1. Context
Humankind has long used plants to meet their healing needs. Medicinal plants remain valuable sources of therapeutic agents for combating diseases (1-4). In recent years, the demand for medicinal plants has been increasing (1, 5). The number of publications in this field has more than tripled over a 10-year period (2008 - 2018), indicating growing attention and interest in research in this area (2). Artemisia is a plant genus that has attracted significant interest due to its diverse biological properties and secondary metabolites. Several species in this genus have been used to prepare herbal remedies for treating major and minor ailments in the forms of herbal teas, beverages, tonics, and cosmetics (6). The genus Artemisia has a long history in traditional medicine and ethnobotanical use worldwide, offering treatments for a broad range of disorders, such as malaria, inflammatory diseases, jaundice, toothache, gastrointestinal problems, menstrual pains, wounds, diarrhea, skin disorders, headache, and intestinal parasites. One of the most important uses of the genus in modern medicine is in the treatment of malaria. Artemisia is a promising plant genus for cancer treatment (7, 8). Artemisia is one of the largest and most widespread genera in the family Asteraceae (9). According to Plants of the World Online (2024), Artemisia includes 499 accepted species. The Artemisia species are widely distributed in temperate regions of the world (10). Asia is home to the highest species diversity, with over 170 species in the ex-USSR, 150 species in China, almost 50 species from Japan, and 35 species of this genus reported in Iran (11). About 15 species of the genus grow in the Southern Hemisphere, of which four species are native to southern Africa and five species to South America. Humans have gradually introduced six species for cultivation in the Southern Hemisphere (12). Artemisia species are adaptable to different habitats (13). They have a wide geographic distribution in Iran (14, 15). One of these species that grows in Iran is Artemisia ciniformis Krasch. & Popov ex Poljakov. In Persian, it is called “Dermaneye talaaie” and “Dermaneye sakhrerooy” (15, 16). Unfortunately, unlike many other species of the genus Artemisia, there are only fragmentary reports about the species A. ciniformis. Different types of extracts, fractions, and the volatile oil of this plant species have interesting biological effects. A number of phytochemical studies have reported the presence of different classes of secondary metabolites in A. ciniformis. There is no complete report on the characteristics of this species. This review attempts to fill this gap and provide comprehensive information on the distribution, botanical characteristics, chemical composition, and biological effects of this beneficial plant species.
2. Evidence Acquisition
A literature search using the keywords "Artemisia ciniformis", "A. ciniformis", "Seriphidium ciniforme", and "ciniformis" was conducted in the databases of Google Scholar, Scopus, Science Direct, ResearchGate, PubMed, Web of Science, Wiley Online Library, SpringerLink, and Plants of the World Online, until December 2024. Available books were used to review some details. We carefully reviewed the available materials. All relevant materials were selected for the preparation of this report. Any data related to biological activity, botanical characteristics, folk medicine, and phytochemical constituents of A. ciniformis were extracted. The chemical structures and CAS registry numbers were retrieved from the PubChem database and drawn using ChemDraw Professional 16.0 software.
3. Results
3.1. Distribution
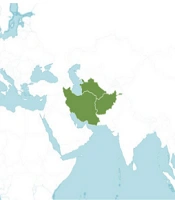
The Artemisia genus is distributed worldwide but is more widespread in the Northern Hemisphere, with a few species growing in the Southern Hemisphere (17). Artemisia species can be found in many ecosystems, from humid environments to desert communities, and from altitudes close to sea level to high mountain peaks with an altitude of about 4000 meters (18). Artemisi ciniformis grows at an altitude of 1200 - 2800 meters above sea level on sandy and clay slopes (19). This species is distributed in the Irano-Turanian region, including Iran, Turkmenistan, and Afghanistan (Figure 1) (20, 21).
Distribution of Artemisia ciniformis (22)
3.2. Botanical Characteristics
3.2.1. Taxonomic Classification and Phylogeny
The taxonomic data related to this plant species (12, 22, 23) are presented in Table 1. Artemisia is the largest genus within the tribe Anthemideae of the Asteraceae family (24). The adaptation of the Artemisia genus to its wide geographical distribution and diverse habitats has been accompanied by changes in morphology, anatomy, cellular structure, and function (25). Therefore, Artemisia has various ploidy levels: Dodecaploid, decaploid, octaploid, hexaploid, tetraploid, and diploid. The basic chromosome number in this genus is usually nine, but sometimes there are fewer chromosomes (x = 8) (26). The genus Artemisia is divided into several subgenera based on morphological characteristics, including pollen type and functional sexual spatial arrangement of the floret within the capitula: Artemisia, Absinthium, Dracunculus, Seriphidium, Tridentatae (26, 27), Pacifica (28). Artemisia ciniformis is included in the subgenus Seriphidium. The characteristic of this subgenus is that the capitulum lacks outer florets, contains fertile and bisexual florets, and the receptacle is glabrous (28). Also, the number of chromosomes in this subgenus is based on x = 9 and different ploidy levels (29). So far, the chromosomes of A. ciniformis have been counted three times: The first time in 1964 in the Turkmen population, where the number of chromosomes was reported as 2n = 36 (30). The second time in 2001, in the Iranian population, where the number of chromosomes was reported as 2n = 18, and the diploid level of this taxon was determined (31), and the third time in 2013 in the Iranian population, where the number of chromosomes was reported as 2n = 2x = 18 + 0 - 1B and a lower ploidy level was mentioned for it (29).
| Kingdom | Plantae |
|---|---|
| Subkingdom | Tracheobionta |
| Super division | Spermatophyta |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Subclass | Asteridae |
| Order | Asterales |
| Family | Asteraceae, Compositae |
| Tribe | Anthemideae |
| Genus | Artemisia L. |
| Species | Artemisia ciniformis Krasch. & Popov ex Poljakov synonyms: Seriphidium ciniforme (Krasch. & Popov ex Poljakov) Poljakov |
3.2.2. Life Form and Morphology
Most species of the Artemisia genus are perennial, with only about 10 species being biennials or annuals (27). General morphological features of the genus Artemisia are described as leaves alternate, capitula small, usually racemose, paniculate or capitate, inflorescence, rarely solitary; involucral bracts in few rows, receptacle flat to hemispherical, without scales and sometimes hirsute; florets all tubular, achenes obovoid, pappus absent or sometimes a tiny scarious ring (32). Artemisia ciniformis is a perennial plant with numerous erect stems. Its stem base is woody. The height of this plant is 20 to 35 centimeters. It has a narrow panicle or spike inflorescence. Its flowers are red and sometimes yellow. The flowering and fruiting time of this species is early to late autumn (33).
