1. Background
Global brain ischemia occurs in events such as cardiac arrest, severe hypotension, and carotid artery stenosis (1, 2). During cerebral ischemia, the activation of multiple toxic biochemical pathways, including excitotoxicity, mitochondrial dysfunction, oxidative stress, neuroinflammation, and apoptosis, leads to blood-brain barrier (BBB) breakdown, neuronal death, and neurological dysfunction (3). Although treatments such as intravenous thrombolysis and mechanical thrombectomy improve stroke outcomes, pharmacological options for ischemic stroke remain limited (1-3), highlighting the need for new therapeutic solutions. Targeting pathological pathways with natural compounds such as flavonoids and polyphenols, which possess antioxidant, anti-inflammatory, and anti-apoptotic properties (4, 5), may protect the BBB and mitigate neuronal damage in cerebral ischemia.
Chrysin, a natural flavonoid found in plants, honey, and propolis, exhibits antioxidant, anti-inflammatory, and anti-apoptotic properties, making it therapeutically significant (6, 7). Preclinical research suggests that chrysin may be beneficial in treating various neurodegenerative disorders, including multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, and traumatic brain injuries (7-9). Furthermore, several recent experimental studies have demonstrated that chrysin reduces brain damage and improves cognitive function in ischemic stroke models (10-13). Despite these benefits, the impact of chrysin on BBB integrity remains unclear, warranting further investigation.
Gallic acid (GA), a natural polyphenol found in plants, has various pharmacological effects, including neutralizing free radicals, modulating inflammation, and inhibiting apoptosis (4). Animal studies have demonstrated the potential of GA to protect the brain from ischemic damage by inhibiting oxidative stress, pro-inflammatory cytokines, and apoptosis (14-16). Moreover, a preclinical investigation showed the ability of GA to preserve BBB integrity following ischemic stroke (17).
The BBB maintains central nervous system homeostasis by controlling substance exchange between the blood and brain (18). Cerebral ischemia often leads to BBB damage, closely associated with structural changes in tight junction proteins, particularly claudin-5, a key component of the intercellular junctions in endothelial cells that preserves barrier integrity (18, 19). Loss of claudin-5 disrupts the BBB, resulting in increased permeability, brain edema, inflammation, and worsened neuronal damage (19). Preserving claudin-5 is essential for minimizing injury in ischemic conditions (18, 19), as it helps maintain tight junctions, protect BBB integrity, and support overall cerebrovascular health.
Chrysin and GA are natural flavonoid and polyphenol compounds that show potential in treating ischemic brain injury by targeting glutamate excitotoxicity, oxidative stress, neuroinflammation, and apoptosis (7, 12, 14, 20). These compounds may act synergistically, enhancing neuroprotection through complementary mechanisms. Combining chrysin and GA could provide greater protection against cerebral ischemia compared to monotherapy in a cerebral hypoperfusion model.
2. Objectives
This study aimed to examine the effects of chrysin, GA, and their combination on neuronal damage in the CA1, CA3, and dentate gyrus (DG) regions of the hippocampus, spatial memory, BBB integrity, and claudin-5 expression in a mouse model of cerebral hypoperfusion induced by bilateral common carotid artery occlusion (BCCAO).
3. Methods
3.1. Animals
In this experimental study, male Swiss albino mice, weighing 30 to 40 grams and aged 3 to 4 months, were obtained from the animal facility at the Semnan Research Center of Physiology. All procedures adhered to established guidelines for the care and use of experimental animals.
3.2. Experimental Approach and Grouping
In this study, we investigated the effects of chrysin (purity: 97%, Cat No. C80105, Sigma-Aldrich, Germany) and GA (purity ≥ 98.5%, Cat No. G7384, Sigma-Aldrich, Germany), both individually and in combination, on neuronal damage, neurological impairments, and spatial memory function. A total of 30 mice were randomly assigned to five equal groups (n = 6 per group) as follows:
(1) Sham-operated group: Animals underwent surgical procedures without BCCAO.
(2) Ischemic control group (BCCAO + DMSO): Dimethyl sulfoxide (DMSO 2%, intraperitoneally; 0.3 mL) was injected at the beginning, 30 minutes, and 1 hour after reperfusion.
(3) Treatment group 1 (BCCAO + chrysin): Chrysin (30 mg/kg, intraperitoneally) was administered at the beginning, 30 minutes, and 1 hour after reperfusion.
(4) Treatment group 2 (BCCAO + GA): GA (50 mg/kg, intraperitoneally) was given at the beginning, 30 minutes, and 1 hour after reperfusion.
