1. Background
Obesity is an epidemic with an increasing prevalence in developing countries, significantly raising the risk of metabolic disorders (1). Prolonged metabolic stress, such as obesity, leads to adipose tissue dysfunction and altered adipokine secretion, which increases lipid flux to the liver and contributes to an altered metabolic state characterized by insulin resistance (2, 3). The relationship between obesity and the development of type 2 diabetes is complex; however, it is evident that obesity is associated with elevated levels of circulating free fatty acids (4). High plasma free fatty acid levels inhibit glucose transport and reduce insulin sensitivity (1, 4). This disruption in metabolic regulation occurs through the accumulation of fat metabolites within cells, negatively impacting insulin signaling. The reduced effectiveness of insulin on its receptors in peripheral tissues promotes triglyceride (TG) synthesis, leading to hyperlipidemia and hyperglycemia (4, 5).
Nicotinamide N-methyltransferase (NNMT) has emerged as a key player in obesity and type 2 diabetes, making it a promising target for treating metabolic diseases (5, 6). The NNMT catalyzes the transfer of a methyl group from S-adenosyl-L-methionine (SAM) to nicotinamide, resulting in the formation of methyl nicotinamide (MNA) and S-adenosyl homocysteine (SAH). This process is closely linked to the cell’s metabolic state, influencing methylation balance and intracellular NAD+ levels (5, 7). The NNMT is primarily localized in adipose tissue and the liver (5, 8). Evidence suggests that NNMT expression is elevated in the liver and white adipose tissue of diabetic and obese mice. Furthermore, reducing NNMT expression has been shown to alleviate insulin resistance and diet-induced obesity (9-11). The preventive effects of NNMT inhibition during the early stages of adipogenesis have also been demonstrated, as it inhibits the differentiation of preadipocytes into adipocytes in response to glucocorticoids (12). Additionally, NNMT gene knockout has been associated with reduced adipogenesis (11).
Previous studies have highlighted the dual role of NNMT in the liver and adipose tissue. While NNMT appears to be detrimental in adipose tissue, it exhibits beneficial effects in the liver (9, 13). In liver tissue, NNMT stimulates gluconeogenesis in primary liver cells through sirtuin 1 and reduces fatty acid and cholesterol synthesis. It also suppresses liver TG levels, thereby enhancing insulin sensitivity (14). In contrast, NNMT expression in adipose tissue is positively correlated with Body Mass Index (BMI) and insulin resistance (15). Elevated NNMT expression and activity have been observed in the adipose tissue of obese mice, particularly those with high-fat diet (HFD)-induced obesity (16, 17). Lifestyle modifications and the regulation of energy metabolism may improve insulin sensitivity by addressing the metabolic disturbances caused by changes in NNMT activity (18).
Previous studies have shown that regular exercise training can improve metabolic outcomes in obesity and type 2 diabetes and regulate energy homeostasis in several tissues, including the liver, adipose tissue, and skeletal muscle (19, 20). Endurance training (ET) has been suggested as a non-pharmacological tool to regulate energy metabolism, promote weight loss, and improve glucose metabolism, insulin sensitivity, and liver TG levels (20, 21). One study reported that eight weeks of ET in HFD-induced obese rats led to an improvement in insulin sensitivity and increased lipid oxidation, demonstrating the regulation of energy homeostasis and glycemic effects of ET (20). Additionally, Kannt et al. reported that 12 weeks of ET (three times per week) significantly reduced NNMT levels in adipose tissue in patients with type 2 diabetes through the regulation of energy metabolism, although the underlying mechanisms remain incompletely understood (22).
Overall, ET is effective in reducing metabolic disorders by regulating energy metabolism and improving insulin sensitivity. However, the results regarding NNMT changes in different tissues, such as adipose tissue and the liver, remain inconclusive. Further research is needed to clarify the effects of ET on this enzyme in conditions of HFD-induced obesity.
In recent years, researchers have increasingly focused on the use of herbal medicines for the treatment and prevention of metabolic diseases (23, 24). For centuries, medicinal plants have played a significant role in disease treatment worldwide. Currently, there is growing interest in medicinal plants, likely due to the side effects and high costs associated with chemical drugs (24, 25).
Anethum graveolens, commonly known as dill, contains valuable nutrients, including vitamin C, carotenoids, and polyphenols such as terpenoids, quercetin, limonene, and other flavonoids (23). This plant has been shown to improve insulin sensitivity and dyslipidemia due to its antioxidant and hypoglycemic properties (24).
