (13). Furthermore, Heredia-Mercado et al. (15) reported the antihyperglycemic effect of EEEs in normoglycemic mice. The effects of the mentioned compounds have been associated with different mechanisms. Persimmon tannins inhibit α-glucosidase (IC50 = 0.35 mg/mL) and α-amylase (IC50 = 0.24 mg/mL), and at 0.20 μg/mL, they decrease glucose absorption by Caco-2 cells, likely through interaction with the SGLT1 and GLUT2 transporters (23). Quercetin (0.1 and 10 nM) and ISO (10 nM) increase glucose assimilation by L6 myotubes through increased GLUT4 translocation, similar to the positive control insulin (100 nM) (18). In pancreatic β-cells (INS-1), Q3G more effectively induces insulin release than I3G (24). In the present study, the DC and ISO groups showed the highest glucose levels throughout the experiment (Table 3). In contrast, Matboli et al. (25) reported that Wistar rats treated with ISO (40 mg/kg b.w.) under fasting conditions showed lower levels of glucose, insulin, and HOMA-IR. This effect was not observed in the present study, which may be due to the lower concentration of ISO used (1.9 mg/kg b.w.); this dose was selected to match the I3G concentration in the EEEs extract that showed in vivo hypoglycemic activity. At the end of the experiment, the MET, EEEs, and MEEs groups reached glycemic levels similar to those of the HC group, corroborating the hypoglycemic effect of the E. subrigida extracts. These results indicate that EEEs and MEEs contain, in addition to I3G, other compounds with additive or synergistic hypoglycemic activity. Methanolic had a better effect on glycemic control than EEEs, and this extract also had higher tannin content, suggesting these compounds may be contributing to the hypoglycemic effect.
5.3. Glucose Tolerance Test
This assay corroborated the antihyperglycemic effect of EEEs and MEEs, which showed patterns similar to those of the MET group (Table 4). The antihyperglycemic effect of EEEs was also observed in normoglycemic mice (15). In fasted mice with STZ-induced diabetes, treatment with a flavonoid-rich W. sinensis extract decreased glucose levels and increased glucose tolerance, showing a lower area under the curve (AUC) than mice in the DC group (9).
5.4. Leptin in Serum
The hormone leptin stimulates the sensation of satiety, and DM2 patients show lower leptin levels (7). Rats in the DM2 groups, except for MEEs, showed lower leptin levels than those in the HC group. All treatments increased leptin concentration compared with the DC group, and this effect was significantly higher in the EEEs and MEEs groups (Table 5). Therefore, treatments with Echeveria extracts contribute to body weight control. Different studies have related high leptin levels to the benefits of controlling obesity and diabetes. Mice on a high-fat diet showed hyperglycemia, hyperinsulinism, increased gluconeogenesis, and decreased leptin levels; treatment with 50 mg/kg body weight (b.w.) of MET improved these conditions (22). Lipodystrophic and obese mice (ob/ob) showed hyperinsulinism and increased levels of SREBP-1c, enzymes of fatty acid synthesis, and gluconeogenesis; leptin treatment helped reduce these negative changes (8). The same authors reported that rats with STZ-induced diabetes showed lower leptin levels, which increased with insulin treatment (8).