(5) Treatment group 3 (BCCAO + chrysin + GA): Mice received a combination of chrysin (30 mg/kg, intraperitoneally) and GA (50 mg/kg, intraperitoneally) at the beginning, 30 minutes, and 1 hour after reperfusion.
Forty-eight hours after reperfusion, the neurological deficit score and spatial memory function were assessed in the groups. Subsequently, four to five animals from each group were randomly selected and deeply anesthetized. Brains were carefully extracted and fixed in a 10% formalin solution for neuronal damage evaluation and claudin-5 expression analysis.
In the second part of the study, Evans blue (EB) dye (Cat No. E2129, Sigma-Aldrich, Germany) extravasation was utilized to assess the integrity of the BBB. To evaluate the extent of EB leakage as an index of BBB permeability, 35 mice were randomly assigned to five equally sized groups (n = 7 per group), following the experimental setup described in the first part of the study.
3.3. Cerebral Ischemia
Transient global cerebral ischemia was induced in mice under anesthesia with ketamine (60 mg/kg, intraperitoneally) and xylazine (10 mg/kg, intraperitoneally) (21). A midline cervical incision was made to expose the common carotid arteries, which were bilaterally occluded for 30 minutes using clamps, followed by reperfusion for 48 hours. Buprenorphine (0.05 mg/kg, intraperitoneally) was administered pre-surgery and post-surgery for pain management.
3.4. Neurological Examination
Sensory and motor dysfunctions were evaluated 48 hours after ischemia/reperfusion using a standardized guideline (21). According to the protocol, animals were assigned scores ranging from 0 to 14. Scores between 10 and 14 indicated severe disability, scores from 5 to 9 reflected moderate disability, and scores from 1 to 4 represented mild disability.
3.5. Radial Arm Water Maze Test
Sensory and motor dysfunctions were evaluated 48 hours after ischemia/reperfusion using a standardized guideline (21). According to the protocol, animals were assigned scores ranging from 0 to 14. Scores between 10 and 14 indicated severe disability, scores from 5 to 9 reflected moderate disability, and scores from 1 to 4 represented mild disability.
3.6. Hematoxylin and Eosin Staining
Hematoxylin and eosin (H&E) staining was used to assess neuronal damage in the hippocampal CA1, CA3, and DG regions. Brain tissue was fixed in formalin, embedded in paraffin, sectioned at 5 µm, deparaffinized, and stained with H&E. Sections were then dehydrated, cleared, and examined under a 400x light microscope. ImageJ software was used to calculate the percentage of living cells in five random fields. Living cells exhibited distinct borders, blue nuclei, and pink cytoplasm, whereas damaged neurons displayed dark, condensed cytoplasm and irregular features.
3.7. Blood-Brain Barrier Permeability
Approximately 60 minutes after BCCAO, a 2% EB dye solution (2 mL/kg) was injected via the tail vein. After 48 hours of reperfusion, animals were euthanized under deep anesthesia, and cardiac perfusion with saline was performed to clear the vascular system. Brains were homogenized in phosphate-buffered saline (PBS) and 60% trichloroacetic acid, then centrifuged. The absorbance of the supernatant was measured at 610 nm to determine the EB concentration. Values were compared to a standard curve and expressed as µg/g of brain tissue (23).
3.8. Immunohistochemistry
The brain was fixed in 10% formalin, embedded in paraffin, and sectioned at a thickness of 5 μm. Sections were deparaffinized, rehydrated, and treated with 5% goat serum and 0.4% Triton X-100 (X100, Sigma-Aldrich Chemie GmbH, Germany) to block non-specific binding. They were incubated overnight with rabbit anti-claudin-5 primary antibodies (1:100, Cat No. GTX00796, GeneTex, Inc., CA 92606, USA), followed by incubation with FITC-conjugated secondary antibodies (1:150, Cat No. orb688925, Biorbyt Ltd., United Kingdom). Cell nuclei were stained with DAPI, and sections were examined under a fluorescent microscope at 400× magnification to detect claudin-5 expression (green fluorescence). ImageJ software was used to quantify claudin-5 positive cells as a percentage of DAPI-stained nuclei.
3.9. Data Analyses
The Shapiro-Wilk test was used to assess data normality. Parametric data, including hippocampal damage, claudin-5 expression, BBB permeability, and target zone time, were analyzed using one-way ANOVA with Tukey's post-hoc test. Non-parametric data, including neurological scores and escape latency, were analyzed using the Kruskal-Wallis test with Dunn's post-hoc tests. The overall experimental procedure was conducted in a blinded manner to minimize bias. Results are presented as mean ± SEM for parametric data and median (IQR) for non-parametric data, with significance set at P < 0.05. Analyses were performed using GraphPad Prism 10.
