1. Background
Mild cognitive impairment (MCI) is a prevalent condition among middle-aged individuals (1). It typically refers to a decline in the ability to learn new information or retrieve previously stored information (2). Among individuals aged 75 - 79, the incidence of MCI is 22.5 per 1000 person-years; this increases to 40.9 for those aged 80 - 84, and 60.1 for individuals over 85 years of age (3). Although MCI can often be reversible, if left untreated, it may progress to dementia (4). One hallmark of this condition is cognitive decline that, while not severe enough to require assistance with daily living activities, remains noticeable and impacts quality of life (5).
Sanford (2), in a review article, identified several key mechanisms underlying the pathophysiology of MCI, including polypharmacy, depression, hypothyroidism, hypotension, vitamin B12 deficiency, dehydration, and infections. Additionally, oxidative stress, inflammation, amyloid beta plaques (6), and mitophagy (7) are among the most frequently reported contributing mechanisms.
Alhagi maurorum, a plant belonging to the Fabaceae family, is a perennial semi-shrub that typically grows to a height of 25 to 40 centimeters. It is characterized by a smooth, hairless stem with fine stripes and oval-shaped leaves. This plant exhibits antioxidant and anti-inflammatory properties (8) and contains various bioactive compounds, including flavonoids, alkaloids, phenolic acids, and polysaccharides (9). The stems of A. maurorum are particularly rich in polysaccharides, which are noted for their potent antioxidant activity (8).
Although few studies have explored the effects of Alhagi on the nervous system, existing research indicates that its extract can mitigate lead-induced neurotoxicity, improving several behavioral parameters such as performance in the open field and forced swim tests (10). The herb’s beneficial effects are primarily attributed to its anti-inflammatory and antioxidant mechanisms. Furthermore, its protective roles against liver (11, 12), kidney (13), and gastric toxicity (14) have also been investigated with favorable outcomes.
Given that oxidative stress and neuroinflammation are key contributors to the pathophysiology of MCI, natural compounds with strong antioxidant and anti-inflammatory properties — such as those found in A. maurorum — present promising therapeutic potential.
2. Objectives
Therefore, based on these considerations, the present study aims to assess the effects of Alhagi extract on memory and cognitive function in a rat model of MCI induced by streptozotocin (STZ).
3. Methods
3.1. Plant Material
The fresh Alhagi plant was purchased in 2023 from local markets in Rafsanjan and was identified by Dr. Valiollah Mozaffarian at the Botany Research Division, Research Institute of Forests and Rangelands, Tehran, Iran. The stems were separated, washed with distilled water, and extracted using 67% ethanol at a material-to-liquid ratio of 1:25. The extraction was performed at 75°C for 75 minutes (8). The plant material was placed into a fiberglass filter and positioned in a Soxhlet apparatus. The solvent was poured into the balloon of the device, which was then placed on a heater. As the alcohol vaporized, it rose to the top of the apparatus and entered the refrigerant section, which was connected to a continuous flow of cold water. The condensed liquid dripped onto the plant sample. As the volume of alcohol in the balloon decreased, negative pressure caused the alcohol to return to the balloon, where it was reheated and vaporized again (15). The total duration of this process was 75 minutes at 75°C (8).
Once the extraction was complete, the extract was dried and weighed to prepare the desired doses, which were then dissolved in normal saline for oral gavage. The yield of the dried Alhagi extract was 32% (w/w), calculated based on the initial weight of the dried plant material.
3.2. Animals
Sixty male Wistar rats, weighing between 250 and 350 g, were obtained from the animal house of Rafsanjan University of Medical Sciences for this investigation. The rats were housed in a temperature-controlled environment (23 ± 2°C) with a 12-hour light/12-hour dark cycle, and had ad libitum access to food and water. Throughout the experiment, all efforts were made to minimize animal discomfort and pain.
All experimental procedures were conducted in accordance with the U.S. National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (Publication No. 23-85), and were approved by the Ethics Committee of Rafsanjan University of Medical Sciences (ethics code: IR.RUMS.AEC.1403.012).
3.3. Groups
The rats were randomly divided into six experimental groups:
Group 1 (control): Normal saline (used as the solvent for the Alhagi extract) was administered via oral gavage at a dose of 3 mL/day/rat for 14 days.
Group 2 (STZ): To induce MCI, STZ was injected intracerebroventricularly (ICV) (3 mg/kg). Two weeks later, the Alhagi solvent (normal saline) was administered via gavage (3 mL/day/rat) for an additional 14 days.
Groups 3, 4, and 5 (STZ + Alhagi at 100, 200, and 300 mg/kg): In these test groups, 14 days after MCI induction, Alhagi extract was administered by oral gavage at doses of 100, 200, and 300 mg/kg, respectively (each dissolved in 3 mL of normal saline/rat/day) for 14 consecutive days.