3.3. Folk Medicine
In the past, people believed that Artemisia plants could protect health and restore strength. Artemisia originated from the Greek word Artemis, which implies 'healthy' (34). Plants of this genus are traditionally used as decoctions, infusions, and tinctures for various diseases, including malaria, gastrointestinal diseases, upper respiratory tract diseases, and rheumatism. In the folk medicine of Central Asia, infusions of Artemisia species flowers are used for hemorrhoids, ulcerative colitis, epilepsy, bad breath, and several other diseases (35). The aerial parts and inflorescences of A. ciniformis have medicinal uses. In Turkmen folk medicine, its inflorescences are used to prepare "polynnogo chaya" ("Artemisia tea"), which has anthelmintic properties. It is also used for malaria, typhoid fever, and convulsions. The oil prepared from Artemisia is also used for fever, dropsy, and scorpion stings (19). In Iran, this plant is used as an appetite stimulant and antiparasitic in folk medicine (36).
3.4. Chemical Composition
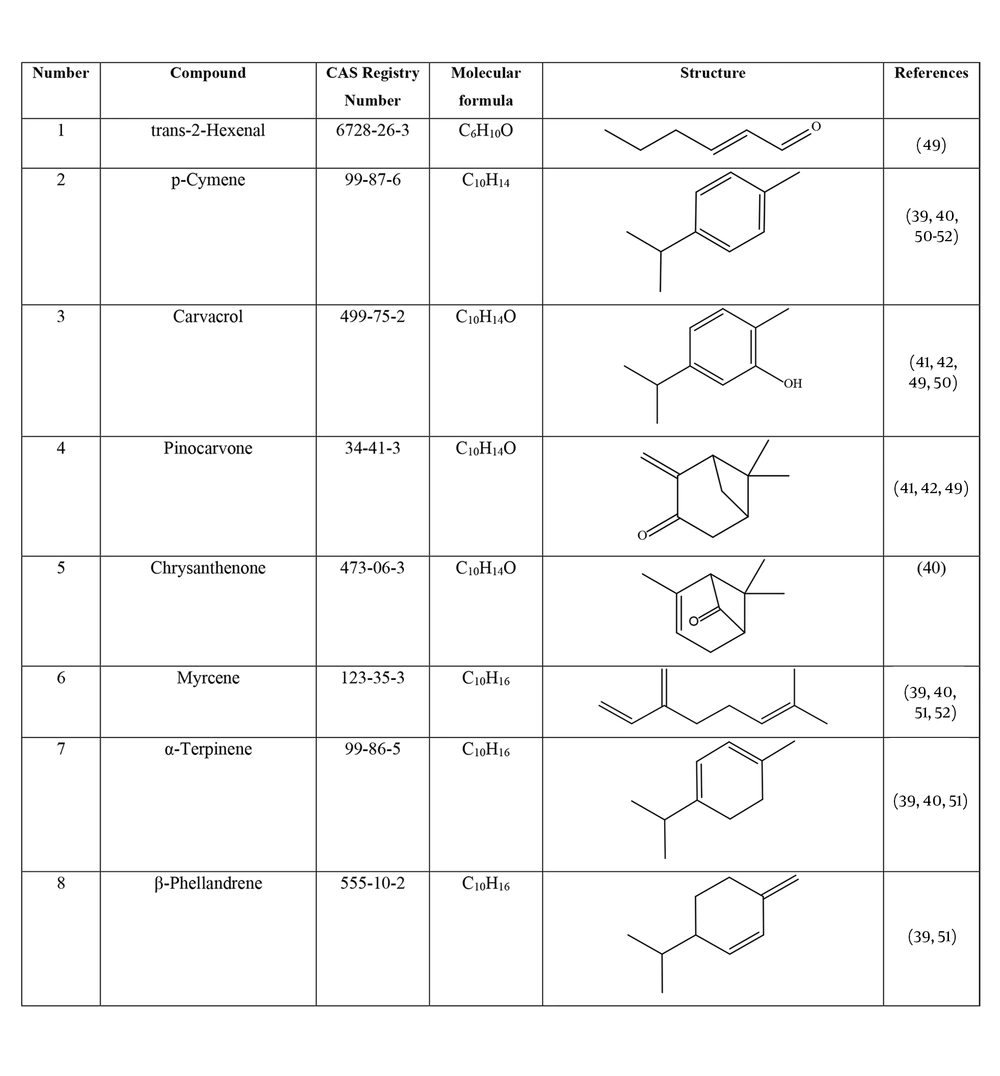
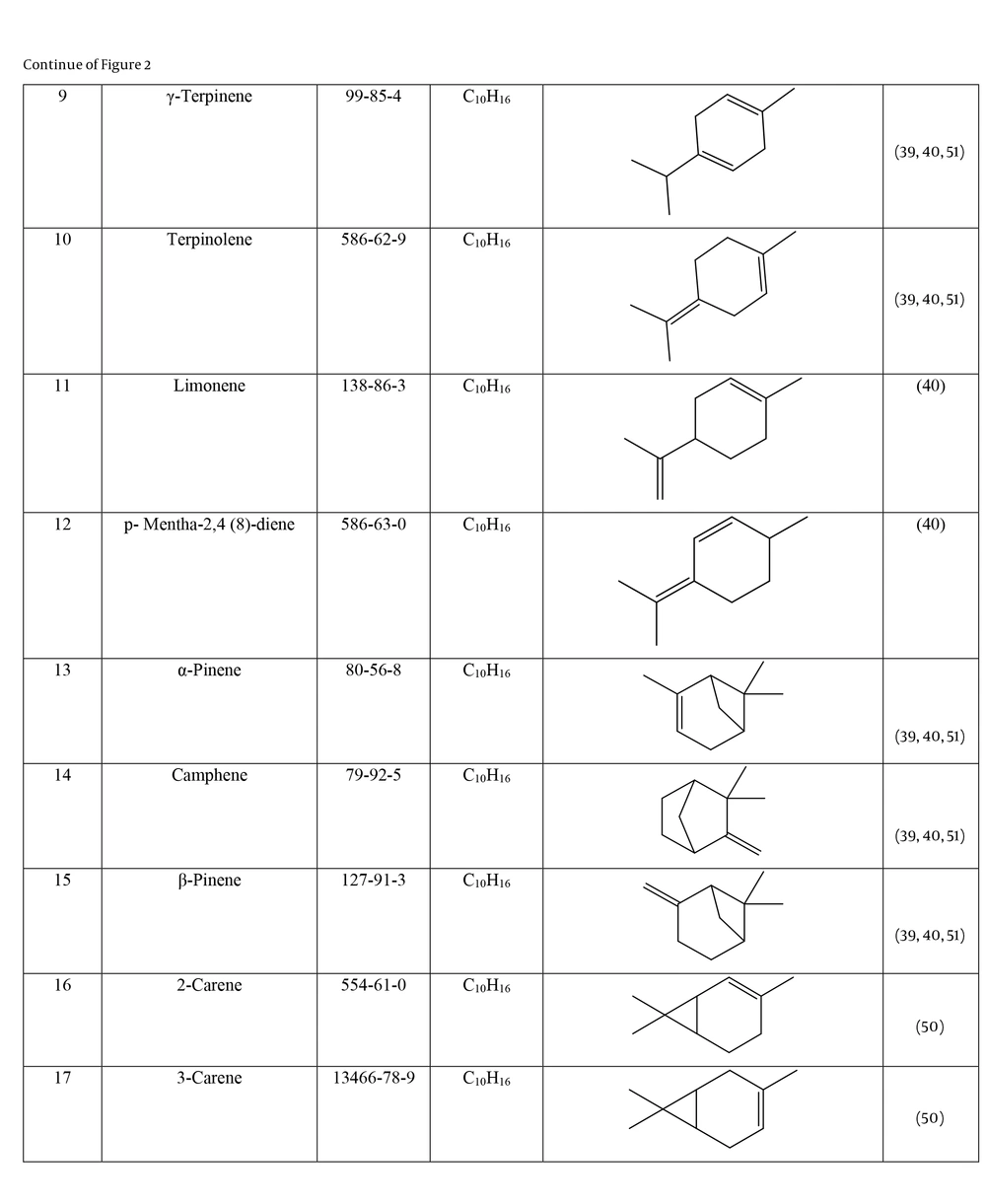
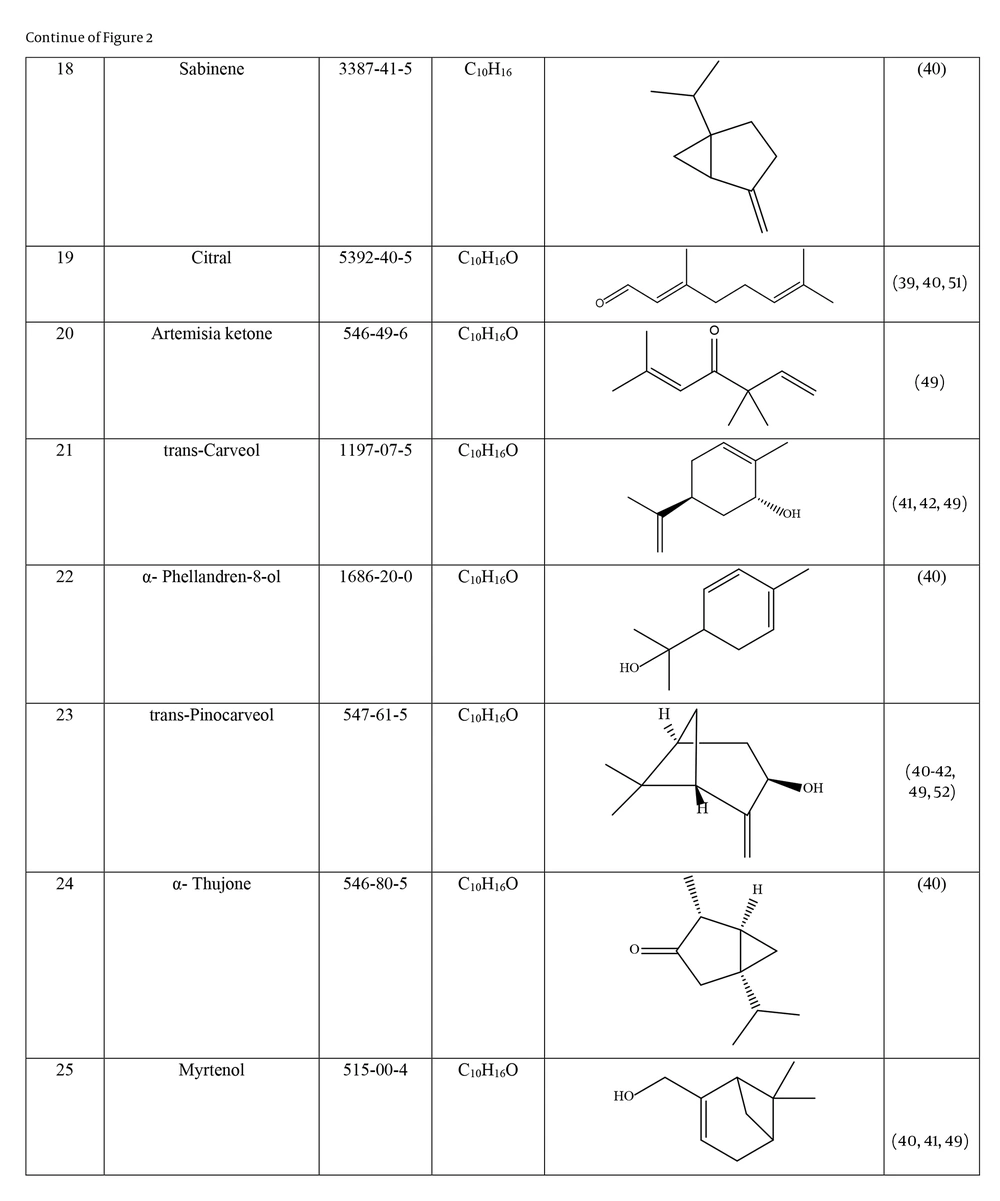
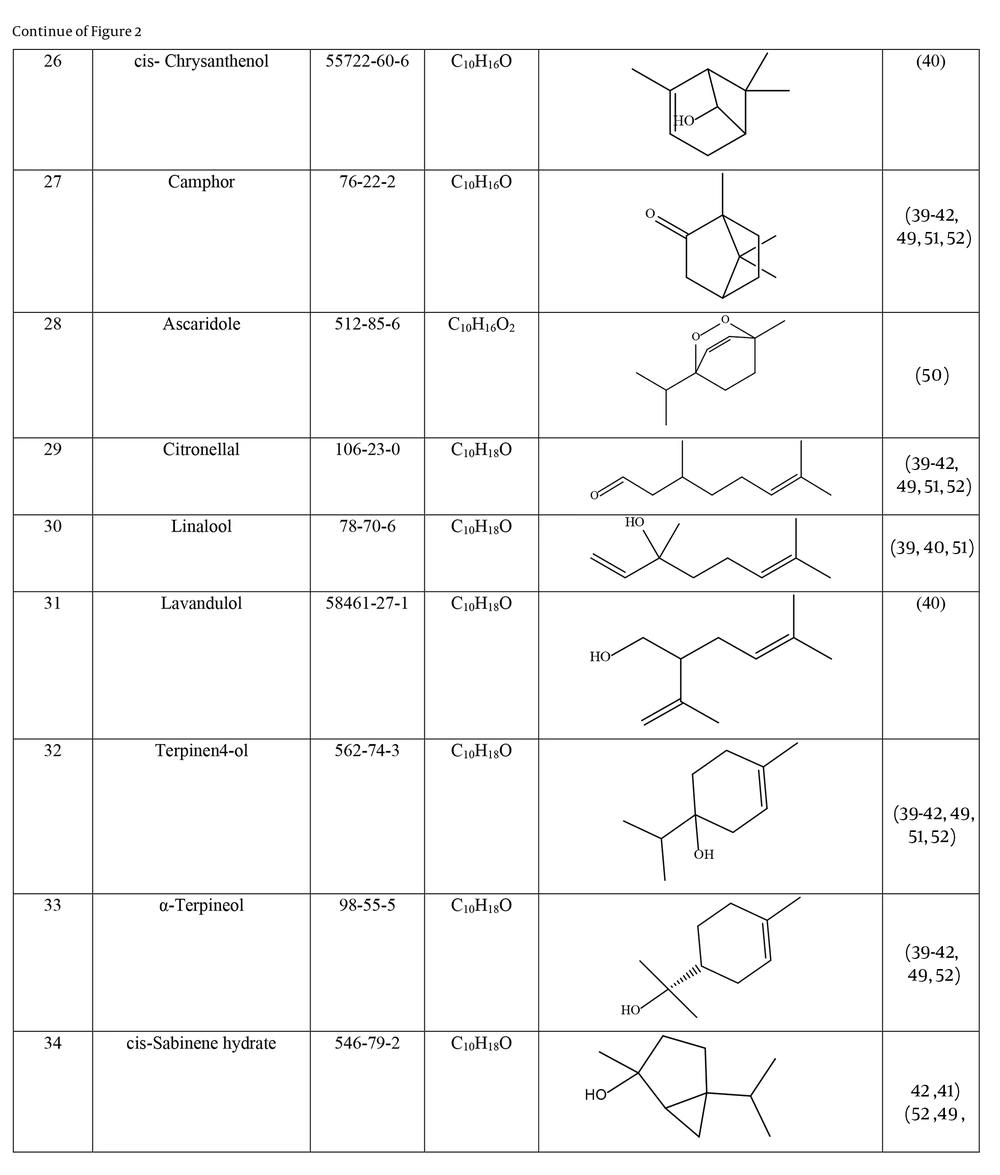
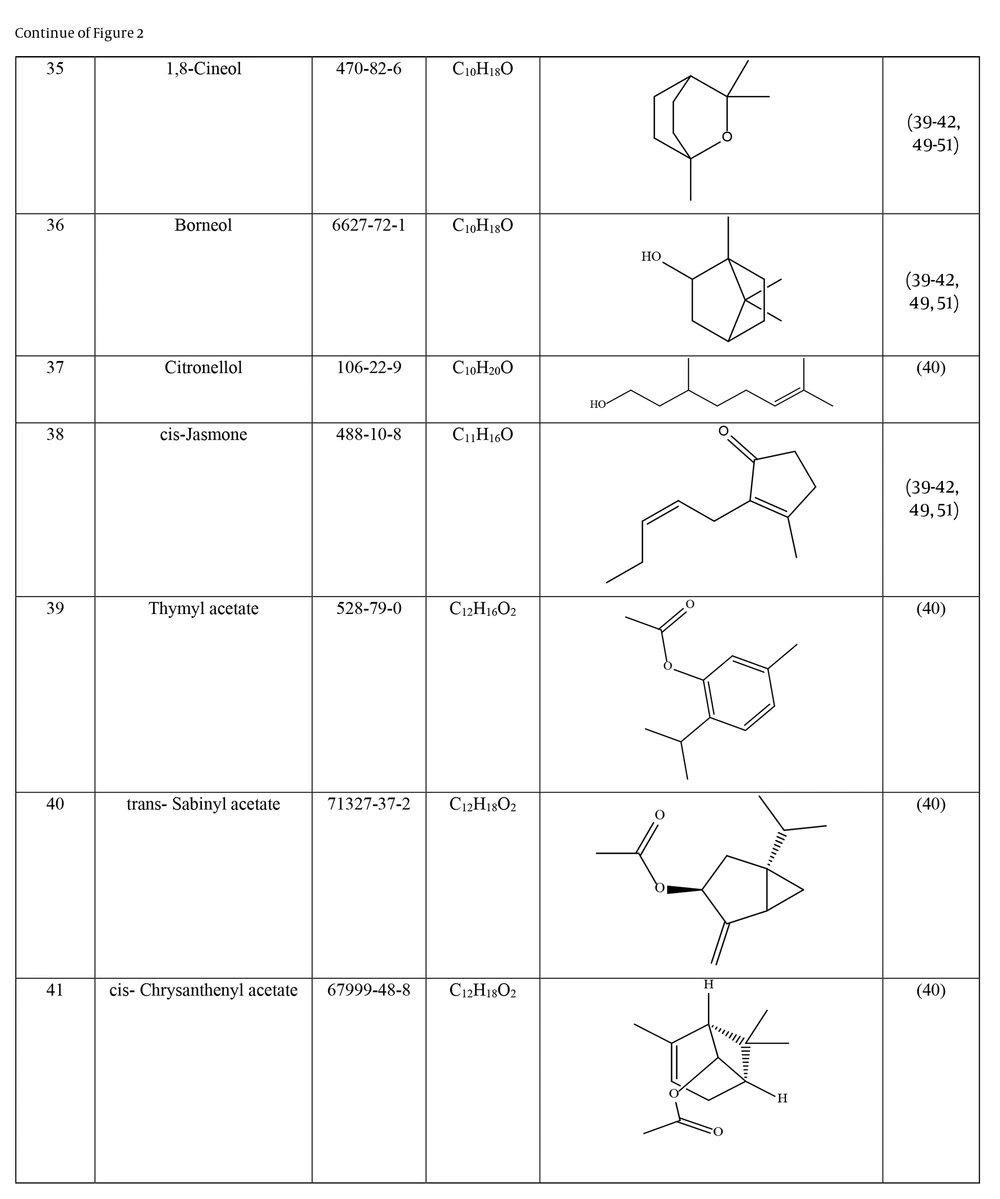
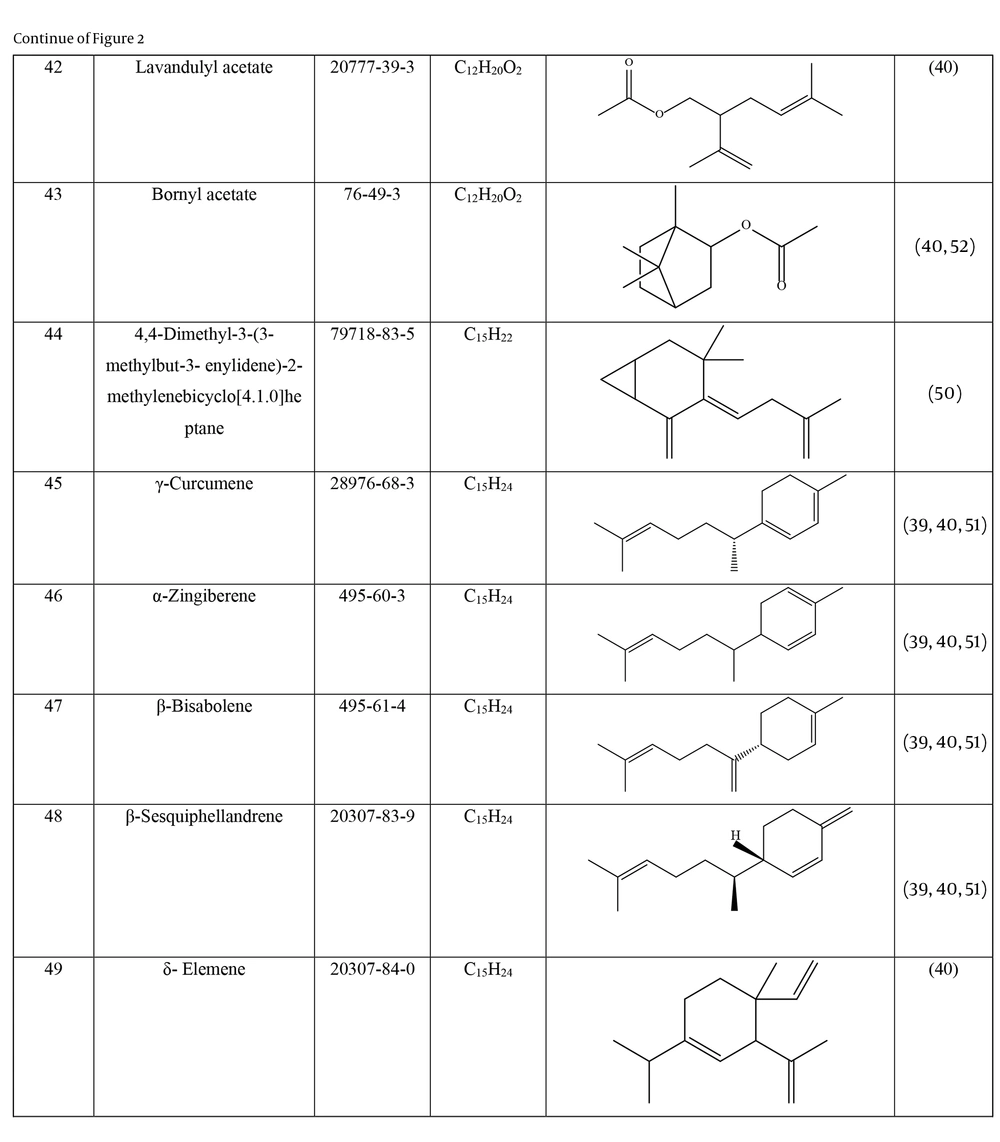
