1. Background
The skin possesses inherent protective mechanisms against aging, which diminish with advancing age (1-3). Solar ultraviolet (UV) radiation is responsible for approximately 80% of skin damage, significantly contributing to skin cancer and aging (4). The UVA radiation generates reactive oxygen species (ROS), leading to indirect DNA damage. In contrast, ultraviolet-B (UVB) radiation is a major factor in skin damage due to its ability to generate intracellular ROS (5-7), directly damaging DNA and triggering inflammation. The UVB photons are 1000 times more energetic than UVA photons, making UVB the primary cause of sunburn, suntanning, and photocarcinogenesis (7, 8). Chronic UV exposure results in photoaging, characterized by wrinkles, roughness, loss of skin tensile strength, fragility, dyspigmentation, and dryness (1, 9-11). Photoaging is also a precursor to photocarcinogenesis.
Skin photoprotection involves both topical formulations and oral supplements (4). While sunscreen is commonly used, it requires frequent reapplication, often leading to inadequate protection. Conversely, oral supplements can repair damage in the deeper skin layers, making exogenous antioxidants in oral supplements more beneficial and practical (12, 13).
This study focuses on pterostilbene (PS), an active compound found in blueberries and Pterocarpus marsupium heartwood (14), known for its anti-aging and anti-photocarcinogenic properties (15, 16). Notably, orally administered PS has higher bioavailability and a longer half-life compared to its analogue, resveratrol (17, 18).
2. Objectives
Therefore, this study aims to investigate the photoprotective effects of oral PS in a UVB-induced skin photoaging BALB/c mouse model. The animal model was selected to mimic human photoaging, providing controlled conditions to explore therapeutic interventions.
3. Methods
3.1. Sample Size Calculation
The number of mice required for the study was calculated using the resource equation approach (19). Based on this calculation, 4 mice per group were needed to maintain a degree of freedom (DF) of 10. The sample size was further adjusted to account for an expected attrition rate of 10% (20), confirming that 4 mice per group were sufficient.
3.2. Animal Maintenance and Experimentation
Sixteen female BALB/c mice, aged six weeks, were procured from the Faculty of Science and Technology animal house at Universiti Kebangsaan Malaysia (UKM) and housed under standard laboratory conditions, which included consistent temperature, humidity, and lighting, at the Faculty of Health Sciences animal house, UKM. The mice were fed standard mouse pellets with water provided ad libitum. Inclusion criteria required healthy mice that met the study requirements, while exclusion criteria included mice exhibiting unrelated illnesses, infections, or skin lesions. Experimental conditions and treatment protocols were standardized to ensure consistency. Ethical approval was obtained from the UKM Animal Ethics Committee (UKMAEC), with the approval code: FSK/2020/AHMAD ROHI/25-NOV./1138-DEC.-2020-AUG.-2022. This study adheres to the ARRIVE guidelines for reporting animal research (21), with a completed ARRIVE guidelines 2.0 checklist available in Appendix 1 in Supplementary File.
3.3. Reagents
The following materials were used in the study: Pterostilbene (J&K Scientific, USA), corn oil (Mazola®, USA), 37% formaldehyde (R&M Chemicals, United Kingdom), sodium phosphate dibasic (Na2HPO4) (Amresco, USA), sodium phosphate monobasic (NaH2PO4) (Merck, Germany), 99.8% absolute ethanol (Chemiz, Malaysia), xylene (Chemiz, Malaysia), paraffin wax (Sigma-Aldrich, USA), Harris hematoxylin powder (Sigma-Aldrich, USA), potassium aluminium sulphate (Sigma-Aldrich, Germany), mercury (II) oxide red (Merck, Germany), glacial acetic acid (Chemiz, Malaysia), eosin Y powder (Sigma-Aldrich, USA), 37% hydrochloric acid (R&M Chemicals, United Kingdom), Bouin's solution (Chemiz, Malaysia), Biebrich scarlet sodium salt (Sigma-Aldrich, Germany), acid fuchsin (Sigma-Aldrich, Germany), Weigert's iron hematoxylin kit (Merck, Germany), aniline blue diammonium salt (Sigma-Aldrich, Germany), phosphotungstic acid hydrate (Sigma-Aldrich, Germany), phosphomolybdic acid hydrate (Sigma-Aldrich, Germany), dibutylphthalate polystyrene xylene (DPX) (Sigma-Aldrich, USA), phosphate buffered saline (PBS) tablets (Oxoid, United Kingdom), Coomassie Blue G-250 (Fisher Scientific, USA), 85% phosphoric acid (Chemiz, Malaysia), bovine serum albumin (Nacalai Tesque, Japan), L-glutathione (GSH) reduced (Sigma-Aldrich, USA), 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB) (Sigma-Aldrich, USA), ethylenediaminetetraacetic acid (EDTA) powder (Chemiz, Malaysia), metaphosphoric acid (Merck, Germany), synthetic melanin (Sigma-Aldrich, USA), sodium hydroxide (Merck, Germany), chloroform (Merck, Germany), and phenol (Merck, Germany). All chemicals were of analytical grade and commercially produced.
