1. Background
Substance dependence and the struggle to overcome it have long been significant concerns for individuals addicted to substances and their families. Each year, substantial resources are allocated in Iran for combating, preventing, and treating addiction, which often incurs not only financial losses but also psychological and physical harm to society (1). The approach of drug producers involves engaging the entire community with this phenomenon, particularly attracting individuals with specific issues. Substance use disorder is a chronic, progressive, and relapsing disease (2). There is a significant relationship between depression and anxiety disorders and substance use disorders (3). Despite existing research, it remains unclear whether substance use causes psychological disorders or if these disorders lead to substance use (4). Individuals with substance abuse problems are twice as likely to experience mood and anxiety disorders compared to the general population (5).
Every increase in pleasure from a stimulant is accompanied by a decrease in pleasure during withdrawal. Withdrawal from drugs eliminates the positive effects of acute drug administration on pleasure, resulting in a significant reduction in pleasure states. This sudden withdrawal leads to mood states resembling depression, similar to those observed in chronic stress. There is a gap in our knowledge regarding how to treat anxiety and depression resulting from substance withdrawal on addictive behaviors such as cravings and relapse over time, as pleasurable states fluctuate. One therapeutic approach to reduce this knowledge gap is the use of traditional medicine and herbal remedies. One of the substances that has garnered significant attention is ginseng, which has been used as a medicine for various diseases and disorders for over 4,000 years (6). Ginseng is a traditional herbal remedy widely used to address various health issues. Recently, its use has expanded to modern diseases such as cardiovascular diseases, cancer, aging, and Alzheimer's disease (7-9). Ginseng has been utilized alone or along with other herbal medications in traditional medicine to prevent the adverse effects of substance abuse and improve their side effects. Empirical studies have provided scientific evidence for this traditional use of ginseng. For example, systemic ginsenosides prevent the development of tolerance to the analgesic and hyperthermic effects of chronic morphine treatment in rodents (10). Older mice treated with ginseng improved cognitive performance through anti-inflammatory activities (11). Some studies have also reported that ginseng reduces addictive responses to drugs such as methamphetamine (METH), morphine, and cocaine (12, 13).
2. Objectives
The impact of ginseng on substance withdrawal has been relatively understudied, particularly in research conducted within Iran. The coexistence of mental disorders and addiction elevates the risk of various diseases, suicide, and poor treatment outcomes for individuals with substance use disorders. Anxiety and depression are significant factors that can increase the likelihood of concurrent substance use in patients undergoing treatment. The use of traditional herbal medicines may be beneficial in enhancing our understanding of the pathophysiology of this complex disorder and in providing options for drug withdrawal. Such novel therapeutic options can potentially reduce anxiety and depression associated with addiction withdrawal. To address the gap in knowledge regarding the treatment of anxiety and depression resulting from substance withdrawal, this study evaluated the effectiveness of ginseng on depression and anxiety symptoms in patients at addiction treatment clinics in Ahvaz.