5.5. Anti- and Pro-inflammatory Molecules
Hyperglycemia leads to the accumulation of glyceraldehyde 3-phosphate in the liver, which can be converted to diacylglycerol (DAG). The DAG activates protein kinase C (PKC), known for its proinflammatory effects, including the activation of nuclear factor kappa B (NF-κB). Moreover, TNF-α also activates NF-κB, which increases the expression of the proinflammatory cytokine IL-6, stimulating the recruitment of macrophages (4, 6). Damaged hepatocytes activate Kupffer cells, inducing the release of proinflammatory molecules [cytokines (e.g., TNF-α, IL-6, TGF-β) and chemokines (e.g., MCP-1)]. Most pancreatic cells increase NF-κB production, leading to the transcription of proinflammatory cytokine and chemokine genes. These inflammatory conditions contribute to insulin resistance and diabetes (4, 6). In this study, rats in the DC group showed increased levels of most proinflammatory and anti-inflammatory (IL-4 and IL-10) cytokines (Table 6). This apparent contradiction is supported by a meta-analysis showing that most patients with DM2 had increased IL-10 levels (26). In another study, DM2 patients presented high levels of both proinflammatory (IL-6, IL-12, and TNF-α) and anti-inflammatory cytokines (IL-4 and IL-10) (27). Patients with DM2 showed hypo-responsiveness to IL-10. This phenomenon was corroborated by experiments on macrophages exposed to high glucose concentration (15 mM), where IL-10 treatment was less effective in inhibiting LPS-induced TNF-α production, possibly due to impaired STAT3 activation by IL-10 (28). The levels of TNF-α and IL-10 in the kidneys and sciatic nerves of diabetic rats were higher compared with non-DC rats. These effects were mitigated in diabetic rats treated with curcumin or captopril and were associated with the inhibition of the angiotensin-converting enzyme (ACE) (29). Obese and alloxan-induced diabetic rats showed higher TNF-α and IL-10 levels compared to control rats; treatment with garlic reduced TNF-α levels in both obese (20%) and diabetic rats (18.3%), and it also increased IL-10 levels in obese rats but failed to increase or maintain IL-10 levels in diabetic rats, probably due to impaired STAT3 activation by IL-10 (30). This research demonstrates that MET and Echeveria (EEEs and MEEs) treatments reversed the increased cytokine levels observed in DC rats, with Echeveria extracts showing the best results (Table 6). Previous research showed that the hydroalcoholic extract of E. subrigida increases mice splenocyte proliferation and inhibits oxidative stress induced by H2O2 in Saccharomyces cerevisiae (14), supporting the results of the present study.
5.6. Histological Analysis of Liver and Pancreas Tissues
The pancreas secretes insulin and glucagon, essential hormones for carbohydrate metabolism. Treating animals with STZ damages the pancreatic β-cells and induces diabetes (31). Diabetic rats on a high-fat diet show damaged pancreatic islets of the β-cells, and insulin immunoreactivity is lost. Treatment of these rats with a W. sinensis extract improved pancreas structure (9). In this study, the pancreas of rats treated with EEEs, MEEs, and ISO showed Langerhans islets of irregular size and shape, indicating no tissue regeneration effect (Figure 2).
In patients with untreated DM2, the liver develops progressive damage, including non-alcoholic hepatic steatosis, due to the accumulation of fat vacuoles in the hepatocyte cytoplasm (32). In this research, the liver slices showed neither steatosis nor morphologic changes due to DM2 progression, likely because of the short time after DM2 establishment (Figure 3). In this regard, mice fed a high-fat diet for four weeks and with diabetes induced by STZ showed steatosis that decreased after treatment with the W. sinensis extract (9).
5.7. Activation of the PI3K and AMPK Pathways in the Liver
The liver is essential in lipid, carbohydrate, and protein metabolism, processes regulated by kinases such as PI3K and AMPK (4, 33). AMPK is a cellular energy sensor that activates metabolic processes to produce energy while inactivating those that require energy (34). The activity of this kinase depends on the evaluated compound and metabolic context (35). For instance, treatment of primary hepatocytes with 75 μM MET increases mitochondrial density and respiratory rate by activating AMPK. Moreover, AMPK mutant mice fed a high-fat diet are MET-resistant (22). As expected, this study showed that AMPK phosphorylation was induced by MET (Figure 4). On the other hand, diabetic rats showed higher AMPK levels and lower p-AMPK levels than healthy rats. In this regard, treating diabetic rats with an extract of Psidium guajava leaves increased AMPK levels, suggesting the importance of this pathway in controlling diabetes (36). The treatments with EEEs and MEEs did not induce AMPK phosphorylation (Figure 4), indicating that the antidiabetic effect of Echeveria extracts is associated with another metabolic pathway. The results showed that Akt was phosphorylated (Figure 4), suggesting the activation of the PI3K/Akt pathway. The p-Akt protein regulates many metabolic processes. For example, many natural products activate the PI3K/Akt pathway in the liver, increasing the synthesis of fatty acids and glycogen and decreasing gluconeogenesis and glycogenolysis. These changes are mediated by the activation of mTORC1, SREBP1c, and AS160 and the inhibition of FOXO1 and GSK3, as reviewed by Savova et al. (35). The antidiabetic effect of the W. sinensis extract in rats is mediated by the PI3K/Akt pathway (9). The adipose tissue of rats with diabetes induced by STZ showed decreased levels of the Akt2 transcript, and treatment with 40 mg/kg body weight (b.w.) of ISO increased the levels of this transcript (25). In L6 myotubes treated with ISO (1 and 10 nM), the increased translocation of GLUT4 was exclusively associated with the PI3K pathway (18). This result agrees with the increase in insulin delivery mediated by the PI3K/Akt pathway, induced by I3G but not by quercetin in INS-1 cells (24). On the other hand, the antidiabetic effect of P. guajava leaves was associated with increased protein levels of PI3K, p-Akt, and GLUT2 (36). Therefore, this study shows that the hyperglycemia control in diabetic rats by Echeveria extracts (EEEs and MEEs) is associated with the PI3K/Akt pathway.