Group 6 (Alhagi toxicity study): This group was included to evaluate the potential side effects of Alhagi on cognitive functions and its possible toxicity to kidney and liver tissues. For this purpose, the maximum dose of Alhagi extract (300 mg/kg) was administered via gavage for 14 days.
3.4. Surgery and Mild Cognitive Impairment Induction
Initially, the rats were weighed and then anesthetized via intraperitoneal injection of ketamine/xylazine (60/4 mg/kg). The animals were then placed on a heating pad in a stereotaxic frame to maintain body temperature. Streptozotocin was injected bilaterally into the lateral ventricles at the following coordinates: 0.8 mm posterior to the bregma, 1.5 mm lateral to the sagittal suture, and 3.6 mm ventral from the brain surface. The STZ dosage was 3 mg/kg, freshly prepared before use by dissolving it in cold sterile citrate buffer (0.1 M, pH 4.5).
Immediately following the surgical procedure and prior to recovery from anesthesia, meloxicam was administered subcutaneously at a dose of 2 mg/kg for postoperative analgesia. Animals were monitored daily for any signs of pain or distress during the initial recovery period. Following the procedure, the animals were allowed a 14-day period for the induction of MCI (16).
3.5. Behavioral Tests
3.5.1. Morris Water Maze
Spatial learning and memory were assessed using the Morris water maze (MWM) apparatus. The setup consisted of a black circular tank measuring 60 cm in height and 140 cm in diameter, geographically divided into four quadrants. The tank was filled with water maintained at 22 ± 1°C. A black platform (10 cm in diameter) was submerged 2 cm below the water surface in one of the quadrants, designated as the target quadrant.
During the training phase, each rat completed three sets of four trials in the maze. In each trial, the rat was placed into the water from a randomly selected starting point in one of the four quadrants, with a different entry point for each of the four trials. Rats were allowed to swim freely in search of the hidden platform. Upon reaching the platform, each rat remained on it for 20 seconds before being returned to its home cage until the next trial. If a rat was unable to locate the platform within 60 seconds, the experimenter guided it to the platform.
Twenty-four hours after training, the rats were subjected to a probe test to evaluate spatial memory. The platform was removed, and each rat was allowed to swim freely for 90 seconds. The time spent and distance traveled in the quadrant where the platform had been located during training were recorded as measures of memory retention.
Following this, a visible platform test was conducted to control for potential sensory or motor impairments caused by experimental treatments. In this test, a visible platform (elevated 2 cm above the water surface and wrapped in aluminum foil) was placed in the same location as the original hidden platform. Rats were assessed for their ability to locate the visible platform.
An automated video tracking system (EthoVision software, Netherlands) was used to monitor and analyze the movement of the rats (17).
3.6. Passive Avoidance Task
To assess passive avoidance memory, a shuttle box apparatus was used (18). This apparatus consists of two chambers — one dark and one light — separated by a guillotine door. The floors of both chambers are equipped with stainless steel bars connected to an electric shock generator.
The test was conducted in three phases.
3.6.1. Habituation Phase
Each rat was placed in the light chamber. Due to their natural preference for dark environments, rats typically moved into the dark chamber. After 2 minutes, the animal was removed from the apparatus. If a rat remained in the light chamber for more than 300 seconds, it was excluded according to the cutoff criterion described by Hadadianpour et al. (18).
3.6.2. Learning Phase
The procedure was repeated; however, once the animal entered the dark chamber, the guillotine door was closed. A mild electric shock (measured in milliamperes) was delivered through the metal bars on the dark chamber’s floor for 5 seconds. The animal's reaction — typically a jump — confirmed receipt of the shock. Afterward, the rat remained in the dark chamber for 20 seconds before being returned to its home cage.
3.6.3. Memory Testing Phase
Twenty-four hours after the learning phase, each rat was reintroduced into the light chamber, and the latency to enter the dark chamber (step-through latency) was recorded. This latency was used as a measure of memory retention.
3.7. Y-maze Alternation Task
The performance of working memory was evaluated using the Y-maze. The Y-maze consists of three arms (40 × 4.5 × 12 cm, 120°). The floor and walls are made of polyethylene plastic. Initially, the animals were placed in one arm, and their entry sequence into the different arms was recorded for 8 minutes. Consecutive entries into a new arm before returning to the two arms that had been previously visited were defined as a successful alternation. To eliminate odors and stains, the chamber was thoroughly cleaned with 50% ethanol at the conclusion of each experiment. Working memory performance was measured by the percentage of accurate alternations (19).
[(number of alternations/total number of arm entries - 2) × 100].
3.8. The Forced Swim Test
One approved technique for evaluating depressive-like behavior in rats is the forced swim test (FST). The procedure was as follows:
Equipment: A cylindrical tank measuring 50 cm in height and 25 cm in diameter, filled to a height of 35 cm with tap water maintained at a temperature of 23 - 25°C.
3.8.1. Protocol
3.8.1.1. Habituation
To familiarize the rats with the swimming environment, they were placed in the water tank for 15 minutes on the day before the test.
