1. Background
Inflammation is a natural defense mechanism the body uses to repair damage and is found in various diseases, such as diabetes, obesity, and cancer (1). During inflammation, changes in substances called cytokines and interleukins lead to symptoms like redness, swelling, itching, pain, and an increase in temperature at the affected area (2). For centuries, traditional medicine has used natural sources to reduce inflammation, with lichens being one of these important sources (3). Lichens are the result of a symbiotic relationship between two or more species, where both benefit from the interaction (4). They consist of a photosynthetic component, typically a green alga or cyanobacterium, encased in fungal threads (5). The photosynthetic part, called the photobiont, produces nutrients through photosynthesis, while the fungus, known as the mycobiont, provides structural support and protection (6).
According to Bergi’s classification (2020), around 20,000 species have been identified, many of which appear dry and crust-like. Lacking roots, stems, or leaves, their body, known as the thallus, is formed by tightly packed fungal threads, with algae typically located near the top (7). These organisms produce a diverse array of primary metabolites, including proteins, amino acids (e.g., alanine, glutamic acid, taurine), polyols (e.g., glycerol, erythritol, ribitol), polysaccharides (e.g., glucose, sucrose, trehalose), and vitamins. They also produce secondary metabolites, including carotenoids, depsides, depsidones, benzyl esters, and phenolic acid derivatives (8). These substances accumulate on the surface of the fungal threads, contributing to their unique biological properties (9).
Valued for their broad applications, these organisms are important in medicine, food production, animal care, and dyeing industries (10). The compounds they produce have shown antibacterial, antiviral, anti-inflammatory, pain-relieving, cancer-fighting, fever-reducing, and antioxidant effects (11). Further research could reveal additional medical and industrial applications of these diverse compounds. With their diverse properties, further assessment could help unlock their full potential. In Khuzestan province, where research on local species is limited, this study was conducted to analyze and compare the polyphenolic content of several species. The primary objective was to evaluate the anti-inflammatory effects of the species with the highest polyphenolic content.
2. Objectives
The present study aimed to investigate the anti-inflammatory effects of lichen extracts at different doses and compare their efficacy with indomethacin in reducing inflammation in a carrageenan-induced rat model.
3. Methods
3.1. Sample Collection
Lichen samples of Squamarina cartilaginea, Diploschistes diacapsis, and Xalocoa ocellata were collected from regions in the north of Khuzestan. The samples were cleaned, crushed, and stored in a refrigerator until the extraction process. The dried powder of the lichens was separately extracted using the maceration method with acetone as the solvent. Extraction was carried out at least three times, each time with a 48-hour interval. Given the low yield and the volatile nature of acetone at ambient temperature, the acetone solvent was evaporated under a laminar hood, and the final dry extract was stored in the refrigerator at 4°C until the start of the experiments.
3.2. Folin-Ciocalteu Reagent
The Folin-Ciocalteu reagent (FCR) was used to determine the phenolic content of the extracts. Gallic acid served as the standard polyphenol compound for the calibration curve. For this, one milliliter of different concentrations of gallic acid solution (25, 50, 75, 100, 125, and 150 mg/L) was mixed with five milliliters of 10% FCR and incubated at room temperature (25°C). After 10 minutes, four milliliters of a 7.5% sodium bicarbonate solution were added, and the mixture was incubated for 60 minutes in the dark at room temperature. The absorbance of each sample was measured at 760 nm using a UV spectrophotometer, with the device calibrated to zero using a blank. This experiment was repeated three times for each dilution, and based on the data obtained, a calibration curve for gallic acid (linear absorbance vs. concentration) was constructed. This method was also applied to the extracts. Finally, the phenolic content was expressed as milligrams of gallic acid equivalent per gram of dry extract (12).
3.3. Assessment of Anti-inflammatory Effect
Among the lichens, X. ocellata, which contains the highest amount of polyphenolic compounds (as determined in the previous experiment), was selected to assess its anti-inflammatory activity. In this study, male albino Wistar rats weighing between 200 to 250 grams were used. The animals were sourced from the Pasteur Institute of Iran. They were housed under standard conditions, including a temperature of 22°C, humidity of 50 - 60%, and a 12-hour light/dark cycle. The animals were randomly selected and divided into five groups of six rats each. Groups one to three received doses of 20, 40, and 80 mg/kg of the extract, respectively. The fourth group, as the positive control, received the non-steroidal anti-inflammatory drug (NSAID), indomethacin, at a dose of 10 mg/kg. The fifth group, as the negative control, received the same volume of the vehicle (normal saline). All groups received their respective treatments before the injection of the inflammatory agent. A 30-minute interval was maintained between treatment administration and carrageenan injection.