3.4. Experimental Design
After a 1-week acclimatization period, the mice were divided into four groups of four mice each (n = 4, with each mouse regarded as an experimental unit). The allocation of mice into each group was conducted using an online random number generator (https://www.graphpad.com/quickcalcs/randomize1/) to generate the randomization sequence. The groups were as follows: Ultraviolet-B (-) as a negative control, UVB (+) as a vehicle control, UVB (+) PS30 (30 mg/kg low-dose PS), and UVB (+) PS60 (60 mg/kg high-dose PS). A positive control group was not included in this study due to the absence of a widely accepted gold standard for oral photoprotection (12). Unlike topical photoprotection, where tretinoin is currently the gold standard for treating photoaging (22), oral approaches remain under exploration. Therefore, comparisons were made with the negative control and vehicle control groups to establish a relative baseline for efficacy.
For two weeks, daily oral gavage was administered without UVB exposure. Mice were fasted for four hours from food before gavage, with volumes set at 0.5% of body weight. The UVB (-) and UVB (+) groups received corn oil, while the UVB (+) PS30 and UVB (+) PS60 groups received PS in corn oil, respectively. After two weeks, a 2.5 × 5 cm area of dorsal fur was shaved clean. Daily oral gavage continued for the next eight weeks, with UVB exposure applied only to the UVB (+), PS30, and PS60 groups.
3.5. Ultraviolet-B Dose Exposure Plan
Photoaging was induced in the mice by exposing them to UVB irradiation using a 15-watt lamp (UVP, USA) that emitted UV light at 312 nm. The irradiation intensity was measured using a UVP UVX radiometer (Analytik Jena, Germany). The dose of UVB exposure was calculated using the following formula (23):
The UVB dose exposure plan, as outlined in Table 1, was adapted from Saito et al. (24). Only the UVB (+), PS30, and PS60 groups were exposed to UVB irradiation three times a week with increasing doses, starting from Week 3 and concluding at Week 10, resulting in a cumulative total of 3702 mJ/cm2 or 3.702 J/cm2. Prior to UVB irradiation, the mice were anesthetized with 0.1 mL/50 g of KTX (a mixture of ketamine, xylazine, tiletamine, and zolazepam) and had their eyes covered with a black polyester waterproof fabric to prevent UVB damage.
| Groups | Weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| UVB (-) | - | - | - | - | - | - | - | - | - | - |
| UVB (+) a | - | - | 48+ | 67+ | 112+ | 133+ | 165+ | 184+ | 213+ | 237+ |
| UVB (+) PS30 | - | - | 48+ | 86+ | 112+ | 156+ | 165+ | 200+ | 213+ | 250+ |
| UVB (+) PS60 | - | - | 67 | 86 | 133 | 156 | 184 | 200 | 237 | 250 |
Abbreviation: UVB, ultraviolet-B.
a Unit: mJ/cm2.
3.6. Macroscopic Evaluation of Photoaging
Pinch tests (25, 26) were conducted once a week throughout the 8-week irradiation period. Mice were anesthetized using KTX (0.1 mL/50 g) before pinching and stretching the skin at the midline of the dorsal area. The time taken (in seconds) for the mice skin to recover to its normal conformation was recorded. Each week, the pinch test was performed four times per mouse.
Upon completion of the treatment, mice were placed under deep anesthesia with a KTX overdose, and photographs of the dorsal skin were taken. Subsequently, the mice were euthanized by cervical dislocation. By first suppressing central nervous system activity with KTX to induce immobilization, cervical dislocation was performed swiftly and effectively, ensuring minimal pain and distress for the mice. The dorsal skin was harvested and divided into equal parts for histopathological observation (the primary outcome measure) and biochemical analyses.
Blinding was not feasible due to visible photoaging effects on the mice's skin, such as thickening, erythema, peeling, and wrinkles. However, other outcome measures, such as histology and biochemical analyses, were objective and quantifiable, reducing the risk of bias. An independent external reviewer was also consulted to validate our findings and further reduce bias.