3.8.1.2. Test Session
On the test day, rats were placed in the tank and allowed to swim freely for 6 minutes.
3.8.1.3. Measurement
The primary measure was immobility time. A rat was considered immobile when it ceased active movements and only made minimal motions necessary to keep its head above the water surface.
3.8.1.4. Interpretation
Prolonged immobility was interpreted as an indicator of depressive-like behavior. A reduction in immobility time was considered to reflect a potential antidepressant effect (19).
3.9. Elevated Plus Maze
Rats' anxious behavior is assessed using the elevated plus maze (EPM). The apparatus consists of four arms arranged in a plus shape.
3.9.1. Structure
Each arm measures 10 × 50 cm and includes two open arms and two closed arms. The closed arms are enclosed by 40 cm high walls on the sides and ends. The open arms are bordered by 1 cm high glass lips. A central platform measuring 10 × 10 cm connects all four arms and is elevated 50 cm above the ground.
3.9.2. Method
Rats are placed in the center zone, facing one of the open arms, and allowed to explore freely for five minutes. Their behavior is recorded on video for later analysis.
The recorded metrics include: The number of entries into open and closed arms, the amount of time spent in each type of arm.
An entry is defined as the placement of all four paws into an arm.
The following formulas are used to calculate anxiety-related behavior: (time in open arms/total time in all arms) × 100; (number of open arm entries/total arm entries) × 100 (20).
3.10. Serum Biochemical Assessments
Blood samples were collected via cardiac puncture under anesthesia. Serum was separated by centrifugation at 3,000 × g for 20 minutes at 4°C. Following separation, the serum was stored at -80°C until further analysis. Liver function was assessed by measuring the serum activity of alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT). For kidney function, blood urea nitrogen (BUN) and serum creatinine (Cr) levels were measured. All biochemical parameters were analyzed using a BT3000-type auto-analyzer.
3.11. Histopathological Evaluations
Four animals were randomly selected from each experimental group for histological analysis. The selected animals were euthanized using an approved method. Liver and kidney tissues were carefully excised. The tissue samples were immediately fixed in 10% neutral buffered formalin. Standard histological protocols were followed for processing, paraffin embedding, sectioning, and hematoxylin and eosin (H&E) staining of the fixed tissues. The pathologist who conducted the histological assessments was blinded to the experimental groups.
Kidney sections were examined for glomerular atrophy, inflammatory cell infiltration, and tubular necrosis. Liver sections were evaluated for congestion, steatosis (fatty change), and pyknosis (nuclear condensation).
3.12. Statistical Analysis
Data were presented as mean ± standard error of the mean (SEM). One-way ANOVA (followed by Bonferroni post hoc test) or two-way ANOVA (followed by Tukey’s post hoc test) was used for statistical analysis. A P-value < 0.05 was considered statistically significant.
4. Results
In the present study, we investigated the potential therapeutic effects of Alhagi herb extract on cognitive and behavioral impairments associated with MCI using an STZ-induced rat model. The STZ was administered ICV to induce cognitive deficits, and the effects of Alhagi extract at varying doses (100, 200, and 300 mg/kg) were evaluated using a battery of behavioral tests.
4.1. Morris Water Maze Test
A two-way repeated measures ANOVA was conducted to analyze spatial learning performance across three learning blocks for six treatment groups: Control, STZ-treated, STZ + Alhagi (100, 200, and 300 mg/kg), and Alhagi alone (300 mg/kg) (Table 1).
| Groups | Learning Phase; Escape Latency (s) | Memory Phase | Visible Phase | |||
|---|---|---|---|---|---|---|
| Block 1 | Block 2 | Block 3 | Time in Target Quadrant (s) | Frequency in Target Quadrant | Distance Moved | |
| Control | 37.1 ± 2.3 | 24.8 ± 2.2 | 16.4 ± 0.9 | 38.6 ± 4.1 | 8.6 ± 0.6 | 788.6 ± 147.6 |
| STZ | 51.1 ± 3.4 b | 36.8 ± 3.0 b | 23.9 ± 2.8 | 22.1 ± 1.8 b | 5.3 ± 0.6 b | 848.9 ± 196.8 |
| STZ + AL (100 mg/kg) | 37.3 ± 2.3 c | 25.6 ± 5.4 | 19.8 ± 4.6 | 37.5 ± 4.4 c | 8.3 ± 0.4 c | 866.7 ± 83.8 |
| STZ + AL (200 mg/kg) | 37.5 ± 2.9 c | 21.7 ± 2.5 | 18.2 ± 3.0 | 35.4 ± 4.1 c | 9.6 ± 0.4 c | 855.3 ± 90.8 |
| STZ + AL (300 mg/kg) | 36.1 ± 3.2 c | 25.1 ± 2.1 | 18.7 ± 5.2 | 35.8 ± 6.2 c | 9.1 ± 0.9 c | 762.0 ± 87.5 |
| AL (300 mg/kg) | 37.8 ± 2.8 | 26.0 ± 3.3 | 20.9 ± 6.3 | 30.7 ± 2.2 | 9.6 ± 0.8 | 728.1 ± 50.1 |
The Effect of Alhagi Treatment on Spatial Learning and Memory in the MWM Test a
4.1.1. Treatment Effects
The results of the study showed a significant main effect of treatment [F (5, 54) = 7.23, P < 0.001], indicating that the groups' overall learning performance differed significantly. According to Tukey's HSD post-hoc pairwise comparisons, the STZ-treated group performed significantly worse than the control group (P < 0.001). Furthermore, significant differences were observed between the STZ group and all STZ + Alhagi-treated groups at all tested doses (P < 0.05 for all comparisons).