To measure the anti-inflammatory effect of the extract, a 1% (w/v) carrageenan solution dissolved in 0.9% normal saline was used to induce local edema. The volume of the rats’ paws was measured using a plethysmometer. The paw volume of all groups was measured immediately before the carrageenan injection, and this value was considered the initial volume at time zero. Then, each of the five groups received 100 µL of 1% carrageenan through intraplantar injection, and the induced edema was measured by the change in paw volume at 1, 2, 3, 4, and 5 hours after injection. The results of paw edema were expressed as the change in paw volume at each time point relative to the initial volume at time zero.
3.4. Measurement of IL-6
Levels of the pro-inflammatory cytokine IL-6 in blood serum were measured using commercially available rat ELISA kits according to the manufacturer's instructions (13).
3.5. Measurement of Malondialdehyde
Malondialdehyde (MDA) levels were determined using the thiobarbituric acid reactive substances (TBARS) assay as previously described (14). Briefly, 100 µL of serum was mixed thoroughly with 200 µL of 20% (w/v) trichloroacetic acid (TCA) and 100 µL of 0.375% (w/v) thiobarbituric acid (TBA). The mixture was incubated in a boiling water bath for 15 minutes. After cooling to room temperature, the samples were centrifuged at 5000 rpm for 10 minutes to remove precipitates. The absorbance of the clear supernatant was measured at 532 nm using a spectrophotometer (BioTek SP2, USA). The MDA concentrations were quantified by comparing the absorbance values to a standard curve generated using 1,1,3,3-tetramethoxypropane (TMP) as the MDA equivalent standard. Results were expressed as nmol/mL of serum (15, 16).
4. Results
4.1. Phenolic Content Analysis
A calibration curve was constructed using gallic acid as the standard, with concentrations of 12.5, 25, 50, 75, and 100 mg/mL. The resulting linear equation had a coefficient of determination (R2 = 0.9816). Using this curve, the total phenolic content (TPC) of three samples was measured and expressed in milligrams of gallic acid equivalents per gram of extract (mg