3. Methods
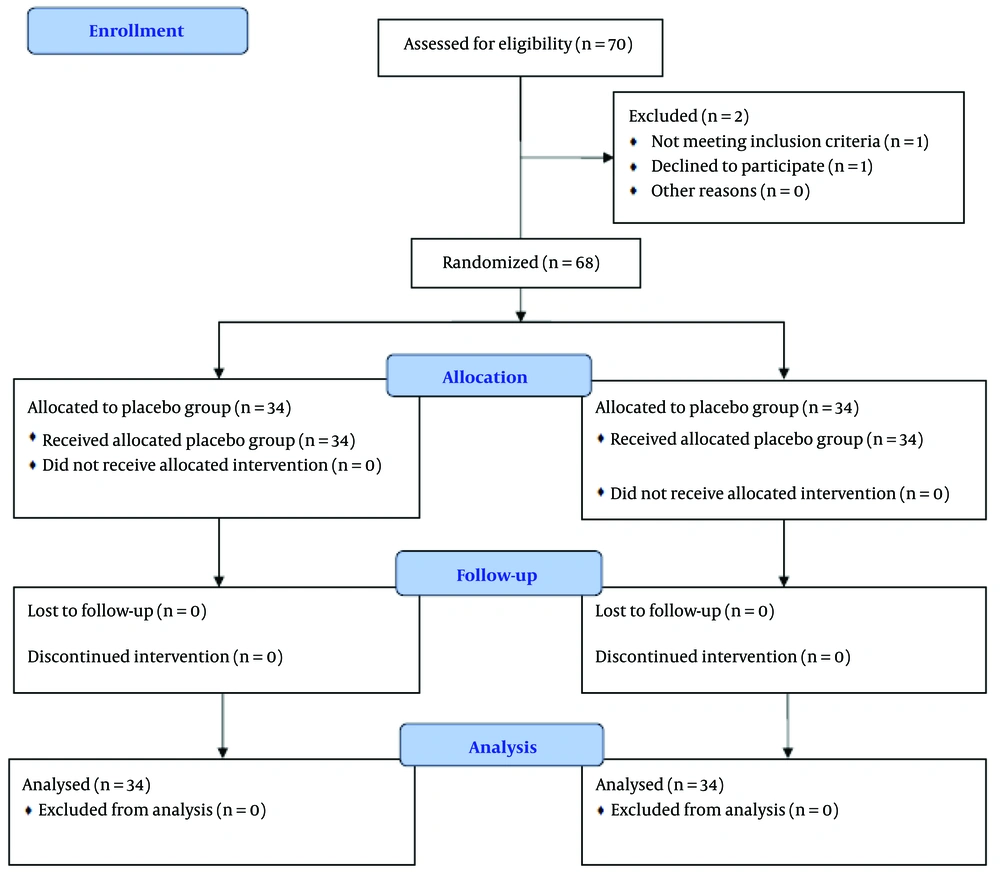
This research employs an experimental design for data collection and is classified as applied research. The study was conducted as a randomized clinical trial with a pre-test and post-test design involving two groups. The statistical population included all patients referred to addiction treatment clinics in Ahvaz during 2023 - 2024. A total of 70 participants met the inclusion and exclusion criteria; however, two participants withdrew, leaving 68 participants who were randomly assigned to two groups (34 participants each). This study was a double-blind clinical trial.
The study was conducted after obtaining an ethical code from the Ethics Committee of Ahvaz Jundishapur University of Ahvaz and receiving the IRCT code. The allocation of patients to the study groups was concealed using random allocation concealment, ensuring that the assigned group was not known before allocation. Random sequences were created using opaque sealed envelopes, with each random sequence recorded on a card placed inside the envelopes in order. To maintain the random sequence, numbering was done on the outer surface of the envelopes in the same order. Finally, the envelopes were sealed and placed in a box in order. At the time of participant enrollment, one of the envelopes was opened sequentially based on the order of eligible participants entering the study, revealing that participant's assigned group.
In one group, patients (34 participants) received ginseng capsules (100 mg), while in the other group (34 participants), a placebo was administered for four weeks. The placebo was prepared in coordination with the Faculty of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, consisting of tablets without active ingredients and containing only inert excipients in the required quantity. During the study period, patients were not deprived of their usual treatment (methadone and buprenorphine).
3.1. Inclusion and Exclusion Criteria
The inclusion criteria for the study were as follows: Participants aged 18 - 60 years, substance dependence for at least one year, stable substance use for at least the past month, no concurrent psychological treatments such as group therapy, cognitive-behavioral therapy, mindfulness, muscle relaxation, or stress management, and completion of a written consent form. The exclusion criteria included pregnant or breastfeeding mothers, patients with psychiatric disorders such as bipolar disorder, schizophrenia, dementia, and intellectual disabilities, as well as serious concurrent medical conditions and severe organic brain disorders based on the history and examination by a psychiatrist of the patient and family.