4.1.2. Learning Block Effects
The learning blocks showed a significant main effect [F (2, 108) = 56.89, P < 0.001], indicating a steady improvement in performance over time. Tukey's HSD pairwise comparisons revealed statistically significant differences between all blocks (P < 0.001), with performance systematically improving from Block 1 to Block 3.
4.1.3. Treatment and Learning Block Interaction
A significant interaction effect between treatment and learning block was observed [F (10, 108) = 2.78, P = 0.004], indicating that the effects of treatment varied across the learning blocks. While all groups showed improvement in performance, the STZ-treated group exhibited a notably slower rate of learning compared to the control group.
4.1.4. Alhagi Extract Effects
Treatment with Alhagi extract appeared to modulate learning performance. The STZ + Alhagi 300 mg/kg group demonstrated the most pronounced improvement across blocks, with performance progressively approaching that of the control group by Block 3. This observation suggests a potential dose-dependent neuroprotective effect of Alhagi extract in mitigating STZ-induced learning impairments.
4.1.5. The Probe Trial
One-way ANOVA revealed significant differences between groups in both the frequency of entries into the target quadrant [F (5, 54) = 5.78, P < 0.001] and the time spent in the target quadrant [F (5, 54) = 6.45, P < 0.001] during the probe trial conducted on the test day. Post-hoc analysis indicated that STZ-treated rats spent significantly less time in the target quadrant and made fewer entries compared to the control group. Alhagi treatment, particularly at higher doses, significantly attenuated these STZ-induced deficits (P < 0.05 for all comparisons with the STZ-treated group).
4.1.6. Visible Platform
Analysis of the visible platform test data showed no significant differences in swimming speed or visual ability among the groups [F (5, 54) = 1.32, P = 0.267], suggesting that the observed differences in maze performance were not due to motor or visual impairments.
Collectively, these findings indicate that Alhagi herb extract effectively reduced the learning and memory deficits induced by ICV administration of STZ in rats.