In one study, the effect of dill extract consumption (300 mg/kg) for 30 days on liver tissue in high-fat, high-cholesterol-fed rats was investigated. The findings indicated that the high polyphenol content in dill extract reduced hepatic lipid production and significantly decreased liver TG levels (25). Investigating the effects of dill extract in controlling metabolic disorders could provide an effective therapeutic strategy against insulin resistance and hyperlipidemia associated with HFD and obesity.
However, scientific evidence on whether dill extract can regulate the levels of enzymes involved in metabolic diseases, such as NNMT, remains limited. This is particularly relevant in liver and adipose tissue, which play a critical role in controlling energy homeostasis and the development of metabolic disorders.
2. Objectives
This study aimed to determine the effects of 10 weeks of ET and dill seed extract (DSE) on insulin sensitivity, liver TG levels, and NNMT levels in the liver and adipose tissue of obese rats.
3. Methods
3.1. Animals and Experimental Design
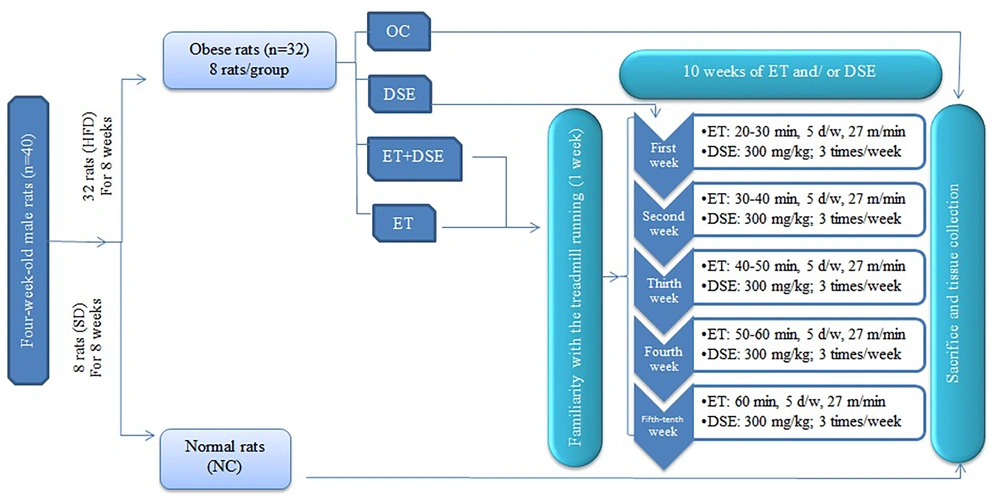
Male Wistar rats (N = 40), aged four weeks, were obtained from the Pasteur Institute in Karaj, Iran. They were subsequently housed in the animal facility under regular monitoring. The sample size was determined based on previous studies (8, 26). Ventilated cages were used to house the animals under controlled conditions (22 - 24°C and a 12:12 h light-dark cycle), with four animals per cage. The animals were provided with food and water ad libitum.
After one week of acclimatization, the rats were randomly assigned to two groups: A standard diet [(SD), n = 8)] and a HFD (n = 32). The SD group was fed a standard diet with the following caloric composition: Fat = 10%, carbohydrate = 70%, and protein = 20%. The HFD group received a diet with the following caloric composition: Fat = 60%, carbohydrate = 20%, and protein = 20% for eight weeks (27) until the start of the main experiment.
Following confirmation of obesity (Lee Index > 310 after eight weeks), the 32 obese rats were randomly divided into four equal groups (n = 8): Obese control (OC), DSE, ET, and ET + DSE (Figure 1).
The HFD was maintained until the end of the study for all obese groups. Additionally, SD-fed rats were used as the NC group. Table 1 presents the fatty acid composition and quantities for both HFD and SD.
| Variables | HFD | SD |
|---|---|---|
| Fatty Acids Composition (mg/g) | ||
| Caproic acid (C10:0) | 0.1 | 0 |
| Lauric acid (C12:0) | 0.2 | 0 |
| Myristic acid (C14:0) | 2.8 | 0.2 |
| Pentadecanoic acid (C15:0) | 0.2 | 0 |
| Palmitic acid (C16:0) | 49.9 | 6.5 |
| Palmitoleic acid (C16:1) | 3.4 | 0.3 |
| Heptadecanoic acid (C17:0) | 0.9 | 0.1 |
| Stearic acid (C18:0) | 26.9 | 3.1 |
| Oleic acid (C18:1) | 86.6 | 12.6 |
| Linoleic acid (C18:2) | 73.1 | 18.3 |
| α-Linolenic acid (C18:3) | 5.2 | 2.2 |
| Arachidic acid (C20:0) | 0.4 | 0 |
| Eicosenoic acid (C20:1) | 1.5 | 0.1 |
| Eicosadienoic acid (C20:2) | 2 | 0.2 |
| Eicosatrienoic acid (C20:3) | 0.3 | 0 |
| Arachidonic acid (C20:4) | 0.7 | 0.1 |
| Docosapentaenoic acid (C22:5) | 0.2 | 0 |
| Total | 254.5 | 43.7 |
| Fatty Acids Proportions (%) | ||
| Saturated fatty acids | 31.72 | 22.65 |
| Monounsaturated fatty acids | 35.95 | 29.74 |
| Polyunsaturated fatty acids | 32.02 | 47.59 |
Abbreviations: HFD, high-fat diet; SD, standard diet.