4. Results
4.1. The Combination of Chrysin with Gallic Acid Protects Hippocampal Neurons Against Cerebral Ischemia
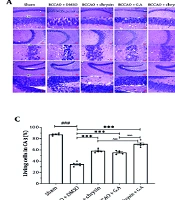
Hematoxylin and eosin staining results showed that cerebral ischemia led to a marked decrease in viable neurons in the CA1 (26 ± 1.6%), CA3 (34 ± 1.7%), and DG (33 ± 1.03%) regions compared to the sham group (Figure 1A and B; P < 0.001). Chrysin treatment significantly improved neuronal survival in the CA1 (46 ± 2.3%), CA3 (55 ± 1.3%), and DG (50 ± 1.3%) regions (Figure 1A - D; P < 0.001). Similarly, GA administration enhanced neuronal survival in the CA1 (37 ± 1.4%), CA3 (58 ± 1.4%), and DG (51 ± 1.32%) regions (Figure 1A - D; P < 0.001). Moreover, the combined treatment with chrysin and GA resulted in a substantial increase in neuronal survival in the CA1 (69 ± 2.5%), CA3 (71 ± 2.18%), and DG (72 ± 1.28%) regions, compared to the BCCAO + DMSO and individual treatment groups (Figure 1A - D; P < 0.001).
A, representative images of tissue sections stained with hematoxylin and eosin (H&E) in the hippocampal CA1, CA3, and D.G regions (400x magnification). The percentages of living cells in the hippocampal regions; B, CA1, C, CA3; and D, D.G are presented for the sham-operated group, the control group [bilateral common carotid artery occlusion (BCCAO) + DMSO], and groups treated with chrysin (BCCAO + chrysin), gallic acid (GA) (BCCAO + GA), and their combination (BCCAO + chrysin + GA). The results are presented as the mean ± SEM (n = 5, each). ### P < 0.001 vs. sham group, *** P < 0.001, vs. BCCAO + DMSO as control group. ^^^ P < 0.001 vs. chrysin and G.A groups, + P < 0.05 vs. chrysin group.
4.2. The Combination of Chrysin with Gallic Acid Reduced Spatial Memory Impairment and Neurological Deficit Score Following Cerebral Ischemia
A two-way repeated measures ANOVA (group × days) indicated no significant differences between the groups (P = 0.95), but a significant difference between days (P < 0.0001), and no interaction (Figure 2A; P = 0.99). The administration of chrysin (P = 0.0048) and its combination with GA (P = 0.002) significantly reduced escape latency time (P = 0.008) and increased the time spent in the target zone (Figure 2B and C; P = 0.027). Moreover, chrysin + GA treatment significantly reduced neurological deficit scores (Figure 2D; P = 0.0346).
A, escape latency time (in seconds) during the four days of training using the RAWM; B, escape latency time in the probe trial; C, time spent in the target zone; and D, neurological deficit scores are shown in the sham-operated group, control group [bilateral common carotid artery occlusion (BCCAO) + DMSO], and groups treated with chrysin (BCCAO + chrysin), gallic acid (GA) (BCCAO + GA), and their combination (BCCAO + chrysin + GA). Parametric results are presented as mean ± SEM, while non-parametric data (escape latency time and neurological deficit scores) are displayed as the median and interquartile range (IQR) (n = 6, each). Statistical significance is indicated as ### P < 0.001, ## P < 0.01, and # P < 0.05 compared to the sham group, and ** P < 0.01 and * P < 0.05 compared to the BCCAO + DMSO group.
4.3. The Combination of Chrysin with Gallic Acid Enhanced the Expression of Claudin-5 and Reduced Blood-Brain Barrier Disruption Following Cerebral Ischemia
Assessment of claudin-5 expression 48 hours after ischemia showed a marked reduction in claudin-5 protein levels (11 ± 1.65%) compared to the sham group (64 ± 1.64%) (Figure 3A and B; P < 0.001). Administration of chrysin (31 ± 2.02%), GA (29 ± 2.13%), and their combination (38 ± 1.29%) significantly enhanced the expression of claudin-5 in the brain compared to the BCCAO + DMSO group (Figure 3A and B; P < 0.001). The combination of chrysin and GA significantly increased claudin-5 expression compared to the GA group (Figure 3B; P = 0.016). The EB dye leakage into brain tissue increased significantly at 48 hours post-ischemia (Figure 3C; P < 0.001). Treatment with chrysin, GA, or their combination significantly reduced EB leakage compared to the BCCAO + DMSO group (Figure 3C; P < 0.001).