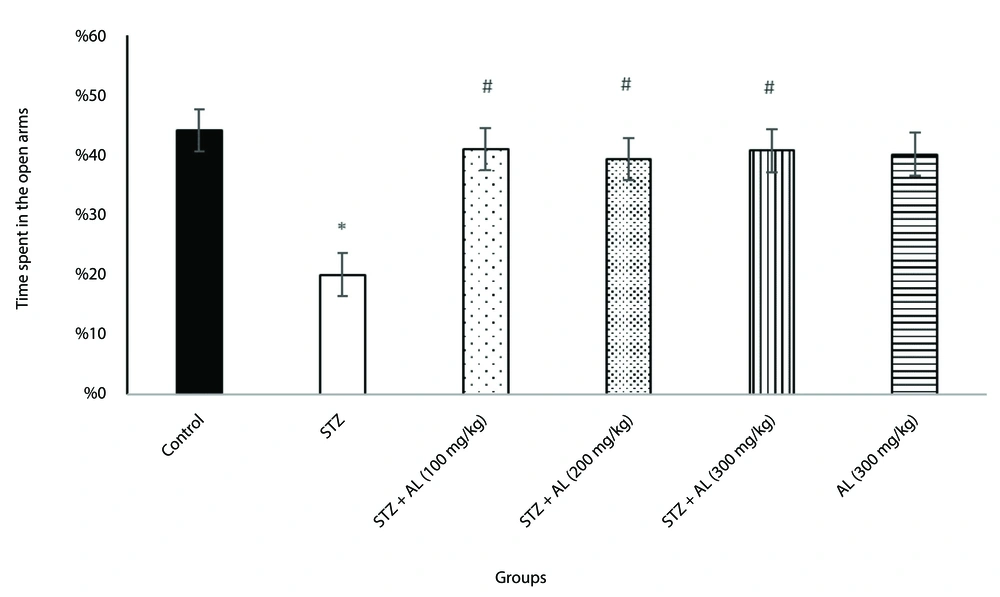
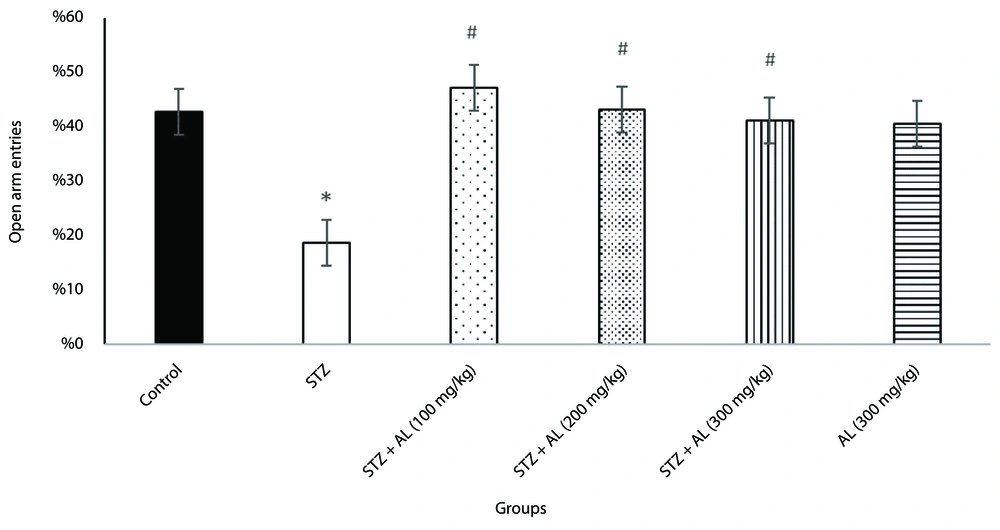
4.2. Elevated Plus Maze Test
To evaluate anxiety-like behavior, the elevated plus maze test was used. A one-way ANOVA revealed significant differences between groups in the proportion of time spent in the open arms [F (5, 38) = 7.92, P < 0.001] and the percentage of open arm entries [F (5, 34) = 3.84, P = 0.007].
Post-hoc analysis showed that the STZ group exhibited significantly more anxiety-like behavior than the control group, spending less time in the open arms and making fewer open arm entries. However, the groups treated with STZ combined with Alhagi extract at doses of 100, 200, and 300 mg/kg spent significantly more time in the open arms and had more open arm entries compared to the STZ group (all P < 0.05), suggesting that Alhagi extract mitigated the anxiety-like behavior induced by STZ (Figures 1 and 2).
The effect of Alhagi treatment on anxiety-like behavior, assessed by time spent in the open arms of the elevated plus maze, in a rat model of STZ-induced MCI. Groups compared are control, STZ, STZ + Al (100 mg/kg), STZ + Al (200 mg/kg), STZ + Al (300 mg/kg), and Al (300 mg/kg). Values represented as mean ± SEM. * P < 0.05 vs control group; # P < 0.05 vs STZ group. STZ, streptozotocin; Al, Alhagi; MCI, mild cognitive impairment.
The effect of Alhagi treatment on anxiety-like behavior, assessed by the percentage of open arm entries in the elevated plus maze, in a rat model of STZ-induced MCI. Groups compared are control, STZ, STZ + Al (100 mg/kg), STZ + Al (200 mg/kg), STZ + Al (300 mg/kg), and Al (300 mg/kg). Values represented as mean ± SEM. Statistical evaluation was obtained by one-way ANOVA followed by Tukey Post-hoc test. * P < 0.05 vs control group; # P < 0.05 vs STZ group. STZ, streptozotocin; Al, Alhagi; MCI, mild cognitive impairment.
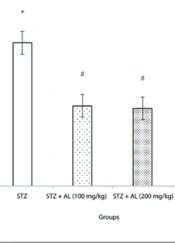
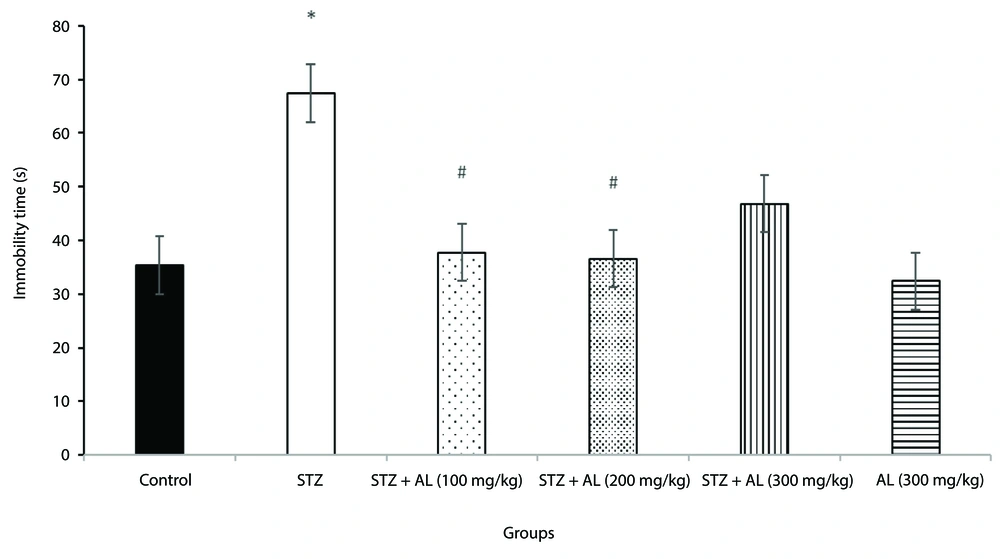
4.3. Force Swimming Test
The forced swim test was employed to evaluate depressive-like behavior. A one-way ANOVA revealed a significant difference in immobility time among the groups [F (5, 38) = 10.94, P < 0.001].