3.2. Exercise Training Protocol
Groups that underwent the training intervention (ET and ET+DSE) ran on a treadmill (Yarmand System, Shomal Company, Iran) with a 0% gradient for one week (5 days per week, 15 minutes per day, at a speed of 15 meters per minute) to familiarize them with treadmill running. After the acclimatization period, the rats were trained on a treadmill 5 days per week for 10 weeks. The training began at 20 - 30 minutes per day, 27 m/min, and 70% - 80% VO₂max during the first week.
Throughout the 10-week training period, the intensity and frequency of training remained constant, but the duration of exercise gradually increased to 60 minutes per day by the fourth week and remained steady from the fifth to the tenth week (28, 29). Additionally, each training session included a 5-minute warm-up and a 5-minute cool-down at a speed of 10 meters per minute (approximately 20% - 30% VO₂max) (30). The same protocol and program design were used in previous studies by the researchers on the same rats (29, 31).
During the initial training sessions, an electric shock (0.5 mA) combined with a sound stimulus (hitting the side of the treadmill) was used to encourage running. In subsequent sessions, only the sound stimulus was used (32).
3.3. Dill Extract Preparation and Consumption Protocol
After purchasing dried dill seeds from the Birjand local market (South Khorasan province, Iran) and confirming their identity with a botanist, the seeds were powdered using an electric grinder. Next, 100 g of the powder was soaked in 96% ethyl alcohol at laboratory temperature (25°C - 28°C) for 24 hours and then filtered through filter paper. The resulting solution was concentrated in two steps using a rotary evaporator (Heidolph, Schwabach, Germany) at 70 rpm and 50°C, reducing its volume to one-third (29, 33). The final solution was dried in an incubator (BD400 - 400 L, Binder, Tuttlingen, Germany) at temperatures below 50°C under sterile conditions.
Subsequently, 3 grams of the dried extract was dissolved in 10 mLof water. Based on a previous study that used the same extraction method, each gram of this extract contained 160 ± 4.0 mg of total phenols (equivalent to gallic acid), 101 ± 4.0 mg of flavonols (equivalent to quercetin), and 120 ± 5.3 mg of flavonoids (equivalent to quercetin) (34).
The extract was administered to the DSE and ET+DSE groups at a dose of 300 mg/kg body weight via gavage, three times a week for 10 weeks. Rats in the other groups received the same volume of saline via gavage three times a week (25, 33). Previous studies have reported that 300 mg/kg is an effective dose for metabolic properties in animal models (34, 35).
3.4. Body Weight and Length Measurements and Lee Index Calculation
Lee's Index was used to assess obesity in this study. Obesity is defined as an index exceeding 310, calculated by dividing the cube root of the rat's body weight (g) by the nose-to-anus length (cm) (36). Obesity in the HFD groups was confirmed based on the Lee Index (DSE = 341.49, ET = 336.17, ET+DSE = 331.61, and OC = 343.62).
Body weight was measured using an automatic scale (Kern, Germany, sensitivity: 0.1 g), and body length was measured using a digital caliper (Insize, China, sensitivity: 0.01 mm).
3.5. Sampling, Preparation, and Biochemical Assays
Forty-eight hours after the final intervention session, all rats were anesthetized with a combination of ketamine and xylazine (70 - 100 mg·kg-¹ and 7 - 10 mg·kg-¹, respectively). Adipose and liver tissues were removed, weighed, frozen in liquid nitrogen (−196°C), and stored at -80°C until further analysis.
For the measurement of NNMT in liver and adipose tissue, as well as liver TG levels, 100 mg of tissue was homogenized in a cold PBS buffer (100 mM, pH 7.4) containing a protease inhibitor cocktail (Problock Goldbio Inc, USA) for 5 minutes (Retsch, Haan, Germany). The homogenate was centrifuged (10,000 rpm for 10 minutes), and the supernatant was stored at -80°C for biochemical analysis. Protein concentration was determined using the Bradford method (Colorimetric, ZellBio GmbH, Lonsee, Germany; sensitivity: 5 μg/mL). The NNMT levels in the liver and adipose tissue were analyzed using an ELISA kit (ZellBio, Germany; sensitivity: 7.5 pg/mL). Liver TG levels were measured using an enzymatic colorimetric method (Pars Azmoon, Iran; sensitivity: < 5 mg/dL).