3.7. Histological Analyses
The harvested dorsal skin was fixed in 10% neutral buffered formalin, processed, embedded in paraffin, and sectioned at 5 μm. Hematoxylin & Eosin (H&E) staining was employed to assess histological features of photoaging, which include changes in the epidermis and dermis, as well as the infiltration of inflammatory cells. Epidermal thickness was measured using Fiji v 2.15.0. Masson's trichrome staining (27) was utilized to evaluate collagen content, which was quantified using color deconvolution in Fiji v 2.15.0.
For the measurement of epidermal thickness, the four best images were selected to represent each mouse, and each image was measured four times at different areas across the epidermis. Similarly, the four best images were chosen for collagen content quantification to represent each mouse.
3.8. Skin Tissue Homogenization
The harvested dorsal skin was washed with cold phosphate-buffered saline (PBS), snap-frozen in liquid nitrogen, and stored at -80°C. After weighing and finely chopping, the skin was homogenized in cold sodium phosphate buffer (pH 6.8). The homogenate was then centrifuged at 8000 rpm for 20 minutes at 4°C, and the supernatant was stored at -80°C for subsequent GSH and melanin content assays.
3.9. Glutathione Assay
Before conducting the assay, the protein concentration of the skin supernatant was determined using the Bradford method (28). The GSH assay, a measure of endogenous antioxidants, was performed using the Ellman method (29). Initially, 50 μL of a 5% metaphosphoric acid solution was mixed with 50 μl of the supernatant. The mixture was vortexed and centrifuged at 3000 rpm for 10 minutes at 4°C. The resulting supernatant was then used for the GSH assay.
Subsequently, 30 μL of reaction buffer solution (containing sodium phosphate dibasic, EDTA, and distilled water, at pH 8.0) was added to the wells, followed by 50 μL of supernatant into each corresponding well. Then, 10 μL of DTNB solution was added to all wells, and the microplate was incubated at room temperature for 15 minutes in the dark. Absorbance was read at 412 nm. The concentration of GSH was determined by substituting the absorbance value into the equation of the GSH standard curve. Four technical replicates per mouse were performed. The final GSH concentration was calculated as shown below and expressed as μmoL/mg:
3.10. Melanin Content Assay
Skin melanin content was determined using a modified method by Iwata et al. (30). Approximately 350 µL of skin homogenate was mixed with 2N sodium hydroxide and incubated for two days at 60°C to solubilize melanin. Subsequently, an equal volume of distilled water was added to the homogenate, followed by the addition of 700 µL of a chloroform:Phenol (1:1) mixture. This mixture was vortexed and centrifuged at 5000 × g for 10 minutes to separate the phases. The resulting supernatant was measured at 400 nm using a microplate reader. The obtained values were expressed as micrograms of melanin per milligram wet weight of skin (µg melanin/mg) (31). Four technical replicates per mouse were performed. The standard curve was generated similarly, using 25 µg/mL of synthetic melanin instead of homogenate.
3.11. Statistical Analysis
Data were analyzed using GraphPad Prism v8.3.0 and are presented as mean ± standard error of the mean (SEM) for quantitative analysis. Normality was confirmed with the Shapiro-Wilk test, validating the use of one-way ANOVA for group comparisons. Statistical significance was set at P < 0.05. All mice (n = 4 per group) were included in all analyses, with no exclusions.
4. Results
4.1. Effect of Ultraviolet-B Irradiation and Pterostilbene on Skin Macroscopic Appearance and Elasticity
Figure 1A illustrates the macroscopic appearance of the mice's skin after the experiment. The UVB (-) group exhibited fine wrinkles without redness, whereas the UVB (+) group displayed coarse wrinkles, erythema, and peeling. Pterostilbene treatment improved the appearance of photoaged skin: The UVB (+) PS30 group showed reduced erythema and coarse wrinkles, while the UVB (+) PS60 group eliminated erythema and resulted in smoother skin, although some coarse wrinkles remained.
In Figure 1B, the pinch test results indicated that the UVB (+) group took significantly longer (4.957 ± 0.115 seconds, P < 0.01) to return to its normal skin conformation compared to the UVB (-) group (4.275 ± 0.154 seconds), suggesting reduced elasticity. Pterostilbene significantly improved skin elasticity (P < 0.0001), as evidenced by the reduced time in the UVB (+) PS30 group (4.049 ± 0.109 seconds) and the UVB (+) PS60 group (3.650 ± 0.062 seconds) compared to the UVB (+) group. Although there was no significant difference between the PS30 and PS60 groups (P > 0.05), the PS60 group showed a further reduction in time below the UVB (-) levels (P < 0.01), demonstrating the greatest improvement in elasticity.