A, immunofluorescence images show claudin-5 expression in the endothelial cells of brain blood vessels (400x magnification). Green indicates claudin-5, while blue represents DAPI (nuclear stain); B, quantitative analysis of claudin-5 expression (n = 4, each); and C, EB leakage (µg/g/tissue, n = 7, each) into brain tissue are presented for the sham-operated group, the control group [bilateral common carotid artery occlusion (BCCAO) + DMSO], and the groups treated with chrysin (BCCAO + chrysin), gallic acid (GA) (BCCAO + GA), and their combination (BCCAO + chrysin + GA). Results are expressed as mean ± SEM, with statistical significance indicated as ### P < 0.001 compared to the sham group, *** P < 0.001 compared to the BCCAO + DMSO group, and ^ P < 0.05 compared to the BCCAO + GA group. Scale bar = 100 μm.
5. Discussion
Our findings indicated that the administration of chrysin or GA alone can effectively protect hippocampal neurons from ischemic injury, consistent with several earlier studies (11, 15-17). Additionally, we demonstrated that the combined intervention synergistically enhanced neuron survival in the hippocampus, which had not been addressed in previous research. The synergy between chrysin and GA might arise from their complementary effects on reducing oxidative stress, inflammation, and apoptosis. The most significant finding of this study was that the combined intervention notably reduced sensory-motor and spatial memory dysfunction. These behavioral improvements were further supported by histological analysis, which revealed a significant increase in neuronal survival in the combined treatment group. These results suggest a positive correlation between improvements in behavioral outcomes and enhanced neuronal survival rates.
Cerebral ischemia disrupts the BBB, worsening brain damage and neurological outcomes. Our findings revealed that treatment with chrysin, GA, or their combination reduced BBB disruption. In line with our results, a recent study demonstrated that GA protected the BBB against cerebral ischemia (17). In the present study, the reduction in BBB disruption was accompanied by improved survival of hippocampal neurons and behavioral outcomes. This data suggests that preserving BBB integrity may help promote neuronal survival and improve overall brain health in stroke patients.
In this study, claudin-5 was chosen as a key marker of tight junction proteins due to its high expression in the BBB (19, 22) and its vital role in maintaining barrier integrity (18, 19). Our results showed a significant decrease in claudin-5 expression 48 hours after ischemia-reperfusion, indicating its role in BBB disruption. Consistent with previous studies (17), we found that GA upregulated claudin-5 expression, suggesting its potential to preserve BBB integrity during ischemia. The mechanism by which GA and Chrysin protect claudin-5 against cerebral ischemia remains unclear. However, evidence suggests that they may enhance claudin-5 expression and maintain BBB integrity by activating the MAPK/ERK and PI3K/Akt pathways. These pathways play a crucial role in mitigating inflammation and oxidative stress (19, 23, 24), ultimately supporting neuroprotection (25).
This study did not fully clarify the molecular mechanisms underlying the neuroprotective effects of chrysin, GA, and their combination. However, their antioxidant, anti-inflammatory, and anti-apoptotic properties, as well as their ability to reduce BBB permeability, may contribute to these effects (6, 12, 16, 20). Additionally, they may promote BDNF expression and mitigate glutamate excitotoxicity (26, 27), further supporting their neuroprotective potential.
5.1. Conclusions
This study found that the combination of chrysin and GA synergistically enhanced neuron survival and improved neurological outcomes, while also reducing spatial memory loss in a non-synergistic manner. The treatment preserved BBB integrity by upregulating claudin-5 expression. We suggest that this combined intervention could serve as a promising therapeutic approach for ischemic stroke patients in clinical practice. Further research is needed to investigate the underlying mechanisms of this synergy and evaluate its long-term effects in both experimental and clinical settings.