GAE/g). The results indicated that the lichen species X. ocellata exhibited the highest phenolic content (116.42 mg GAE/g extract), followed by D. diacapsis (100.63 mg GAE/g extract) and S. cartilaginea (83.8 mg GAE/g extract). Consequently, further experiments were conducted on X. ocellata due to its higher phenolic content.
4.2. Assessment of Paw Volume Changes
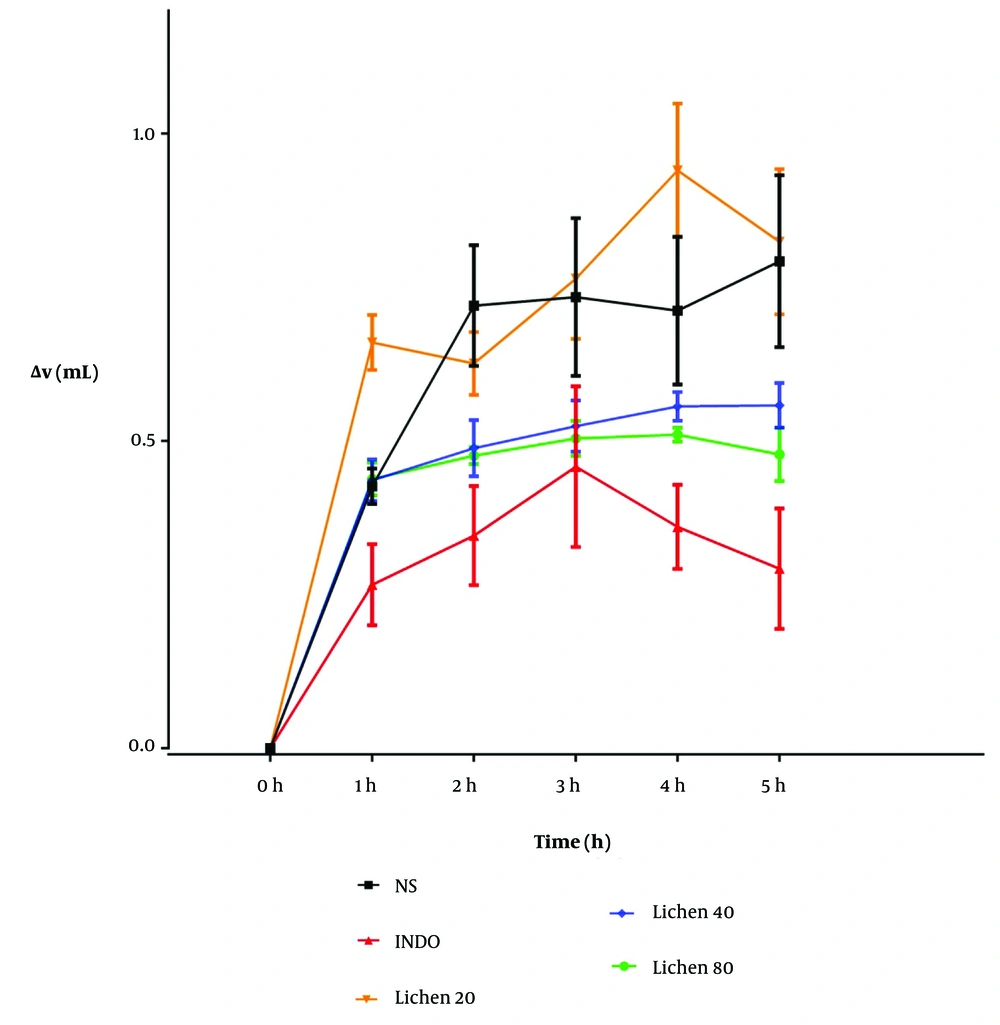
After preparing and processing the X. ocellata extract, various doses of the extract, indomethacin, and physiological saline were administered intraperitoneally to groups of six male rats. Thirty minutes after the injections, a 1% carrageenan solution was subcutaneously injected into the plantar region of the rats. Paw volume changes were measured using a plethysmometer at 1, 2, 3, 4, and 5 hours post-injection (Table 1 and Figure 1). The zero time point in Figure 1 corresponds to the time of carrageenan injection. Changes in paw volume (mL) were recorded at hours 1 - 5 following subcutaneous carrageenan injection, after prior intraperitoneal administration of indomethacin (10 mg/kg), the extract (20, 40, and 80 mg/kg), and physiological saline (5 mL/kg) (Table 1 and Figure 1).
| Hour | Normal Saline | Indomethacin | Xalocoa ocellata (20 mg/kg) | Xalocoa ocellata (40 mg/kg) | Xalocoa ocellata (80 mg/kg) |
|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0.426 ± 0.029 | 0.266 ± 0.066 | 0.66 ± 0.044 a | 0.436 ± 0.034 | 0.438 ± 0.026 |
| 2 | 0.720 ± 0.098 | 0.346 ± 0.080 b | 0.626 ± 0.051 | 0.488 ± 0.045 | 0.476 ± 0.014 |
| 3 | 0.734 ± 0.128 | 0.458 ± 0.130 | 0.764 ± 0.098 | 0.524 ± 0.041 | 0.504 ± 0.028 |
| 4 | 0.712 ± 0.120 | 0.360 ± 0.068 b | 0.94 ± 0.110 c | 0.556 ± 0.023 d | 0.510 ± 0.011 e |
| 5 | 0.792 ± 0.140 | 0.292 ± 0.097 | 0.824 ± 0.120 c | 0.558 ± 0.036 | 0.478 ± 0.043 |
a Significant difference compared to the indomethacin group.
b Significant difference compared to the normal saline group (* P < 0.01).
c P < 0.001.
d Significant difference between the groups receiving 20 mg/kg and 40 mg/kg of the extract.
e Significant difference between the groups receiving 20 mg/kg and 80 mg/kg of the extract.