After coordinating and obtaining an ethical code from Jundishapur University of Medical Sciences in Ahvaz (IR.AJUMS.REC.1402.644) and a clinical trial code (IRCT20240310061238N1), the researcher obtained permission to enter the research environment. The research was conducted at the psychiatric clinic of Golestan Hospital in Ahvaz. Before preliminary assessments, all conditions for participation in the study were explained to applicants so that they could voluntarily enter the evaluation process. After explaining the research and its conditions along with other ethical considerations, participants were asked to complete an informed consent form.
Regarding sample selection, it should be noted that the sample size was determined using results obtained from other studies, such as, Lee et al., Braz et al., and Jeong et al. (14-16).
Considering a power of 80% and a type I error rate of 0.05, and accounting for a 10% dropout rate in each group, 34 participants were calculated for each group. In total, 70 individuals were assessed for eligibility. Two participants did not participate, resulting in a total of 68 individuals completing the study, with the final analysis conducted on these 68 participants. It is noteworthy that no costs were imposed on the participants in this research. All subjects were assessed at the beginning of the study and after the intervention using the Hamilton Depression Rating Scale (HDRS) and the Hamilton Anxiety Rating Scale (HARS), as illustrated in CONSORT Figure 1.
For data analysis, in addition to descriptive statistics, analysis of covariance, chi-square tests, and independent t-tests were employed. All analyses were performed using SPSS version 26 software.
3.2. Instruments
3.2.1. Hamilton Anxiety Rating Scale
The HARS was introduced by Hamilton in 1959 to measure the severity of anxiety symptoms in patients with various psychiatric issues (17). Each item is scored on a scale from 0 (not present) to 4 (severe, grossly disabling), with a total score range of 0 - 56. A total score of less than 17 indicates mild anxiety, a score of 18 - 24 indicates mild to moderate anxiety, and a score of 25 - 30 indicates moderate to severe anxiety (18).
3.2.2. Hamilton Depression Rating Scale
The HDRS, introduced by Hamilton in 1960, is used to measure the severity of depression symptoms in patients with various psychiatric conditions (19). More than half of the items on the HDRS are rated on a scale from 0 to 4, while the remaining items are scored from 0 to 2, except for weight loss, which is scored from 0 to 3. Patients with a score of 0 to 7 are considered normal or in a period of recovery. A total score greater than 20 indicates at least moderate to severe depression (20). The HDRS, along with the HARS, has been accepted as a gold standard in psychiatric practice for over 40 years due to its psychometric properties (21).
4. Results
In this study, 68 participants aged between 28 and 59 years, with a mean age of 43.22 ± 8.89 years, were included. Among these participants, 34 were assigned to the intervention group and 34 to the placebo group. The mean age of the intervention group was 42.23 ± 8.83 years, while the mean age of the placebo group was 44.20 ± 9.44 years. According to Table 1, results from the independent t-test indicated no significant difference in age between the two groups (P > 0.05). Additionally, the chi-square test results showed no significant differences in education level, gender, occupation, and medication use between the groups (P > 0.05).
| Variables | Groups | χ2 | P-Value | |
|---|---|---|---|---|
| Intervention | Placebo | |||
| Occupation | 0.77 | 0.38 | ||
| Employed | 25 | 28 | ||
| Unemployed | 9 | 6 | ||
| Marital status | 1.18 | 0.55 | ||
| Married | 28 | 30 | ||
| Single | 5 | 4 | ||
| Widowed | 1 | 0 | ||
| Academic status | 3.5 | 0.47 | ||
| Diploma | 11 | 13 | ||
| Associate degree | 6 | 2 | ||
| Bachelor | 1 | 2 | ||
| Master | 0 | 1 | ||
| Undergraduate | 16 | 16 | ||
| Medication | 2.34 | 0.30 | ||
| Opioid | 6 | 10 | ||
| Multidrug | 16 | 17 | ||
| Just methadone therapy | 10 | 7 | ||
| Psychiatric medication | 0.09 | 0.75 | ||
| Using psychiatric medication | 7 | 6 | ||
| Not using psychiatric medication | 27 | 28 | ||
As shown in Table 2, the mean post-test scores compared to the pre-test scores in the intervention group did not demonstrate a greater reduction in anxiety and depression variables compared to the placebo group. Prior to conducting the analysis of covariance, the assumptions of the method were verified, including the independence of observations, normality of the distribution of the dependent variable, homogeneity of variances, homogeneity of regression slopes, and homogeneity of the variance-covariance matrix across different groups (Tables 3 and 4).