5.8. Analysis of the Transcriptional Expression of SREBP-1c and PPARα in the Liver
The expression analysis showed that the treatment of diabetic rats with EEEs and MEEs activated the PI3K/Akt pathway (Figure 5). The Akt protein regulates the expression of the transcription factor SREBP-1c and promotes the expression of glycolytic and lipogenic enzymes (35). Reports on SREBP-1c expression have shown contrasting results (8, 37). In rats with STZ-induced diabetes, the mRNA levels of SREBP-1c decreased simultaneously with insulin levels, and the mRNA levels of glucokinase and fatty acid synthase were also reduced (8). Concurrently, there was an increase in transcript levels of gluconeogenic enzymes, such as glucose 6-phosphatase, fructose 1, 6-bisphosphatase, and phosphoenolpyruvate carboxykinase (8). Conversely, increased SREBP-1c levels were found in diabetic mice (37). This study showed the Akt protein expression in the rats of the DC group but not its phosphorylation. Despite this, the transcriptional expression of SREBP-1c in these animals indicates that hyperglycemia leads to hyperinsulinism and activation of fatty acid synthesis (35).
In the liver, the PGC1α/PPARα signaling pathway depends on the PI3K/Akt pathway and responds to changes in the feeding-fasting cycle. During fasting, PGC1α/PPARα activates the genes involved in fatty acid transport and β-oxidation (35). The function of PPARα was demonstrated in fasted PPARα-null mice, which showed lipid accumulation in the liver and impairments in gluconeogenesis and β-oxidation (38). Moreover, mice fed a high-fat diet showed higher transcriptional and translational expression of liver PPARα. Therefore, PPARα expression should be observed in groups where SREBP-1c is not expressed; however, PPARα was only detected in the HC group because they were fasting and needed to use their energy reserves (38). The absence of PPARα expression in the EEEs and MEEs groups could be due to their antidiabetic effects not being mediated by the AMPK pathway (39).
5.9. Conclusions
The extracts of E. subrigida (EEEs and MEEs) exhibited antidiabetic activity in rats, effectively limiting body weight gain and reducing hyperglycemia. The results indicate that EEEs and MEEs inhibit gluconeogenesis and glycogenolysis while promoting fatty acid synthesis in the liver. These effects are likely due to a reduction in pro-inflammatory cytokine serum levels and the positive regulation of the PI3K/Akt pathway in the liver, which affects the transcriptional expression of SREBP-1c and PPARα. Consequently, extracts of E. subrigida may be developed into food supplements or pharmaceutical preparations for the prevention or treatment of obesity and DM2.