Blood samples were collected from the heart using syringes coated with ethylenediamine tetraacetic acid (EDTA) and transferred to EDTA-containing tubes. The samples were immediately centrifuged (3000 rpm for 15 minutes) to separate plasma for glucose and insulin measurements. Blood glucose concentration was measured using a Pars Azmoon kit (sensitivity: < 5 mg/dL) and a Selectra-2 autoanalyzer (Netherlands). Insulin concentration was measured using an ELISA kit (Mercodia, Uppsala, Sweden; sensitivity: < 0.1 µg/L).
The Quantitative Insulin Sensitivity Check Index (QUICKI) was calculated using the formula: QUICKI = 1/[log(fasting insulin in µU/mL) + log(fasting glucose in mg/dL)] (37).
3.6. Statistical Analysis
After confirming the normality of data distribution using the Shapiro-Wilk test and equality of variances with Levene’s test, repeated measures analysis of variance (ANOVA) and Tukey's post hoc tests were used to analyze body weight data. Blood glucose, TG, and NNMT data were analyzed using one-way ANOVA and Tukey's post hoc tests, while insulin and QUICKI data were analyzed using the Kruskal-Wallis and Mann-Whitney U tests.
All statistical analyses were performed using SPSS 20.0 (SPSS, Chicago, IL, USA) at a significance level of P < 0.05. Graphs were created using GraphPad Prism 9 software.
4. Results
4.1. Body Weight
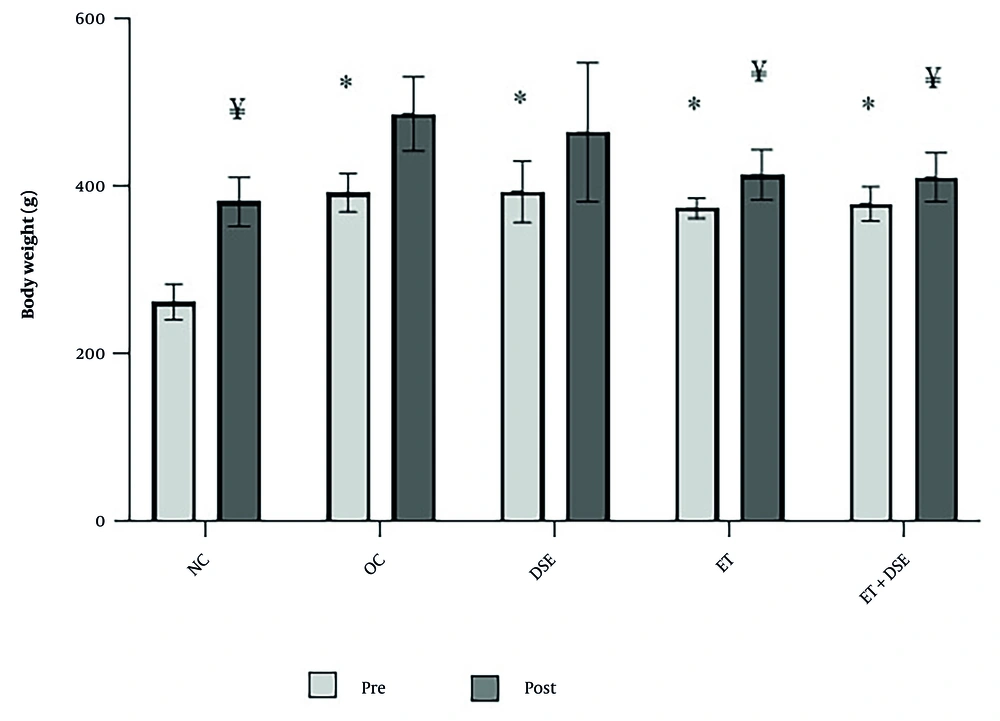
The repeated measures ANOVA revealed significant effects for time [F (1, 35) = 196.03, P = 0.01], group (F [4, 35] = 14.46, P = 0.01), and time × group interaction [F (4, 35) = 10.40, P = 0.01]. The results showed that body weight in the ET, DSE, ET+DSE, and OC groups was significantly higher than in the NC group before the 10-week intervention, with percentage increases of approximately 42.65%, 50.13%, 44.58%, and 49.70%, respectively (P < 0.001 for all comparisons). No significant differences were observed between other paired comparisons (P ≥ 0.05).