Post-hoc analysis indicated that the STZ group exhibited significantly longer immobility time compared to the control group (P < 0.05) (Figure 3), confirming increased depressive-like behavior in the MCI model.
The effect of Alhagi treatment on depressive-like behavior, assessed by immobility time in the forced swim test (s), in a rat model of STZ-induced MCI. Groups compared are control, STZ, STZ + Al (100 mg/kg), STZ + Al (200 mg/kg), STZ + Al (300 mg/kg), and Al (300 mg/kg). Values represented as mean ± SEM. * P < 0.05 vs control group; # P < 0.05 vs STZ group. STZ, streptozotocin; Al, Alhagi; MCI, mild cognitive impairment.
Treatment with Alhagi extract mitigated this effect; specifically, the groups receiving STZ plus Alhagi extract at doses of 100 mg/kg and 200 mg/kg showed significantly shorter immobility times compared to the STZ group (P < 0.05 for both doses), (Figure 3).
However, the 300 mg/kg dose did not show a statistically significant difference from the STZ group in this test.
These findings suggest that Alhagi extract, particularly at 100 and 200 mg/kg doses, alleviated the depressive-like behavior induced by STZ.
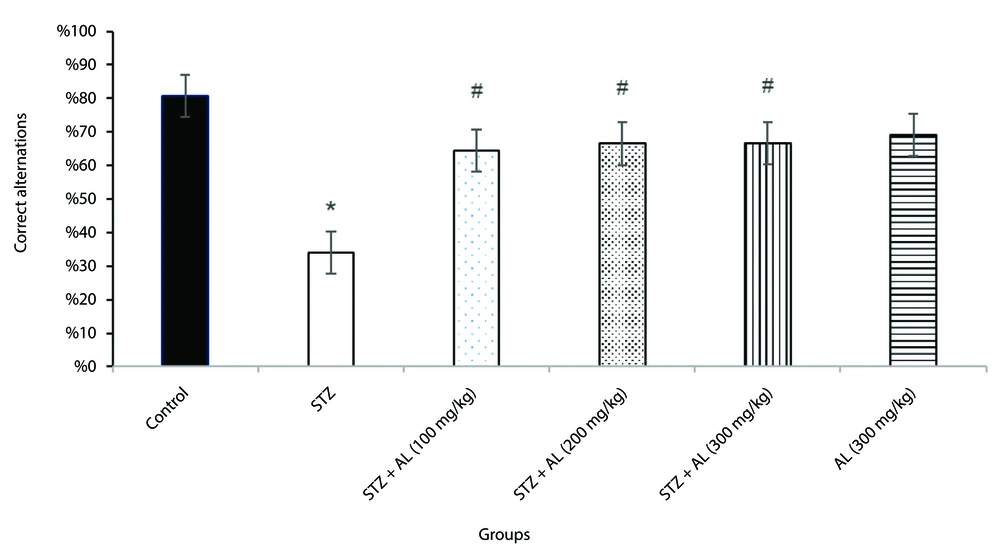
4.4. Y-maze Test
Spatial working memory was evaluated using the Y-maze test. A one-way ANOVA showed that the proportion of accurate alternations varied significantly across the groups [F (5, 38) = 17.94, P < 0.001].
Tukey's HSD post-hoc pairwise comparisons revealed that the STZ-treated group had significantly fewer accurate alternations than the control group (P < 0.001), indicating a deficit in spatial working memory.
Notably, the groups treated with STZ in combination with Alhagi extract at doses of 100, 200, and 300 mg/kg exhibited significantly more correct alternations compared to the STZ group (all P < 0.05).
These findings suggest that Alhagi extract alleviated the spatial working memory impairment induced by STZ administration (Figure 4).
The effect of Alhagi treatment on spatial working memory, assessed by the percentage of correct alternations in the Y-maze test, in a rat model of STZ-induced MCI. Groups compared are control, STZ, STZ + Al (100 mg/kg), STZ + Al (200 mg/kg), STZ + Al (300 mg/kg), and Al (300 mg/kg). Values represented as mean ± SEM. * P < 0.05 vs control group; # P < 0.05 vs STZ group. STZ, streptozotocin; Al, Alhagi; MCI, mild cognitive impairment.