4.2. Skin Histopathological Changes of Ultraviolet-B Irradiation and Pterostilbene Treatment
Figure 2A illustrates increased epidermal thickness and inflammatory cell infiltration in the UVB (+) group compared to the UVB (-) group. The UVB (+) PS30 and UVB (+) PS60 groups demonstrated reduced epidermal thickness, although inflammation persisted. Figure 2B confirms a significant increase in thickness for the UVB (+) group (67.388 ± 1.688 µm, P < 0.0001) compared to the UVB (-) group (15.993 ± 0.441 µm). The UVB (+) PS60 group significantly reduced thickness (43.640 ± 2.080 µm) compared to both the UVB (+) group (P < 0.0001) and the UVB (+) PS30 group (61.869 ± 3.577 µm, P < 0.001), indicating that PS60 provided the greatest improvement.
Figure 3A highlights collagen content (blue), which is quantified in Figure 3B. The UVB (+) group (1.105 ± 0.013) showed a slight, non-significant increase in collagen compared to the UVB (-) group (1.000 ± 0.026, P > 0.05). Pterostilbene significantly increased collagen in the UVB (+) PS30 group (1.407 ± 0.040, p < 0.0001) and the UVB (+) PS60 group (1.266 ± 0.020, P < 0.01) when compared to the UVB (+) group. However, the PS30 group showed a greater increase than the PS60 group (P < 0.05), suggesting that PS30 was more effective at boosting collagen content.
4.3. Effect of Ultraviolet-B Irradiation and Pterostilbene on Skin Glutathione and Melanin
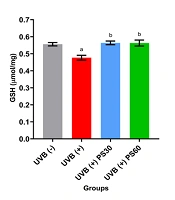
Figure 4A demonstrates a significant decrease in GSH levels in the UVB (+) group (0.477 ± 0.014 µmol/mg, P < 0.01) compared to the UVB (-) group (0.556 ± 0.010 µmol/mg). Pterostilbene treatment significantly increased GSH levels in the UVB (+) PS30 group (0.564 ± 0.011 µmol/mg) and the UVB (+) PS60 group (0.563 ± 0.018 µmol/mg) when compared to the UVB (+) group (P < 0.01), with no significant difference between the doses (P > 0.05).
In Figure 4B, the UVB (+) group (0.387 ± 0.042 µg/mg) showed a significant increase in melanin content compared to the UVB (-) group (0.271 ± 0.005 µg/mg, P < 0.05). The UVB (+) PS30 group (0.337 ± 0.007 µg/mg) and the UVB (+) PS60 group (0.347 ± 0.008 µg/mg) showed no significant difference (P > 0.05) compared to other groups, indicating that PS had no effect on melanin levels in photoaged skin.
5. Discussion
Only one in vivo study has demonstrated that topical PS is more effective than resveratrol against UVB-induced photoaging (32). To date, no studies have investigated the effects of oral PS in preventing photoaging. In our study, the UVB (+) PS60 group exhibited smooth skin with no redness and only coarse wrinkles, while the UVB (+) group showed significant UVB-induced damage. Pterostilbene mitigated UVB-induced oxidative stress and preserved skin integrity, unlike the UVB (+) group, where endogenous skin antioxidants alone were insufficient to prevent damage.
The pinch test revealed that the UVB (+) PS60 group demonstrated improved elasticity, as evidenced by faster skin recovery time compared to both the UVB (+) and UVB (-) groups. This suggests that PS at a higher dose restored elasticity more effectively than untreated skin.
Hematoxylin & Eosin staining showed significant epidermal thickening in the UVB (+) group. This thickening is attributed to UV irradiation, which causes sunburnt cells (apoptotic keratinocytes), followed by increased cell division to replace lost cells, temporarily thickening the epidermis to shield basal stem cells. However, chronic UV exposure may eventually deplete stem cells, leading to atrophy (8). In our study, the UVB (+) PS60 group significantly decreased epidermal thickness compared to the UVB (+) and UVB (+) PS30 groups, demonstrating its superior ability to ameliorate photoaging-induced epidermal thickening.