| Variables and Groups | Pre-test | Post-test |
|---|---|---|
| Depression | ||
| Ginseng | 29.85 ± 6.19 | 15.44 ± 11.37 |
| Placebo | 27.79 ± 7.21 | 11.91 ± 10.83 |
| Anxiety | ||
| Ginseng | 25.00 ± 6.06 | 12.26 ± 10.21 |
| Placebo | 23.91 ± 4.82 | 13.32 ± 8.10 |
a Values are expressed as mean ± SD.
| Variable | Source of Change | SS | df | F | P-Value | Effect Size | Statistical Power |
|---|---|---|---|---|---|---|---|
| Depression | Group | 151.924 | 1 | 1.24 | 0.27 | 0.019 | 0.19 |
a The effectiveness of ginseng on depression is not significant (P > 0.05).
| Variable | Source of Change | SS | df | F | P-Value | Effect Size | Statistical Power |
|---|---|---|---|---|---|---|---|
| Anxiety | Group | 39.920 | 1 | 0.496 | 0.484 | 0.008 | 0.107 |
5. Discussion
In the past three decades, the comorbidity of substance use disorders and mental disorders has garnered extensive attention. Substantial evidence indicates that substance use disorders are more prevalent among individuals with mental health issues compared to the general population (22). Mental disorders and substance use disorders can directly influence each other (5), with anxiety and depression being significant factors in this interaction. Negative emotions are recognized as risk factors for addiction and vulnerability to relapse (23). Conversely, the disorders themselves can lead to treatment resistance and result in a return to substance use (24). Unresolved questions remain regarding the underlying mechanisms of ginseng's effect as a therapeutic intervention for treating withdrawal symptoms related to substances, such as anxiety and depression. Therefore, this study was conducted to evaluate the effectiveness of ginseng on the symptoms of depression and anxiety in patients attending addiction treatment clinics in Ahvaz. Our study results, comparing the two groups, showed that the mean post-test scores compared to pre-test scores in the intervention group did not demonstrate a significant reduction compared to the placebo group.
Nah et al. investigated the effects of ginseng extract (ginseng saponin) on the brain's response to cocaine in rats. They found that when administered with cocaine, ginseng saponin reduced the excessive release of dopamine induced by the drug. This effect also helped prevent the abrupt increase in dopamine levels that occurs during cocaine withdrawal. The strength of this effect was dose-dependent on the amount of ginseng saponin used. However, ginseng saponin did not appear to influence cocaine's blockade of dopamine reuptake (25).
Kim et al. investigated the effects of Korean red ginseng (KRG) on alcohol's impact on the brain. Their research, conducted on mice, suggests that KRG may help reduce alcohol-related memory problems and addictive behaviors (26). This beneficial effect appears to result from KRG's ability to suppress brain inflammation, which is often triggered by alcohol consumption. Specifically, KRG seems to prevent the decrease in a crucial brain protein called brain-derived neurotrophic factor (BDNF), which is important for brain health. While these findings are promising, a separate review by Smith et al. found that although ginseng may have positive effects on cognitive function in humans, more conclusive evidence is still needed (27).