After the intervention period, body weight was significantly lower in the ET+DSE (15.52%, P = 0.003) and ET (14.91%, P = 0.005) groups compared to the OC group. Additionally, body weight in the ET+DSE (11.56%, P = 0.03) and ET (10.91%, P = 0.04) groups was significantly lower than in the DSE group.
Higher body weight after the intervention was observed in the DSE (21.76%, P ≤ 0.001) and OC (27.47%, P ≤ 0.001) groups compared to the NC group (Figure 2). No significant differences were observed between other paired comparisons (P ≥ 0.05).
Changes in body weight during the 10 weeks of the intervention. NC, normal control; OC, obese control; DSE, dill seed extract; ET, endurance training. * Significant difference compared to the NC group at baseline; ¥ significant difference compared to the OC and DSE groups at the end of intervention (P < 0.05).
4.2. Glycemic Indices
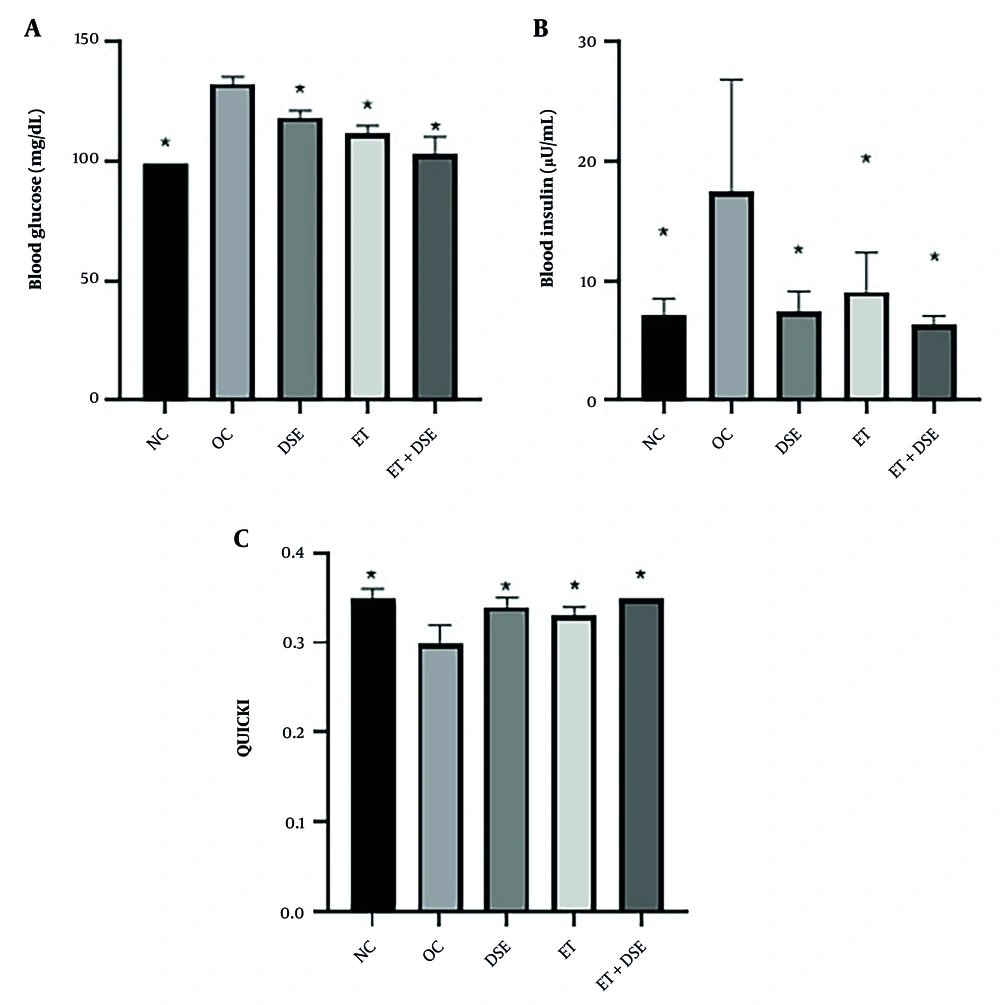
A significant difference in blood glucose levels across the groups was observed using one-way ANOVA [F(4, 35) = 8.04, P = 0.001]. Tukey's post hoc test showed that blood glucose was significantly lower in the DSE (10.63%, P = 0.001), ET (15.89%, P = 0.001), ET+DSE (22.09%, P = 0.001), and NC (25.30%, P = 0.001) groups compared to the OC group (Figure 3A).