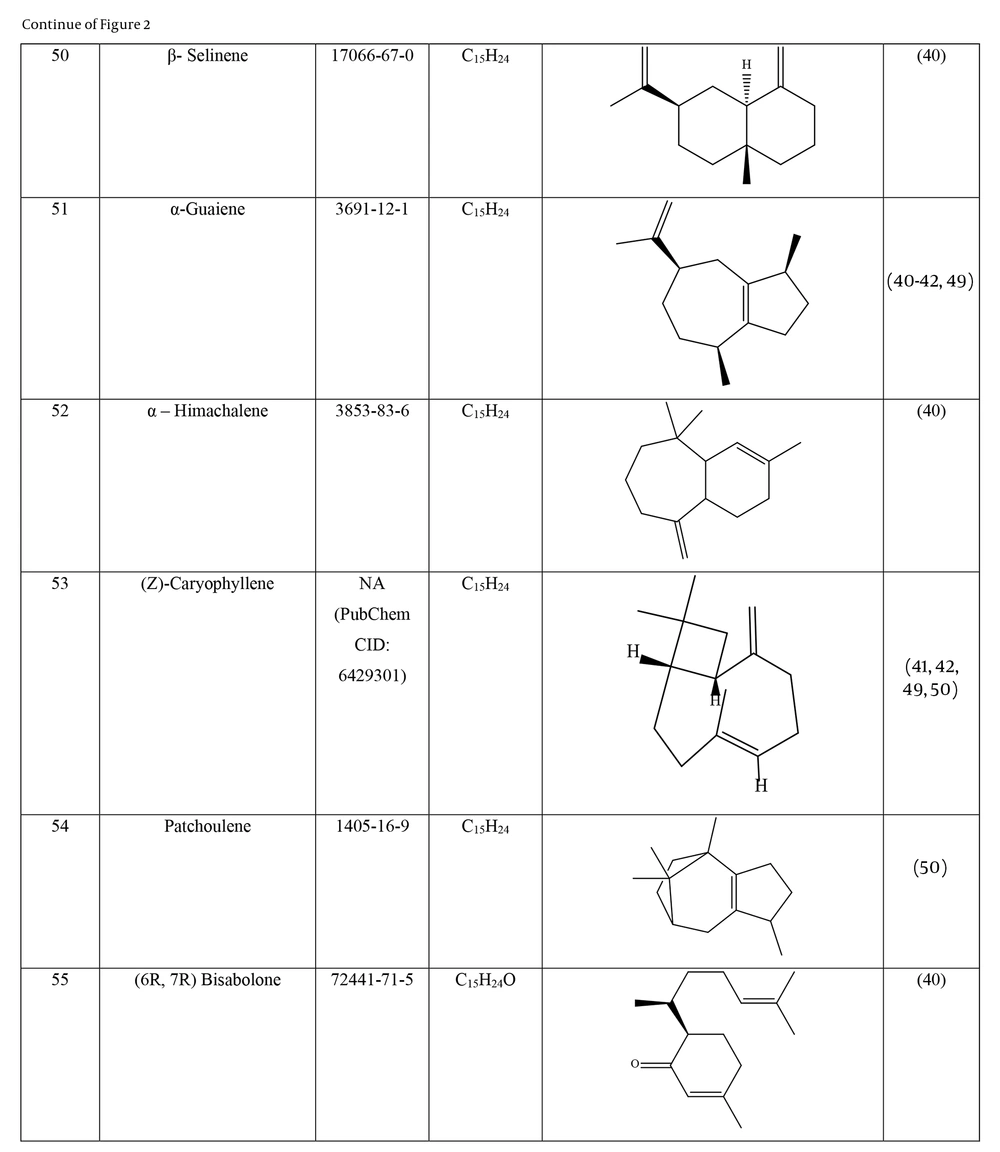
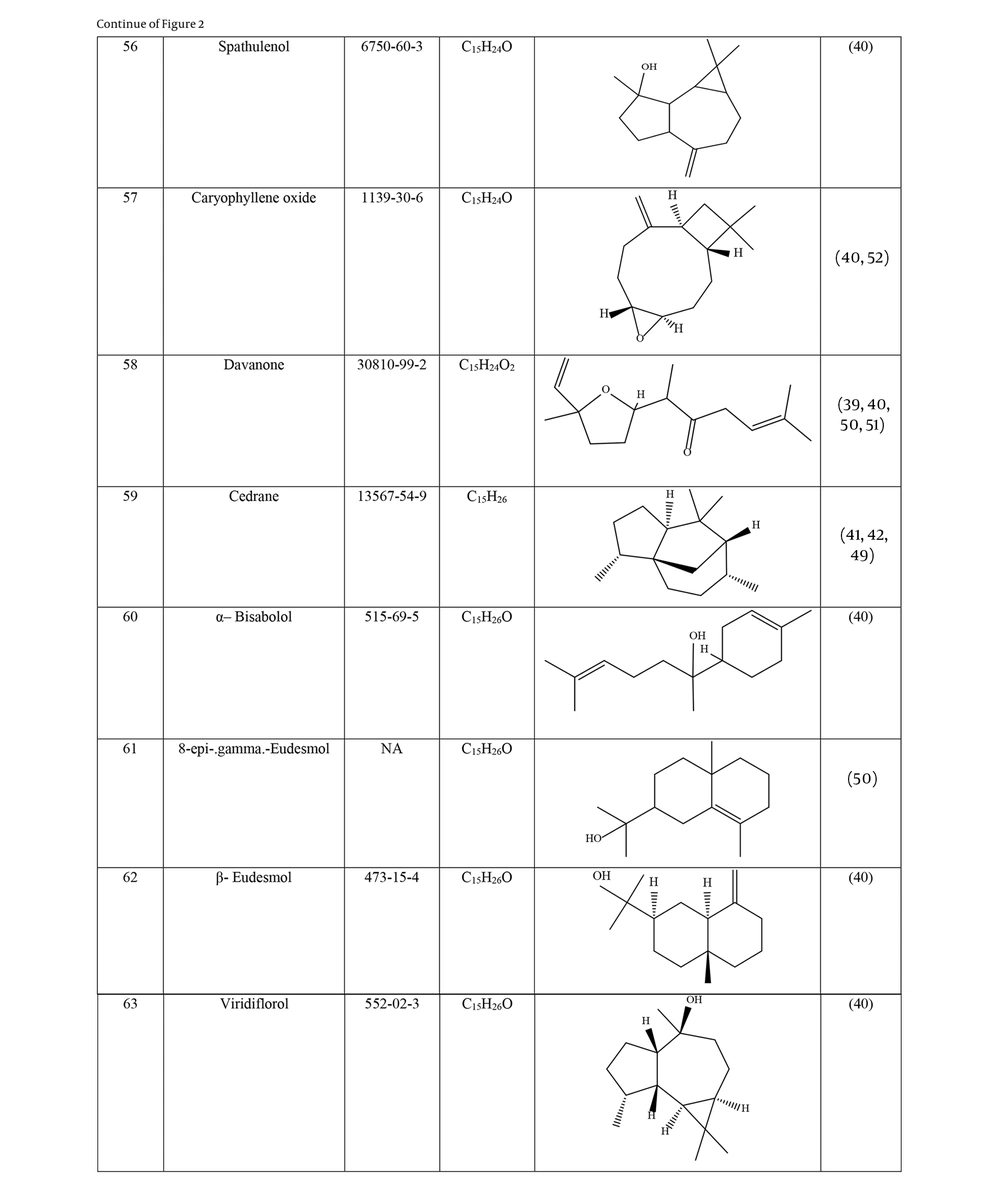
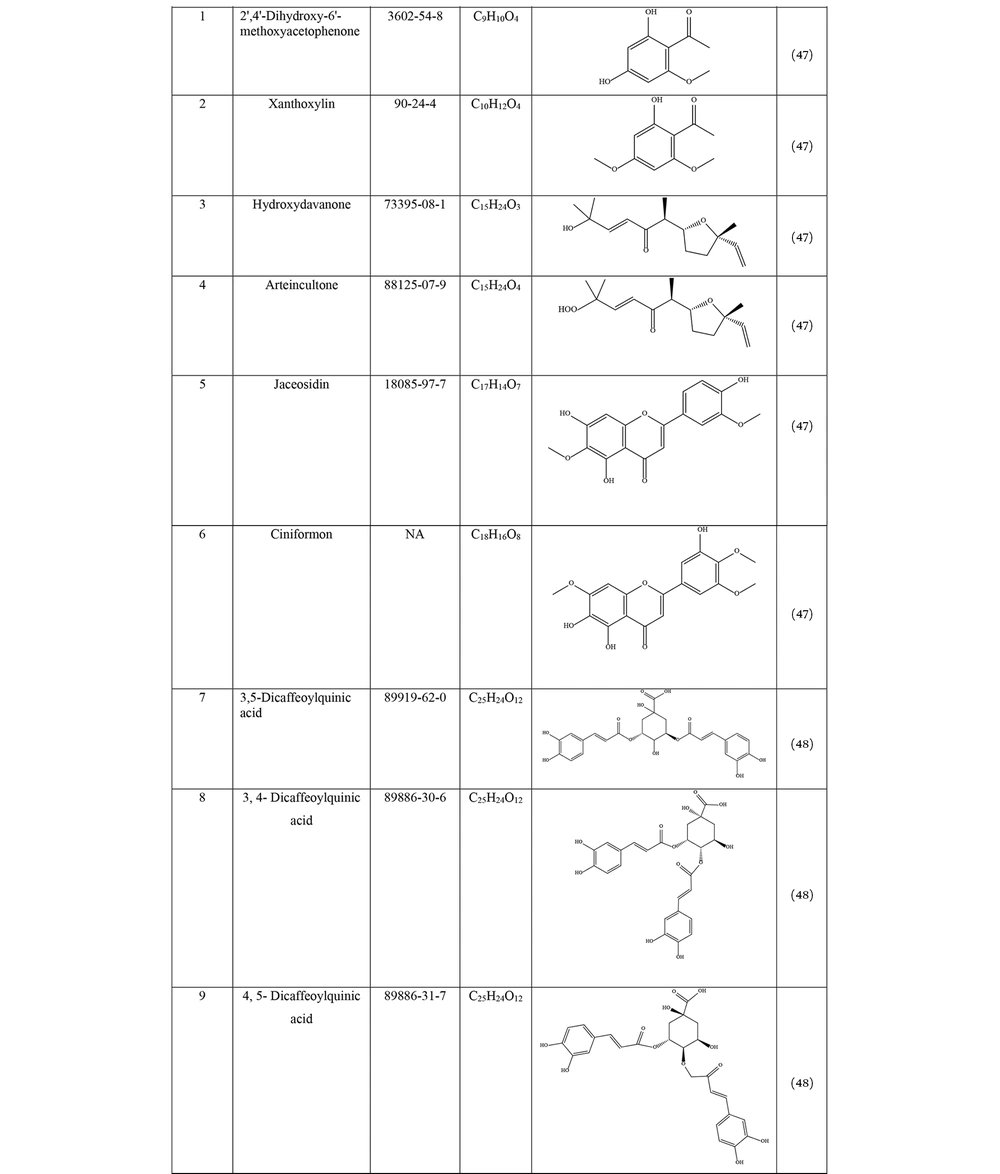
Many species of Artemisia are aromatic; therefore, they are rich in volatile and aromatic compounds. However, they also contain non-volatile and non-aromatic compounds. Phytochemical studies on Artemisia have shown that this genus mainly contains terpenoids, coumarins, and flavonoids, although other compounds are also present (37). The presence of numerous sesquiterpenoids in this genus is notable (38). Several studies have investigated and identified the essential oil compounds of A. ciniformis, which indicates the presence of various monoterpenes and sesquiterpenes (39-42). The total phenolic content of the oil was 206.20 ± 4.58 mg GAE/g (43). Figure 2 lists the reported compounds of A. ciniformis essential oil. 1,8-cineol, camphor, borneol, and α-terpineol have been reported more frequently than other compounds. In studies conducted on A. ciniformis, the amount of flavonoids in the surface raw materials was measured and found to be 1.50% (44), and the flavonoid content of its methanolic extract was estimated to be 131.81 ± 6.83 mg/g CE (43). It has also been found that in this species, the amount of unsaturated sesquiterpene lactones is higher than saturated ones (45). The presence of artemisinin in the leaves of A. ciniformis has been reported during the vegetative stage of the plant (46). The phytochemical investigation of A. ciniformis extract revealed the presence of isochlorogenic acid isomers, tetrahydrofuran-type sesquiterpenoids, acetophenone derivatives, polymethoxylated flavone, and flavonoids (47, 48). Figure 3 shows the compounds isolated from non-volatile extracts of the species.
3.5. Biological Effects
Artemisia species have a very long history of being used to treat human diseases in various parts of the world. This important medicinal genus has promising therapeutic potential for multiple problems because there are numerous reports on a broad range of bioactivities, such as antimalarial (53), hepatoprotective (54), analgesic (55), antimicrobial (56), antileishmanial (57), antiparasitic (58), antitumor (59), antiulcer (60), and antidiabetic (61).
3.5.1. Antimicrobial Effects
Investigation of the in vitro antimicrobial effects of A. ciniformis essential oil showed moderate inhibitory activity against Escherichia coli (MIC = 1000 µg/mL), Staphylococcus aureus (MIC = 2500 µg/mL), Candida albicans (MIC = 1000 µg/mL), Acinetobacter baumannii (MIC = 20 µg/mL), Pseudomonas aeruginosa (MIC = 1000 µg/mL), and clinical isolates of Vibrio