Lee et al. conducted research on a red ginseng (RG)-based drink designed to combat hangovers. Their findings suggest that this drink is effective in reducing hangover symptoms, as evidenced by decreased blood alcohol levels, reduced alcohol in the breath, and a significant improvement in overall hangover severity (28). Furthermore, Lee et al. (14) explored the use of KRG as an augmentation therapy for patients with treatment-resistant depression. Their study indicated that adding RG to standard treatments may be an effective and well-tolerated approach for improving outcomes in these challenging cases. Additionally, Lee et al. (as cited by Kim et al.) (26) examined whether wild ginseng (WG) reduces anxiety- and depression-like behaviors during morphine withdrawal. Daily administration of WG significantly reduced anxiety- and depression-like behaviors and suppressed the expression of corticotropin-releasing factor (CRF) while stimulating the expression of neuropeptide Y (NPY) in the hypothalamus. The results indicated that WG extract may be effective in inhibiting anxiety and depression responses associated with morphine withdrawal by modulating the hypothalamic CRF and NPY systems. Furthermore, these findings suggest that WG extract could be utilized for developing new medications to treat or alleviate morphine withdrawal symptoms and to prevent relapse in morphine use.
The potential antidepressant effects of RG have been consistently reported in numerous preclinical studies and a few clinical trials for depression and related disorders. An acceptable mechanism for KRG augmentation (RGA) as an antidepressant may involve its modulation and regulation of various neurotransmitters, neuroendocrine pathways, neural signaling pathways, neuroinflammatory pathways, and neuroprotective processes, among others (29). Stress and depression disrupt the expression and function of BDNF in the hippocampus and prefrontal cortex, which are critical for synaptic plasticity (30). Red ginseng positively restores BDNF signaling pathways in patients with major depressive disorder (MDD) (31). Additionally, saponins have reversing effects on the chronic reduction of monoamine neurotransmitters, including norepinephrine, dopamine, and homovanillic acid, induced by mild stress (32). Red ginseng improved aimlessness, hopelessness, and sleep disorders through the modulation of corticosterone, testosterone, androgen receptors, and glucocorticoid receptors (GR) (33). In the first double-blind randomized clinical trial (63 participants) (34), RG demonstrated better cognitive responses and stabilization of the sympathetic nervous system through the regulation of epinephrine and steroids, which aligns with the positive effects of GR on stress control. This also corresponds with the findings of a remote study that showed improvements in anxiety, depression, fatigue, and physical symptoms, evidenced by a reduction in the cortisol to DHEA-S ratio.
Recent studies indicate that Panax ginseng (PG) reduces symptoms of depression and anxiety disorders in humans. Some studies have reported that PG demonstrates antidepressant activities in the forced swimming test (FST) and also reduces anxiety-like behavior in the elevated plus maze (EPM) in animal models. Ossoukhova et al. (35) investigated a single dose of American ginseng (Panax quinquefolius L.) in healthy middle-aged adults and found no significant effect on mood or blood glucose levels. The main strength of the present study is that it is the first to investigate ginseng as an alternative therapeutic option for anxiety and depression related to addiction withdrawal in Iran. The level of improvement and response was quite disappointing and lower than reported in other trials, which may be attributed to the lack of initial therapeutic effects of ginseng, the pharmacological characteristics of ginseng, the small sample size, external factors, lack of sample diversity, and low sensitivity of the scales used. Ginseng was well-tolerated in this study, and no side effects were reported. The sample size was small, although the power was sufficient to detect symptomatic changes during the study. The pharmacokinetics of ginseng were not controlled, and biomarkers were not examined. Due to time constraints, it was not possible to conduct a follow-up phase. Another limitation of the research relates to the generalizability of the results; therefore, caution should be exercised when generalizing to non-experimental conditions and other clinical groups. Accordingly, it is suggested that future research be conducted with larger sample sizes and follow-up phases. However, randomized controlled trials with sufficient power are needed for confirmation.
5.1. Conclusions
The results of our study comparing the two groups indicated that the mean post-test scores, compared to pre-test scores in the intervention group, did not demonstrate a significant reduction compared to the placebo group. However, randomized controlled trials with larger sample sizes and sufficient power are needed for confirmation. The results of this study should be interpreted with caution.