![A, representative images of tissue sections stained with hematoxylin and eosin (H&E) in the hippocampal CA1, CA3, and D.G regions (400x magnification). The percentages of living cells in the hippocampal regions; B, CA1, C, CA3; and D, D.G are presented for the sham-operated group, the control group [bilateral common carotid artery occlusion (BCCAO) + DMSO], and groups treated with chrysin (BCCAO + chrysin), gallic acid (GA) (BCCAO + GA), and their combination (BCCAO + chrysin + GA). The results are presented as the mean ± SEM (n = 5, each). ### P < 0.001 vs. sham group, *** P < 0.001, vs. BCCAO + DMSO as control group. ^^^ P < 0.001 vs. chrysin and G.A groups, + P < 0.05 vs. chrysin group. A, representative images of tissue sections stained with hematoxylin and eosin (H&E) in the hippocampal CA1, CA3, and D.G regions (400x magnification). The percentages of living cells in the hippocampal regions; B, CA1, C, CA3; and D, D.G are presented for the sham-operated group, the control group [bilateral common carotid artery occlusion (BCCAO) + DMSO], and groups treated with chrysin (BCCAO + chrysin), gallic acid (GA) (BCCAO + GA), and their combination (BCCAO + chrysin + GA). The results are presented as the mean ± SEM (n = 5, each). ### P < 0.001 vs. sham group, *** P < 0.001, vs. BCCAO + DMSO as control group. ^^^ P < 0.001 vs. chrysin and G.A groups, + P < 0.05 vs. chrysin group.](https://services.brieflands.com/cdn/serve/3170b/b21e9c6bd1f899bd50559a19a8666da2e8f541f6/jjnpp-159908-i001-F1-preview.webp)
![A, escape latency time (in seconds) during the four days of training using the RAWM; B, escape latency time in the probe trial; C, time spent in the target zone; and D, neurological deficit scores are shown in the sham-operated group, control group [bilateral common carotid artery occlusion (BCCAO) + DMSO], and groups treated with chrysin (BCCAO + chrysin), gallic acid (GA) (BCCAO + GA), and their combination (BCCAO + chrysin + GA). Parametric results are presented as mean ± SEM, while non-parametric data (escape latency time and neurological deficit scores) are displayed as the median and interquartile range (IQR) (n = 6, each). Statistical significance is indicated as ### P < 0.001, ## P < 0.01, and # P < 0.05 compared to the sham group, and ** P < 0.01 and * P < 0.05 compared to the BCCAO + DMSO group. A, escape latency time (in seconds) during the four days of training using the RAWM; B, escape latency time in the probe trial; C, time spent in the target zone; and D, neurological deficit scores are shown in the sham-operated group, control group [bilateral common carotid artery occlusion (BCCAO) + DMSO], and groups treated with chrysin (BCCAO + chrysin), gallic acid (GA) (BCCAO + GA), and their combination (BCCAO + chrysin + GA). Parametric results are presented as mean ± SEM, while non-parametric data (escape latency time and neurological deficit scores) are displayed as the median and interquartile range (IQR) (n = 6, each). Statistical significance is indicated as ### P < 0.001, ## P < 0.01, and # P < 0.05 compared to the sham group, and ** P < 0.01 and * P < 0.05 compared to the BCCAO + DMSO group.](https://services.brieflands.com/cdn/serve/3170b/8503ecdf05db60eceabe6e56372ecf6a8244c61c/jjnpp-159908-i002-F2-preview.webp)
![A, immunofluorescence images show claudin-5 expression in the endothelial cells of brain blood vessels (400x magnification). Green indicates claudin-5, while blue represents DAPI (nuclear stain); B, quantitative analysis of claudin-5 expression (n = 4, each); and C, EB leakage (µg/g/tissue, n = 7, each) into brain tissue are presented for the sham-operated group, the control group [bilateral common carotid artery occlusion (BCCAO) + DMSO], and the groups treated with chrysin (BCCAO + chrysin), gallic acid (GA) (BCCAO + GA), and their combination (BCCAO + chrysin + GA). Results are expressed as mean ± SEM, with statistical significance indicated as ### P < 0.001 compared to the sham group, *** P < 0.001 compared to the BCCAO + DMSO group, and ^ P < 0.05 compared to the BCCAO + GA group. Scale bar = 100 μm. A, immunofluorescence images show claudin-5 expression in the endothelial cells of brain blood vessels (400x magnification). Green indicates claudin-5, while blue represents DAPI (nuclear stain); B, quantitative analysis of claudin-5 expression (n = 4, each); and C, EB leakage (µg/g/tissue, n = 7, each) into brain tissue are presented for the sham-operated group, the control group [bilateral common carotid artery occlusion (BCCAO) + DMSO], and the groups treated with chrysin (BCCAO + chrysin), gallic acid (GA) (BCCAO + GA), and their combination (BCCAO + chrysin + GA). Results are expressed as mean ± SEM, with statistical significance indicated as ### P < 0.001 compared to the sham group, *** P < 0.001 compared to the BCCAO + DMSO group, and ^ P < 0.05 compared to the BCCAO + GA group. Scale bar = 100 μm.](https://services.brieflands.com/cdn/serve/3170b/2af58e84b83313e62cbaf9289b4592c0d232b876/jjnpp-159908-i003-F3-preview.webp)