The anti-inflammatory effects of the extract of Xalocoa ocellata at doses of 20, 40, and 80 mg/kg were compared with groups receiving physiological saline (5 mL/kg) and indomethacin (10 mg/kg), administered intraperitoneally in a carrageenan-induced inflammation model (n = 6). Abbreviations: NS, normal saline; INDO, indomethacin; Lichen, X. ocellata extract.
4.3. Assessment of Malondialdehyde Levels
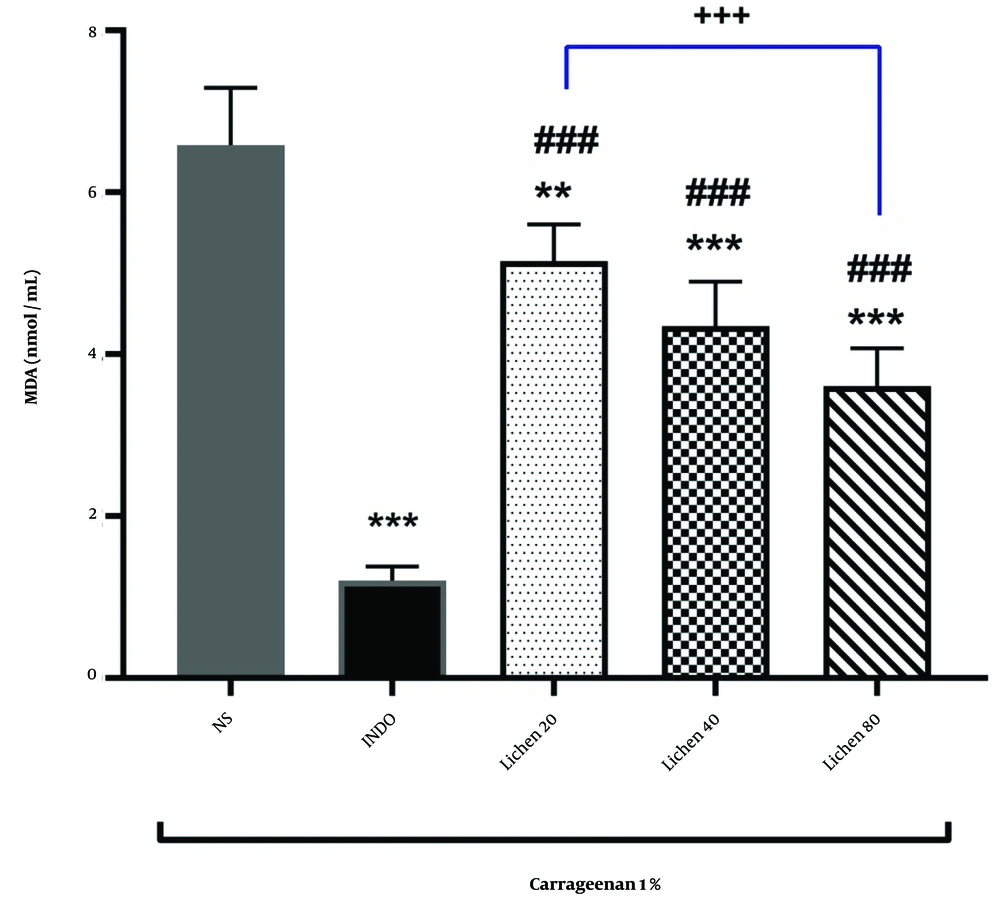
The results of MDA measurements in different groups are presented in Figure 2. The findings indicated that indomethacin administration significantly reduced MDA levels compared to the normal saline group (P < 0.001). Administration of the extract of X. ocellata at doses of 20, 40, and 80 mg/kg also led to a significant reduction in MDA levels in rat paw blood serum compared to the normal saline group. This reduction highlights the extract’s effect in mitigating oxidative damage induced by carrageenan injection. The decrease in MDA levels was statistically significant at all extract doses (P < 0.01 and P < 0.001, respectively). Furthermore, MDA levels in the groups receiving 20, 40, and 80 mg/kg of the extract of X. ocellata were significantly different from the indomethacin group (P < 0.001). Additionally, a significant difference was observed between the group receiving 20 mg/kg of the extract and the group receiving 80 mg/kg of the extract (P < 0.001).