The Kruskal-Wallis test revealed significant differences in insulin levels (χ² = 15.80, P = 0.003) and the QUICKI Index (χ² = 21.24, P = 0.001) between the groups. The Mann-Whitney U test showed significantly lower insulin levels and higher QUICKI indices in the DSE (57.30%, P = 0.01 and 11.87%, P = 0.003, respectively), ET (48.07%, P = 0.02 and 10.66%, P = 0.01, respectively), ET+DSE (63.88%, P = 0.002 and 16.82%, P = 0.001, respectively), and NC (59.00%, P = 0.007 and 15.18%, P = 0.001, respectively) groups compared to the OC group (Figure 3B and C).
No significant differences were observed in blood glucose, insulin levels, or QUICKI Index between the DSE, ET, and ET+DSE groups (P ≥ 0.05).
4.3. NNMT Levels in Liver and Adipose Tissue and Liver TG
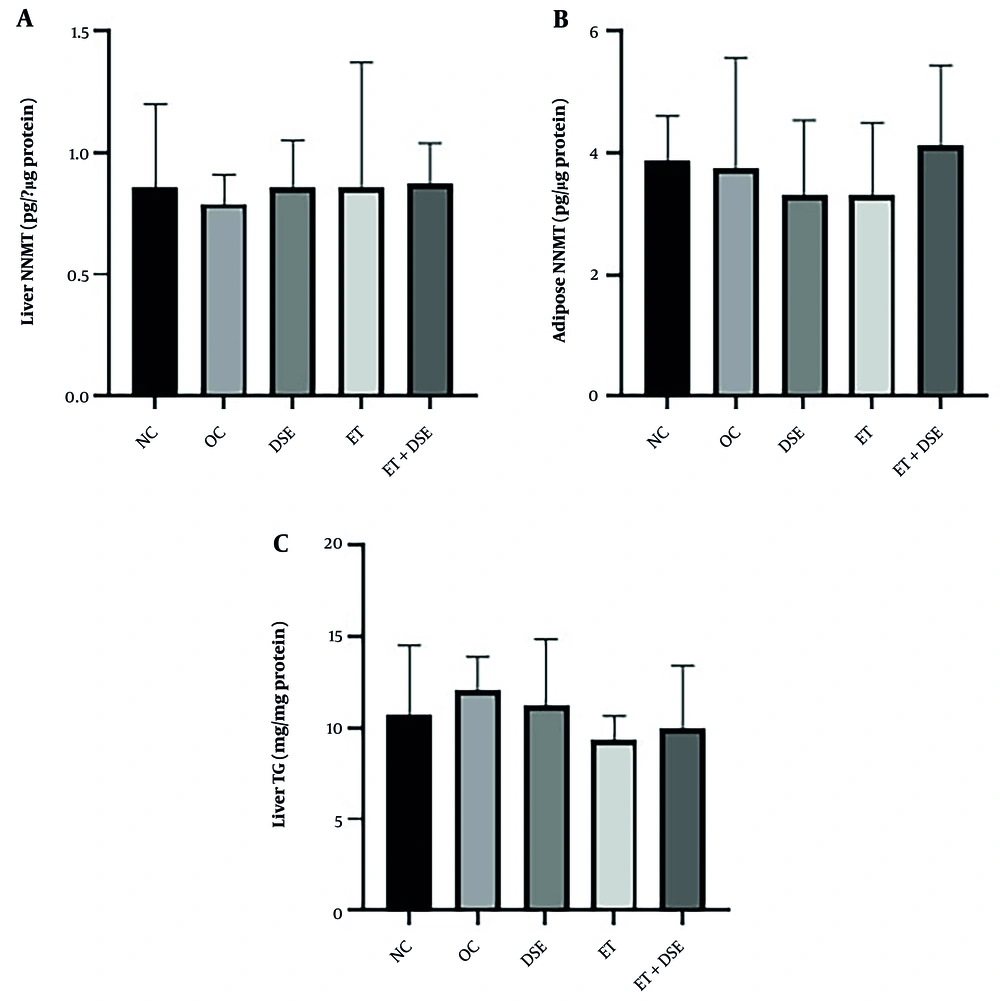
The ANOVA test showed no significant differences in NNMT levels in the liver [F (4, 35) = 0.08, P = 0.98] or adipose tissue [F (4, 35) = 0.62, P = 0.64] between the groups (Figure 4A and B). Additionally, no significant differences were observed in liver TG levels between the groups [F (4, 35) = 1.79, P = 0.15] (Figure 4C).
5. Discussion
This study examined the effects of 10 weeks of ET and DSE on body weight, blood glucose, insulin levels, the QUICKI Index, NNMT levels in the liver and adipose tissue, and liver TG levels in HFD-induced obese rats. Compared to the OC group, the ET, DSE, ET+DSE, and NC groups exhibited significantly lower blood glucose and insulin levels, as well as a higher QUICKI Index. However, NNMT levels in the liver and adipose tissue, as well as liver TG levels, remained unchanged after the 10-week intervention in all groups.