cholerae (the diameter of inhibition zone = 24 mm) and S. aureus (the diameter of inhibition zone = 58 mm) (39, 41). Hydroethanolic extract of A. ciniformis has inhibitory activity against bacterial strains of P. aeruginosa (MIC = 2000 µg/mL), Streptococcus pyogenes (MIC = 2000 µg/mL), S. epidermidis (MIC = 1000 µg/mL), Micrococcus luteus (MIC = 500 µg/mL), Acinetobacter sp. (MIC = 2000 µg/mL) and S. aureus (MIC = 2000 µg/mL), and aqueous extracts (infusion and decoction) have shown in vitro antimicrobial activity against S. epidermidis (MIC = 750 µg/mL), and Acinetobacter sp. (MIC = 750 µg/mL) (62, 63). The findings of Ghomi et al. showed that A. ciniformis extract can significantly reduce the gene expression of the norA efflux pump in ciprofloxacin-resistant S. aureus and prevent the release of antibiotics and other drugs (64).
3.5.2. Antioxidant and Cytoprotective Effects
Various studies have investigated and proven the cytoprotective and antioxidant effects of A. ciniformis. Taherkhani evaluated the antioxidant and anti-tyrosinase capacity, iron chelation, superoxide anion, and NO radical scavenging activity of A. ciniformis leaf volatile oil. The essential oil exhibited a dose-dependent scavenging of DPPH, NO, and superoxide radicals with IC50 values of 10.75 mg/mL, 10.63 μg/mL, and 