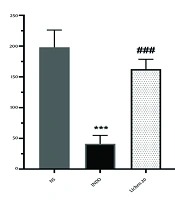
4.4. Assessment of IL-6 Levels
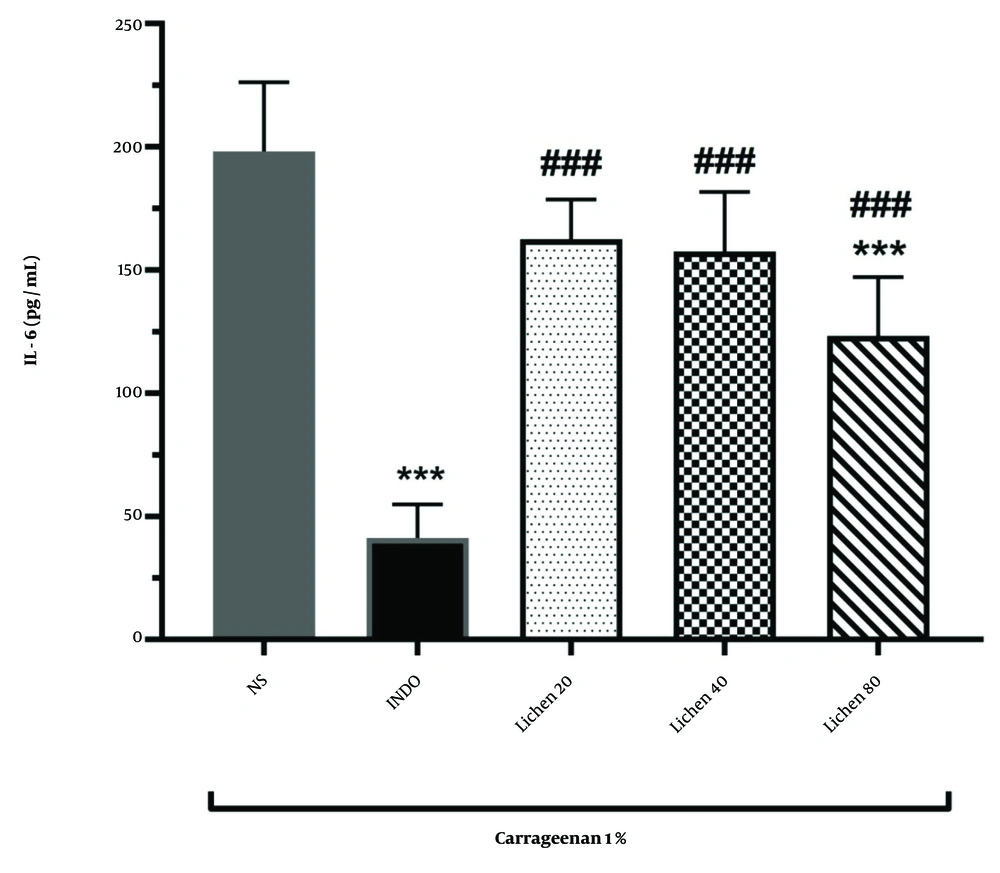
The results of IL-6 measurements in different groups are depicted in Figure 3. The findings showed that administration of indomethacin significantly reduced IL-6 levels compared to the normal saline group (P < 0.001). Administration of the extract of X. ocellata at a dose of 80 mg/kg significantly decreased IL-6 levels in rat paw blood serum compared to the normal saline group, indicating the extract's effectiveness in reducing carrageenan-induced inflammation (P < 0.001). Additionally, IL-6 levels in the groups receiving 20, 40, and 80 mg/kg of the extract of X. ocellata were significantly different from the indomethacin group (P < 0.001). However, no significant differences were observed between the extract-treated groups (P > 0.05).
5. Discussion
The immune system protects the body against infections and injuries through the activity of white blood cells and the release of chemical mediators, whose accumulation can lead to inflammation (1). Non-steroidal anti-inflammatory drugs are widely used to manage inflammation but are associated with gastrointestinal and hematological side effects (17). Therefore, the search for alternative anti-inflammatory agents with fewer adverse effects remains crucial (18). Lichens, rich in polyphenolic compounds, have emerged as promising candidates due to their antioxidant and anti-inflammatory properties (19).