Previous studies have reported that HFD decreases insulin sensitivity and glucose tolerance (20, 38). Consistent with these findings, our results showed that HFD affected glycemic indices, as evidenced by the differences between the NC and OC groups. Additionally, exercise training has been recognized as a therapeutic and preventive intervention for insulin resistance (39). As expected, the DSE, ET, and ET+DSE groups had lower blood glucose and insulin levels, as well as higher QUICKI indices, compared to the OC group. In line with our findings, Horii et al. reported that eight weeks of aerobic training reduced fasting blood glucose and improved insulin sensitivity in rats with type 2 diabetes (40). Endurance training has been shown to enhance glucose transport through glucose transporter type 4 (GLUT4) and modulate enzymes involved in glucose phosphorylation and oxidation (21, 40). Furthermore, ET increases energy expenditure, promotes weight loss, enhances glucose delivery to muscles, stimulates fatty acid oxidation, and ultimately improves insulin sensitivity (39, 40). In this study, rats in the ET and ET + DSE groups had lower body weight compared to the OC group at the end of the 10-week intervention. The improvements in blood glucose, insulin levels, and the QUICKI Index in the training groups are likely attributable to weight loss induced by exercise training.
Interestingly, the DSE group also exhibited lower blood glucose and insulin levels, as well as a higher QUICKI Index. This may be attributed to the antioxidant and anti-inflammatory properties of dill, which can improve insulin sensitivity and reduce blood lipid levels (23, 24). The findings of Haidari et al. align with our results, demonstrating reductions in fasting blood glucose, insulin levels, and insulin resistance following dill extract consumption (24). The glucose-lowering effect of dill extract is likely mediated by its flavonoid content, particularly quercetin-3-O-β-D-glucuronide, which exhibits insulin-like effects and enhances glucose uptake in peripheral tissues (23, 24).
According to recent studies, one mechanism influencing glycemic indices is changes in the NNMT enzyme in the liver and adipose tissue. The NNMT is a cytosolic enzyme primarily expressed in the liver and adipose tissue, catalyzing the N-methylation of nicotinamide (NAM) and regulating lipid, cholesterol, and glucose metabolism (5, 7). In this context, Kannt et al. demonstrated that NNMT expression in adipose tissue is higher in diabetic patients than in healthy individuals and that there is an inverse relationship between insulin sensitivity and NNMT expression in adipose tissue (22). Although NNMT expression in adipose tissue correlates with adiposity in both mice and humans (12), one study suggested that NNMT upregulation can differentially regulate liver fat accumulation (10).
However, a key finding in the present study is that despite improvements in glycemic indices following ET and DSE, no significant changes were observed in NNMT levels in the liver and adipose tissue of obese rats. It has been reported that NNMT levels and activity in different organs, particularly the liver and adipose tissue, as well as circulating MNA concentrations, vary under different physiological conditions, such as disease, exercise, and HFD (6, 22). One study showed that NNMT levels in adipose tissue decreased significantly after 12 weeks of ET in diabetic patients with a high BMI, although the underlying mechanisms remain unclear (22).
The role of NNMT in aerobic and anaerobic energy metabolism also differs (22, 41, 42). For instance, anaerobic exercise has been shown to enhance NNMT expression in skeletal muscles more than aerobic exercise, especially when glucose is the primary energy source (41). Additionally, inhibiting NNMT activity with MNA impaired the performance of rats during anaerobic ET (42). The endurance nature of the exercise training used in the present study may explain the lack of significant changes in NNMT levels in the liver and adipose tissue.
It has been reported that feeding mice with an HFD selectively increases NNMT activity in adipose tissue while leaving liver activity unchanged (5). Interestingly, we observed no changes in NNMT levels in the liver or adipose tissue under HFD (no difference between the NC and OC groups), which may explain why this enzyme remained unaffected by training and extract consumption. Some studies suggest that the composition of dietary fat and the use of different fatty acids can produce varying metabolic responses, particularly in fat oxidation rates across tissues (43, 44). Diets with a higher proportion of polyunsaturated and monounsaturated fatty acids (e.g., oleic acid) compared to saturated fatty acids increase fat oxidation in adipose tissue and reduce fat deposition in other tissues, such as the liver, which is critical for preventing HFD-induced metabolic dysfunction (44).
In the present study, the higher ratio of polyunsaturated and monounsaturated fatty acids to saturated fatty acids in the HFD may have increased beta-oxidation of fats, thereby reducing the impact of HFD on NNMT and TG levels. The presence of compounds like oleic acid in HFD can also enhance beta-cell activity and insulin sensitivity (44). Thus, not only the quantity of dietary fats but also their composition plays a fundamental role in metabolic changes, including fat accumulation and lipid metabolism.