Masson's Trichrome staining indicated a slight, non-significant increase in collagen in the UVB (+) group, possibly as a photoprotective mechanism before collagen degradation or due to UV-induced collagen cross-linking (33). Interestingly, the UVB (+) PS30 group exhibited significantly higher collagen density than the UVB (+) PS60 group. This finding parallels a clinical study (34) where high-dose beta-carotene exhibited pro-oxidant effects, whereas a low dose demonstrated antioxidant properties. However, further investigations are needed to confirm the specific effects of PS on collagen.
Reduced GSH is a ubiquitous antioxidant that counteracts the oxidizing effects of reactive species (35, 36). In the UVB (+) group, GSH levels significantly decreased compared to the UVB (-) group, likely due to the need to counteract ROS from UV exposure. In contrast, GSH levels in both PS-treated groups were restored to levels similar to the UVB (-) group. Despite this, no significant difference between the doses was observed, indicating that either dose could have a beneficial effect.
Finally, melanin content was significantly increased in the UVB (+) group compared to the UVB (-) group. Increased melanin synthesis is vital for skin photoprotection (37, 38). However, melanin can also increase intracellular ROS, which causes DNA damage and p53 activation (39, 40). Hence, antioxidants are crucial to counter the ROS produced. In this study, both PS doses showed a non-significant decreasing trend in melanin, suggesting that higher doses may be required for notable effects.
There are several limitations in our study. First, pinch test results should be paired with an objective measurement of elastin to further reduce bias. Additionally, investigating other antioxidants, such as superoxide dismutase and GSH peroxidase, would broaden the understanding of PS's antioxidant profile. Finally, pairing melanin results with tyrosinase measurement would better elucidate PS's role in melanogenesis.
5.1. Conclusions
Overall, our study highlights the potential of oral PS to combat oxidative stress and complement topical treatments to mitigate photoaging. In UVB-induced skin photoaging, oral PS exhibited significant photoprotective properties. Notably, 60 mg/kg PS proved most effective in enhancing skin appearance and elasticity as well as reducing epidermal thickness, while 30 mg/kg was best at increasing collagen content. Both doses successfully restored GSH levels. However, neither dose significantly reduced melanin.
![A, Skin macroscopic appearance. The arrows indicate (black) coarse wrinkles, (red) skin erythema and (blue) skin peeling; B, Skin recovery time via pinch test. [a = significant vs. ultraviolet-B (UVB) (-), b = significant vs. UVB (+)]. A, Skin macroscopic appearance. The arrows indicate (black) coarse wrinkles, (red) skin erythema and (blue) skin peeling; B, Skin recovery time via pinch test. [a = significant vs. ultraviolet-B (UVB) (-), b = significant vs. UVB (+)].](https://services.brieflands.com/cdn/serve/3170b/fb10909376082ab8277fb7c362dbd4b595b16d2e/jjnpp-158908-i001-F1-preview.webp)
![A, Hematoxylin & Eosin (H&E) staining of skin at 10x magnification; B, Epidermal thickness length. [a = significant vs. Ultraviolet-B (UVB) (-), b = significant vs. UVB (+), c = significant vs. UVB (+) PS30]. A, Hematoxylin & Eosin (H&E) staining of skin at 10x magnification; B, Epidermal thickness length. [a = significant vs. Ultraviolet-B (UVB) (-), b = significant vs. UVB (+), c = significant vs. UVB (+) PS30].](https://services.brieflands.com/cdn/serve/3170b/06db941cefc5ff5044bb127e43486e220e78de25/jjnpp-158908-i002-F2-preview.webp)
![A, Masson's trichrome staining of skin at 10x magnification; B, relative expression of collagen content. [a = significant vs. ultraviolet-B (UVB) (-), b = significant vs. UVB (+), c = significant vs. UVB (+) PS30]. A, Masson's trichrome staining of skin at 10x magnification; B, relative expression of collagen content. [a = significant vs. ultraviolet-B (UVB) (-), b = significant vs. UVB (+), c = significant vs. UVB (+) PS30].](https://services.brieflands.com/cdn/serve/3170b/be06fb62683169c60648d88e87c01dad02b0ee7d/jjnpp-158908-i003-F3-preview.webp)
![A, Skin glutathione (GSH) level; B, skin melanin content. [a = significant vs. ultraviolet-B (UVB) (-), b = significant vs. UVB (+)]. A, Skin glutathione (GSH) level; B, skin melanin content. [a = significant vs. ultraviolet-B (UVB) (-), b = significant vs. UVB (+)].](https://services.brieflands.com/cdn/serve/3170b/296ca23a5d1b3f5c3c0604e415a44f6d51b15e79/jjnpp-158908-i004-F4-preview.webp)