![Histological analysis of pancreas from healthy and diabetic rats. Optical micrographs of pancreas sections from the different study groups stained with hematoxylin and eosin (40X). Diabetic + metformin (MET) [500 mg/kg b.w.]; diabetic + isorhamnetin (ISO) [1.9 mg/kg b.w.]; diabetic + ethanol extract of <i>Echeveria subrigida</i> (EEEs) [400 mg/kg b.w.]; diabetic + methanol extract of <i>E. subrigida</i> (MEEs) [284.7 mg/kg b.w.]. Islets of langerhans (arrows); acinar cells (delimited by the circle). HC, healthy control; DC, diabetic control. Histological analysis of pancreas from healthy and diabetic rats. Optical micrographs of pancreas sections from the different study groups stained with hematoxylin and eosin (40X). Diabetic + metformin (MET) [500 mg/kg b.w.]; diabetic + isorhamnetin (ISO) [1.9 mg/kg b.w.]; diabetic + ethanol extract of <i>Echeveria subrigida</i> (EEEs) [400 mg/kg b.w.]; diabetic + methanol extract of <i>E. subrigida</i> (MEEs) [284.7 mg/kg b.w.]. Islets of langerhans (arrows); acinar cells (delimited by the circle). HC, healthy control; DC, diabetic control.](https://services.brieflands.com/cdn/serve/3170b/e74ad5c3aadb66cc00e2545be4ca7eba6bed046b/jjnpp-158548-g001-F2-preview.webp)
![Histological analysis of liver from healthy and diabetic rats. Optical micrographs of liver sections from the different study groups stained with hematoxylin and eosin (40X). Diabetic + metformin (MET) [500 mg/kg b.w.]; diabetic + isorhamnetin (ISO) [1.9 mg/kg b.w.]; diabetic + ethanol extract of <i>Echeveria subrigida</i> (EEEs) [400 mg/kg b.w.]; diabetic + methanol extract of <i>E. subrigida</i> (MEEs) [284.7 mg/kg b.w.]. Sinusoids (arrows); liver parenchyma (delimited by the circle). HC, healthy control; DC, diabetic control; CV, central vein. Histological analysis of liver from healthy and diabetic rats. Optical micrographs of liver sections from the different study groups stained with hematoxylin and eosin (40X). Diabetic + metformin (MET) [500 mg/kg b.w.]; diabetic + isorhamnetin (ISO) [1.9 mg/kg b.w.]; diabetic + ethanol extract of <i>Echeveria subrigida</i> (EEEs) [400 mg/kg b.w.]; diabetic + methanol extract of <i>E. subrigida</i> (MEEs) [284.7 mg/kg b.w.]. Sinusoids (arrows); liver parenchyma (delimited by the circle). HC, healthy control; DC, diabetic control; CV, central vein.](https://services.brieflands.com/cdn/serve/3170b/c761ee43444004a0ab414c2f47684f181a714fb0/jjnpp-158548-g002-F3-preview.webp)
![Western blot analysis of protein phosphorylation levels of the PI3K and AMPK signaling pathway in livers from the different study groups. Diabetic + metformin (MET) [500 mg/kg b.w.]; diabetic + isorhamnetin (ISO) [1.9 mg/kg b.w.]; diabetic + ethanol extract of <i>Echeveria subrigida</i> (EEEs) [400 mg/kg b.w.]; diabetic + methanol extract of <i>E. subrigida</i> (MEEs) [284.7 mg/kg b.w.]. HC, healthy control; DC, diabetic control. Western blot analysis of protein phosphorylation levels of the PI3K and AMPK signaling pathway in livers from the different study groups. Diabetic + metformin (MET) [500 mg/kg b.w.]; diabetic + isorhamnetin (ISO) [1.9 mg/kg b.w.]; diabetic + ethanol extract of <i>Echeveria subrigida</i> (EEEs) [400 mg/kg b.w.]; diabetic + methanol extract of <i>E. subrigida</i> (MEEs) [284.7 mg/kg b.w.]. HC, healthy control; DC, diabetic control.](https://services.brieflands.com/cdn/serve/3170b/9b8c0b94742e5c6003f93fe0cc0359b1f7bc1d3e/jjnpp-158548-i002-F4-preview.webp)
![A, gene expression analysis of SREBP-1c and PPARα by RT-PCR; B, relative expression of SREBP-1c and PPARα. Diabetic + metformin (MET) [500 mg/kg b.w.]; diabetic + isorhamnetin (ISO) [1.9 mg/kg b.w.]; diabetic + ethanol extract of <i>Echeveria subrigida</i> (EEEs) [400 mg/kg b.w.]; diabetic + methanol extract of <i>E. subrigida</i> (MEEs) [284.7 mg/kg b.w.]. M, marker; NC, negative control; HC, healthy control; DC, diabetic control. A, gene expression analysis of SREBP-1c and PPARα by RT-PCR; B, relative expression of SREBP-1c and PPARα. Diabetic + metformin (MET) [500 mg/kg b.w.]; diabetic + isorhamnetin (ISO) [1.9 mg/kg b.w.]; diabetic + ethanol extract of <i>Echeveria subrigida</i> (EEEs) [400 mg/kg b.w.]; diabetic + methanol extract of <i>E. subrigida</i> (MEEs) [284.7 mg/kg b.w.]. M, marker; NC, negative control; HC, healthy control; DC, diabetic control.](https://services.brieflands.com/cdn/serve/3170b/b9daf3feb167df9746935405ee8f2ba0170a161c/jjnpp-158548-i003-F5-preview.webp)