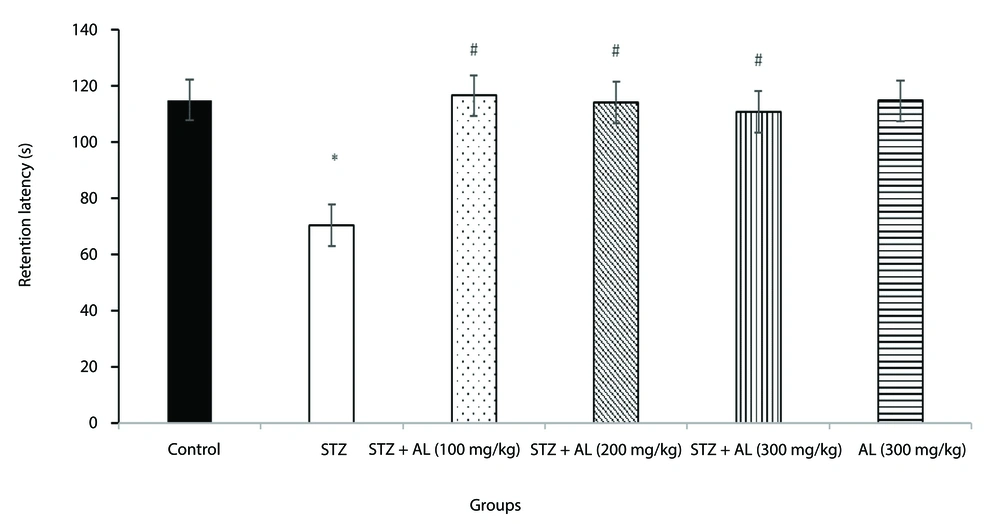
4.5. Passive Avoidance Test
The effect of Alhagi herb extract on long-term memory in rats was further evaluated using the passive avoidance test (shuttle box). A one-way ANOVA revealed a significant difference between the groups in retention latency during the passive avoidance test [F (5, 35) = 17.86, P < 0.001].
Tukey's HSD post-hoc pairwise comparisons indicated that the STZ-treated group exhibited significantly lower retention latency compared to the control group (P < 0.001).
Notably, the groups treated with STZ in combination with Alhagi extract at doses of 100, 200, and 300 mg/kg showed significantly higher retention latency compared to the STZ group (all P < 0.05) (Figure 5).
The effect of Alhagi treatment on long-term memory, assessed by retention latency in the passive avoidance test, in a rat model of STZ-induced MCI. Groups compared are control, STZ, STZ + Al (100 mg/kg), STZ + Al (200 mg/kg), STZ + Al (300 mg/kg), and Al (300 mg/kg). Values represented as mean ± SEM. * P < 0.05 vs control group; # P < 0.05 vs STZ group. STZ, streptozotocin; Al, Alhagi; MCI, mild cognitive impairment.
4.6. Biochemical Parameters
In rats with STZ-induced cognitive impairment, the levels of BUN [F (4, 15) = 0.382, P > 0.05], Cr [F (4, 15) = 2.14, P > 0.05], SGOT (AST) [F (4, 15) = 0.54, P > 0.05], SGPT (ALT) [F (4, 15) = 1.23, P > 0.05], and ALP [F (4, 15) = 1.02, P > 0.05] were not significantly altered by the administration of Alhagi extract at varying dosages (Table 2).
| Biochemical Factors and Groups | Mean ± SD | F-Value | P-Value |
|---|---|---|---|
| Blood urea (MG/DL) | 0.382 | 0.819 | |
| STZ | 32.75 ± 1.03 | ||
| STZ + AL (100 MG/KG) | 31.25 ± 1.4 | ||
| STZ + AL (200 MG/KG) | 35.75 ± 3.1 | ||
| STZ + AL (300 MG/KG) | 33.50 ± 4.1 | ||
| AL (300 MG/KG) | 31.5 ± 2 | ||
| Cr (MG/DL) | 2.14 | 0.112 | |
| STZ | 0.67 ± 0.01 | ||
| STZ + AL (100 MG/KG) | 0.71 ± 0.03 | ||
| STZ + AL (200 MG/KG) | 0.71 ± 0.01 | ||
| STZ + AL (300 MG/KG) | 0.70 ± 0.01 | ||
| AL (300 MG/KG) | 0.71 ± 0.01 | ||
| SGOT (AST) (U/L) | 0.54 | 0.708 | |
| STZ | 118.7 ± 6.0 | ||
| STZ + AL (100 MG/KG) | 156.7 ± 53.4 | ||
| STZ + AL (200 MG/KG) | 121.2 ± 9 | ||
| STZ + AL (300 MG/KG) | 163.0 ± 54.0 | ||
| AL (300 MG/KG) | 124.8 ± 20.1 | ||
| SGPT (ALT) (U/L) | 1.23 | 0.332 | |
| STZ | 49.8 ± 3.2 | ||
| STZ + AL (100 MG/KG) | 49.7 ± 11.9 | ||
| STZ + AL (200 MG/KG) | 42.3 ± 8.5 | ||
| STZ + AL (300 MG/KG) | 32.3 ± 5.3 | ||
| AL (300 MG/KG) | 32.8 ± 2.3 | ||
| ALP (U/L) | 1.02 | 0.421 | |
| STZ | 317.8 ± 12.8 | ||
| STZ + AL (100 MG/KG) | 314.0 ± 24.4 | ||
| STZ + AL (200 MG/KG) | 331.0 ± 25.9 | ||
| STZ + AL (300 MG/KG) | 329.0 ± 23.5 | ||
| AL (300 MG/KG) | 297.0 ± 11.6 |
Effect of Alhagi Administration on Biochemical Factors
These results suggest that under the studied conditions, Alhagi extract did not have a significant impact on these biochemical markers.