16.81 μg/mL, respectively. In the β-carotene-linoleic acid test system, oxidation of linoleic acid was effectively inhibited (86.39 ± 2.53%, 0.625 mg/mL). Anti-tyrosinase activity of the essential oil was also reported (IC50 = 6.53 mg/mL) (42). In a study that examined the antioxidant properties of A. ciniformis essential oil in the pre-and flowering stages, it was found that the antioxidant properties and phenolic content were higher in the flowering stage (52). Results of a study showed that ethyl acetate, ethanolic, and hydroethanolic extracts of A. ciniformis protected H9c2 cardiac muscle cells against H2O2-induced cell death (increase in the cell viability to 76 ± 4.53, 72 ± 1.25, and 82 ± 3.21% of control, respectively), possibly via the mechanism of reactive oxygen species (ROS) inhibition and/or free radical scavenging (65). An in vitro study showed that the hydroethanolic extract of A. ciniformis has significant antioxidant activity and high content of total phenolics (134.67 ± 0.49 mg GAE /g). The pretreatment of PC12 cells with some fractions of this extract reduced DOX-induced cytotoxicity and ROS production. An increase in superoxide dismutase (SOD) activity was also observed. One of the fractions significantly reduced the activity of caspase-3 and increased the mitochondrial membrane potential (MMP) in the PC12 cell line (66). It has also been found that dichloromethane, ethyl acetate, and petroleum ether extracts of A. ciniformis can inhibit the cytotoxicity of H2O2 in the PC12 cells. Also, an increase in cell survival in the group of cells pretreated with the extracts was observed. The rate of H2O2-induced apoptosis was also affected. ROS scavenging, preservation of MMP, suppression of caspase-3 activity, and inhibition of SOD activity reduction were all exerted by these three extracts. The ethyl acetate extract exerted the highest antioxidant activity against H2O2-induced oxidative damage. This extract reduced ROS accumulation to 70%, H2O2-induced cell death to 35%, and caspase 3 activity to about 175%. It also increased SOD activity to 47% and mitochondrial membrane potential levels by 27% (67). In a study that examined sesquiterpene fractions from several Artemisia species, including A. ciniformis, it was found that the level of nitric oxide produced by lipopolysaccharide-primed macrophages was significantly reduced by all prepared fractions in a dose-dependent manner. However, in this study, the unsaturated sesquiterpene lactones of A. ciniformis had a less modulating effect on COX-2 expression and Prostaglandin E2 (PGE2) production (68).