In 2019, Mendili et al. investigated the phenolic, flavonoid, tannin, and proanthocyanidin content of methanolic and acetonic extracts from the lichen Diploschistis ocellatus. The results indicated that the acetonic extract contained the highest phenolic content (286 μg GA/g DW) and flavonoid content (24.3 μg CE/g DW), while the methanolic extract had the highest tannin (5.5 μg TAE/g DW) and proanthocyanidin content (12.35 μg CE/g DW). Additionally, the antioxidant capacity of the extracts was evaluated using three methods: DPPH, FRAP, and iron chelation assays. The methanolic extract exhibited the strongest antioxidant activity in the DPPH (IC50 = 0.29 mg/mL) and iron chelation (IC50 = 0.425 mg/mL) assays, whereas the acetonic extract showed the highest antioxidant activity in the FRAP assay (IC50 = 0.118 mg/mL) (20).
In a subsequent study, Mendili et al. again analyzed the secondary metabolites of four different lichen genera, including S. cartilaginea. The results revealed that this lichen had the highest proanthocyanidin content (31.77 μg CE/g DW). Additionally, the acetonic extract of S. cartilaginea showed the strongest antioxidant capacity using the DPPH assay (IC50 = 0.9 mg/mL). FT-IR and NMR spectroscopy of the extracts confirmed the presence of various phenolic, aromatic compounds, and fatty acids with potential biological effects (21).
In another study, Mendili et al. also investigated the antimicrobial activity of several lichen species, including D. ocellatus and S. cartilaginea. The results demonstrated that methanolic and acetonic extracts of S. cartilaginea exhibited the strongest activity against Enterococcus faecalis, with inhibition zones of 29.5 mm and 27.5 mm, respectively (22). Furthermore, the methanolic extract of S. cartilaginea demonstrated the most potent inhibitory effect in MIC assays against Enterobacter cloacae and Staphylococcus aureus (MIC = 125 μg/mL) (22).
In 2022, Sedrpoushan et al. investigated the secondary metabolites of the lichen D. diacapsis using LC-ESI-MS/MS analysis. They successfully identified several new compounds, including four depsides, one depsidone, one tridepside, and one phenolic compound (23). These polyphenols can neutralize reactive oxygen species (ROS), reduce oxidative stress, and inhibit key inflammatory mediators such as IL-6 and TNF-α (24).
In this study, we examined three lichen species from Khuzestan province and identified X. ocellata as having the highest polyphenolic content. Using the carrageenan-induced paw edema model, we demonstrated that this species significantly reduced paw swelling and inflammatory markers such as MDA and IL-6 in a dose-dependent manner (25). Among the three doses of the extract tested (20, 40, and 80 mg/kg), the anti-inflammatory effect was more pronounced with increasing doses, with the highest effect observed at the 80 mg/kg dose. This suggests that in future experiments, doses higher than 80 mg/kg could also be examined. However, the 80 mg/kg dose was not as effective as the drug indomethacin. Nonetheless, it can be suggested that the long-term use of this natural extract may have fewer side effects compared to chemical anti-inflammatory drugs like indomethacin.
Based on our findings and previous studies on anti-inflammatory pathways, it is likely that these effects are mediated through the suppression of oxidative stress-related pathways and the regulation of inflammatory mediators such as NF-κB, MAPK, PI3K/Akt, COX-2, TNF-α, and IL-1β (26-29). Additionally, the involvement of NF-κB gene and protein expression, as well as PGE2 production, may play a critical role in this process (30). Moreover, evaluating antioxidant factors such as glutathione (GSH) and antioxidant enzymes like superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) could provide deeper insights into the mechanisms underlying the anti-inflammatory and antioxidant effects of lichens (31). These aspects warrant further assessment in future studies to elucidate the precise molecular and cellular pathways involved.
5.1. Conclusions
The present study demonstrated that lichen extracts have notable anti-inflammatory effects, with the strongest results observed at the 80 mg/kg dose. Although not as effective as indomethacin, these natural extracts may have fewer long-term side effects, making them a safer option for treating inflammation. Further research on higher doses, active compounds, and improved formulations is needed to enhance their effectiveness. Lichen extracts hold promise as a natural and safe alternative for managing inflammation, especially in chronic conditions.