5. Discussion
To date, few studies have examined the biological effects of Alhagi. Previous research has investigated the protective effects of Alhagi hydroalcoholic extract on various tissues, including the liver (11, 12), kidneys (13), and stomach (14). It has been shown that the extract possesses both anti-inflammatory and antioxidant properties. To the best of our knowledge, this study is the first to investigate the effects of the hydroalcoholic extract of this plant on a rat model of cognitive impairment induced by ICV injection of STZ. The results demonstrated that Alhagi hydroalcoholic extract could alleviate MCI, as evidenced by improved animal behavior in the Morris water maze, Y-maze, shuttle box, elevated plus maze, and forced swim tests.
The most commonly reported mechanisms involved in STZ-induced cognitive impairment include impaired insulin signaling in the brain (21), increased oxidative stress and neuroinflammation, alterations in tau protein (abnormal N-terminal tau cleavage) (22), and changes in synaptic plasticity and dynamics (23).
In earlier research, the primary components of Alhagi hydroalcoholic extract were identified through GC-MS analysis. These include Beta-D-glucopyranose, 1,6-anhydro-(levoglucosan) (28.91%), 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl (13.56%), 3-methyl-2-(2-methyl-2-butenyl)-furan (13.56%), and 3-methyl-2-(2-methyl-2-butenyl)-furan (rosefuran) (12.84%). These substances have demonstrated strong antioxidant and free radical scavenging properties (24-26).
The possible mechanisms underlying the ameliorative effects of A. maurorum hydroalcoholic extract on STZ-induced MCI observed in this study include:
Antioxidant activity: The extract contains phenolic and flavonoid compounds that exhibit potent antioxidant properties. These compounds may reduce oxidative stress in the brain, a key factor in STZ-induced cognitive impairment (10).
Neuroprotection: The extract has shown neuroprotective effects by attenuating apoptosis in brain tissues, helping to preserve neuronal function and prevent cognitive decline.
Anti-inflammatory action: Alhagi maurorum extract has been shown to reduce neuroinflammation, commonly associated with cognitive impairment in the STZ-induced model (10).
Regulation of neurotransmitters: While not directly confirmed for A. maurorum, plant extracts with similar properties have been shown to modulate neurotransmitter systems, potentially including acetylcholinesterase (AChE) activity (27).
Reduction of diabetes-related effects: In STZ-induced diabetic rats, the extract has demonstrated antidiabetic effects, such as lowering blood glucose levels. This may indirectly improve cognition by mitigating the adverse effects of diabetes on brain function (25).
Protection against oxidative damage: The extract has been shown to enhance the activity of antioxidant enzymes in the brain, including glutathione peroxidase (GPx) and superoxide dismutase (SOD), which may help protect against oxidative damage associated with STZ-induced MCI.
Reduction of lipid peroxidation: The extract significantly reduces malondialdehyde (MDA) levels, indicating decreased lipid peroxidation in neural tissues (27).
These mechanisms collectively contribute to the improvement of STZ-induced MCI in rats treated with A. maurorum hydroalcoholic extract, supporting its potential as a neuroprotective agent.
However, several limitations should be noted in this study:
The exact molecular targets of the extract within the brain were not identified.
The study primarily focused on behavioral outcomes; biochemical and molecular analyses could further elucidate the mechanisms of action.
Long-term studies are required to evaluate the chronic safety and efficacy of the extract.
The extract lacked chemical standardization (e.g., HPLC quantification of flavonoids or polysaccharides).
For future research, it is recommended to conduct preliminary clinical trials to evaluate the safety and efficacy of Alhagi in individuals with MCI or early-stage Alzheimer's disease.
5.1. Conclusions
The present study demonstrates the promising neuroprotective effects of A. maurorum hydroalcoholic extract in ameliorating STZ-induced MCI in rats. The extract’s beneficial effects can be attributed to its potent antioxidant, anti-inflammatory, and neuroprotective properties, which are likely mediated by its bioactive components, such as levoglucosan, 4H-pyran-4-one derivatives, and rosefuran. The multifaceted action of A. maurorum extract targets several key pathological mechanisms involved in STZ-induced cognitive impairment, including oxidative stress, neuroinflammation, and impaired insulin signaling.