3.5.3. Cytotoxic and Apoptotic Effects
Studies have shown the cytotoxic effects of various extracts of A. ciniformis. In a study that investigated the cytotoxic effects of native Iranian Artemisia species, dichloromethane (IC50 value: 35 µg/mL) and methanolic (IC50 value: 60 µg/mL) extracts of A. ciniformis had the highest growth inhibitory effects on the human gastric adenocarcinoma cell line (AGS), and dichloromethane extract (IC50 value: 29 µg/mL) had the highest inhibitory effects on human breast carcinoma (MCF-7) cells compared to other extracts studied. The human cervix carcinoma cell line (HeLa) showed high sensitivity to ethyl acetate extract of A. ciniformis (IC50 value: 73 µg/mL). Also, the ethyl acetate extract of this species (IC50 value: 94 µg/mL) was one of the most potent extracts studied in inhibiting the human colon adenocarcinoma cell line (HT-29) (69). In another study, the dichloromethane fraction of A. ciniformis exerted the highest toxicity against AGS cells (IC50 value: 35 µg/mL) compared to six other Artemisia species. Its hexane fraction also had the lowest toxicity on normal fibroblast (L929) cells (70). The petroleum ether extract of this plant showed apoptotic and anti-proliferative effects on HL-60 (IC50 value: 105.20 µg/mL) and K562 human leukemic cancer cell lines (IC50 value: 25.53 µg/mL). The dichloromethane extract possessed the highest anti-proliferative effects on HL-60 cells with an IC50 value of 31.33 μg/mL (71). Some fractions of these two extracts exhibited potent cytotoxic and apoptotic effects on the mouse skin cancer (B16/F10) (IC50 value: 53.69 ± 2.9 µg/mL), the human prostate carcinoma (PC3) (IC50 value: 1.54 ± 0.7 µg/mL), and MCF-7 cell lines (IC50 value: 4.15 ± 1.09 µg/mL) (72). The cytotoxic effects and apoptosis induction of free extract and alginate nanogel encapsulating A. ciniformis extract were compared in the AGS cell line. The nanogel was more potent in the induction of apoptosis. Flow cytometric results showed that it could inhibit cell proliferation and arrest the cell cycle at the G0/G1 phase. The up-regulation of expression levels of pro-apoptotic genes and the down-regulation of anti-apoptotic and metastatic genes were detected (73).
3.5.4. Antileishmanial Activity
The leishmanicidal activity of A. ciniformis was investigated in a study by Emami et al. This study investigated the leishmanicidal activity of different extracts of 11 Artemisia species in vitro. According to the results obtained, various extracts of A. ciniformis had a leishmanicidal effect. The ethanol extract of this species was one of the most effective extracts among the studied species and showed a strong leishmanicidal effect (IC50 value: 25 ± 0.4 μg/mL) (74).
3.5.5. Antimalarial Activity
Based on the results of a study on the antimalarial activity of different extracts of three Artemisia species, including A. ciniformis, using the in vitro β-hematin formation method, the extracts with medium polarity had antimalarial activity. Among all the extracts, the dichloromethane extract of A. ciniformis (IC50 value: 0.92 ± 0.01 mg/mL) had the highest antimalarial activity (75).
3.5.6. Antifungal Effects
In a study, the antifungal effects of different extracts of three Artemisia species against Microsporum canis, Trichophyton verrucosum, Trichophyton rubrum, and Epidermophyton floccosum were investigated. According to the results of this experiment, petroleum ether extracts of all three species were the most active extracts, which showed inhibitory effects against all four fungi. Among the extracts examined, the lowest minimum inhibitory concentration (MIC) was recorded for the petroleum ether extract of A. ciniformis (78.12 μg/mL) against T. rubrum (76).
3.5.7. Miscellaneous Effects
In a study, Baniasadi et al. showed that an electrospun wound dressing containing polyvinyl alcohol, nanochitosan, and an extract of A. ciniformis had the essential characteristics of a good wound dressing due to its significant antibacterial properties against E. coli and S. aureus, support for human fibroblast cell attachment, and rapid water absorption within the first 2 hours. Hence, the A. ciniformis extract can be used for biomedical applications, especially as a dressing in wound healing (77). In a study, the green synthesis of silver nanoparticles (AgNPs) was investigated using A. ciniformis leaf extract and their anti-proliferative and apoptotic effects on the AGS cell line were evaluated. Green synthesized AgNPs inhibited the proliferation of human gastric carcinoma cells through apoptosis (78). In a study conducted in Turkmenistan, the use of a decoction containing Juniperus turcomanica and A. ciniformis was found to be beneficial in treating complications of oral cancer (79).
4. Conclusions
Artemisia ciniformis is a valuable plant species with a broad range of reported bioactivities such as antimicrobial, cytotoxic, cytoprotective, antioxidant, antileishmanial, antimalarial, and antifungal effects. The mono- and sesquiterpenoids comprise the majority of volatile constituents of A. ciniformis. So far, flavonoids, isochlorogenic acid isomers, tetrahydrofuran-type sesquiterpenoids, and acetophenone derivatives have been isolated from the non-volatile extracts of the plant species. Perhaps, the cytotoxic and antifungal effects of tetrahydrofuran-type sesquiterpenoids, and the fungicide and fungiostatic activities of acetophenone derivatives (80-82) justify the biological effects of A. ciniformis, to some extent. The prominent cytoprotective and antioxidant effects of A. ciniformis are at least partly due to the presence of isochlorogenic acid isomers (6, 83, 84). Considering the diversity of phytochemicals and biological effects, this plant species is a good candidate for more extensive studies.