The duration of HFD exposure may also influence metabolic responses. For example, fat mass increases continuously over 6, 10, and 20 weeks of HFD consumption. However, the expression of adipogenesis regulatory genes (e.g., PPAR, adiponectin, and leptin) peaks early in HFD exposure and gradually declines over time (45). In the current study, prolonged HFD exposure may explain the lack of significant effects on NNMT levels in the liver and adipose tissue, and improvements in insulin sensitivity and glycemic indices may have occurred through mechanisms other than NNMT. Definitive conclusions in this area should be made cautiously, and further research is needed with varying durations of HFD and different fatty acid compositions.
Another possible reason for the lack of change in NNMT levels in adipose tissue and liver, despite improved insulin sensitivity, is the small sample size and large standard deviation of NNMT data. Larger sample sizes reduce the standard deviation of the distribution of sample means, enhancing confidence in the results and increasing the likelihood of detecting statistical significance (46).
Furthermore, NNMT knockdown has been shown to significantly reduce body weight, fat mass, and insulin levels in female mice fed a Western diet (47% kcal from fat and 34% kcal from carbohydrates). However, no changes were observed in body weight, fat mass, fasting blood glucose, insulin levels, or glucose tolerance in HFD-fed NNMT knockdown male mice (60% kcal from fat and 20% kcal from carbohydrates). Gender differences have been proposed as a potential explanation for this discrepancy (47). The exclusive use of male rats in the present study may explain the lack of HFD effects on NNMT. Including both male and female rats in future studies could provide a more comprehensive understanding of this phenomenon.
Although previous studies have reported that ET and dill extract reduce lipid accumulation in liver tissue (20, 24), our findings showed no significant differences in liver TG levels between groups after HFD intervention (no difference between NC and OC groups) or following DSE and ET (no difference between DSE, ET, ET+DSE, and OC groups). One related study found that using an HFD with MNA decreased serum and liver cholesterol and liver TG levels in mice. By stabilizing the SIRT1 protein through its fatty acid β-oxidation properties, MNA effectively reduces liver lipid levels, highlighting the beneficial role of NNMT in hepatocyte gluconeogenesis (6). The lack of change in liver TG levels in our study may be due to the absence of changes in NNMT and, consequently, the lack of MNA and SIRT1 activity.
The results regarding NNMT levels in the liver and adipose tissue and their physiological effects are contradictory (5, 10, 22). While inhibiting or reducing NNMT in adipose tissue can enhance insulin sensitivity (5, 22), the precise mechanisms by which NNMT influences insulin sensitivity and blood glucose regulation remain unclear. In the present study, the increase in the QUICKI Index may have been independent of changes in NNMT levels in the liver and adipose tissue. Therefore, additional research is necessary to better understand the role of NNMT in glucose regulation.
5.1. Limitations
This study has several limitations. First, we only investigated NNMT levels in the liver and adipose tissue of rats and did not assess transcriptomic data, which complicates inferences about changes in protein activity and the regulation of targeted mechanisms. Second, we did not measure plasma levels of MNA or essential intermediates, such as NAD+ and SAM, which are substrates for the NNMT reaction. Future studies should focus on transcriptional factors and the upstream and downstream pathways of NNMT. Additionally, the lack of measurement of total phenols, flavonoids, and flavonols in the DSE, as well as the small sample size, are limitations of this study. To draw more accurate conclusions regarding the effects of ET and DSE on research indicators, particularly NNMT, it is recommended to use a larger sample size and quantify the total phenols, flavonoids, and flavonols in the DSE.
Another limitation is the method of dill extraction and administration, which may have influenced the results related to insulin sensitivity and NNMT levels in the liver and adipose tissue. Techniques such as ultrasonic-assisted extraction can enhance the efficiency of extracting beneficial compounds, while hydrodistillation provides an alternative method to isolate essential oils with distinct chemical compositions. In this study, hydrodistillation was used, which is a practical and common extraction method. Future studies should compare different extraction methods (e.g., steam distillation versus solvent extraction) and their effects on insulin sensitivity and NNMT activity in both animal models and humans.
5.2. Conclusions
Based on our results, 10 weeks of ET and DSE, either alone or in combination, reduced blood glucose and insulin levels and increased the QUICKI Index in obese rats. However, no changes were observed in NNMT levels in the liver and adipose tissue or in liver TG levels, likely due to the lack of effect of HFD on these indicators. Further research is needed to clarify the effects of ET and DSE on NNMT levels in the liver and adipose tissue.