1. Background
Cosmeceuticals are a class of cosmetic products that incorporate biologically active ingredients with medicinal or drug-like benefits. They aim to present physiologically relevant benefits without the incorporation of prescription drugs. Although the efficacy and mechanism of action of cosmetics remain incompletely understood, they have gained more attention among the public and the dermatological community (1, 2). Skin-lightening cream, as a cosmetic product, contains a variety of active ingredients that may exhibit toxic properties. Numerous topical agents are utilized to treat hyperpigmentation, which can interfere with the pigmentation process at multiple stages, such as hydroquinone (HQ), kojic acid (KA), ellagic acid, arbutin, ascorbic acid (AA), linoleic acid, azelaic acid, mercury, acerola fruit extract, methimazole, dioic acid, rucinol, licorice, N-acetylglucosamine, niacinamide, etc. (3, 4).
The HQ (benzene-1, 4-diol) is a widely utilized depigmenting agent in clinical studies for the management of hyperpigmentation (5, 6). Topical HQ can result in various adverse effects. Common immediate side effects include irritant or hypopigmentation, allergic contact dermatitis, and post-inflammatory hyperpigmentation. Prolonged use is often associated with exogenous ochronosis, a condition caused by the accumulation and deposition of homogentisic acid in the skin. This condition typically presents with symptoms such as erythema, papulonodules, colloid milia, and symmetric blue-black or gray-brown hyperpigmentation in sun-exposed regions. Chronic users of HQ may also experience trimethylaminuria, commonly known as "fish odor syndrome", characterized by the emission of a foul fish-like smell due to trimethylamine secretion in bodily fluids like sweat, urine, and saliva. Nail hyperpigmentation, although rare, has been documented as a side effect, manifesting as brown pigmentation of the nails, which usually resolves upon discontinuation of HQ usage. Additional side effects may include reduced skin elasticity, impaired wound healing, and peripheral neuropathy. While there have been concerns about the potential carcinogenicity of HQ based on DNA damage observed in animal studies and isolated reports of squamous cell carcinoma in human users, there is currently insufficient evidence to definitively link topical HQ use to cancer or malignant conditions in humans (7).
The U.S. Food and Drug Administration (FDA) has recommended the removal of over-the-counter (OTC) HQ products due to safety concerns, including potential adverse effects such as cutaneous rashes, ochronosis, and facial edema (8). Meanwhile, the World Health Organization (WHO) maintains a 2% maximum permissible concentration in skin-lightening products (9). Arbutin (p-hydroxyphenyl-β-D-glucopyranoside), a bioactive hydrophilic polyphenol and glucoside derivative of HQ, exists in two isomeric forms (alpha-arbutin and beta-arbutin). This compound finds extensive application in cosmetic products as a skin-lightening agent. Some research indicates that the alpha isomer exhibits approximately 10-fold greater efficacy compared to natural arbutin (10). To demonstrate the skin-lightening effects of arbutin in hyperpigmented skin, deoxyarbutin, as a potent tyrosinase inhibitor, inhibits melanogenesis and tyrosine hydroxylase activity in a dose-dependent manner (1).
Due to the absence of notable adverse reactions associated with arbutin and its derivatives, they represent a valuable substitute for HQ (11). It has been reported that various symptoms of contact dermatitis (including erythema, burning, and irritation) are the main adverse effects of topical arbutin application, particularly when combined with other skin-lightening agents (10). It is advisable to exercise caution when utilizing products containing arbutin, particularly due to the possibility of HQ formation during product application (12). The European Union's Scientific Committee on Consumer Safety (SCCS) recommended 0.5% and 2% as a safe and effective dose of arbutin for body lotions and facial creams, respectively (10).
The KA [5-Hydroxy-2-(hydroxymethyl)-4H-pyran-4-one], a fungal-derived skin-lightening agent, exhibits multiple mechanisms of action, including tyrosinase inhibition and suppression of interleukin-6 production in keratinocytes (13). Although topical products containing KA offer a wide range of advantages, drawbacks such as contact dermatitis and potential photo-induced skin damage should be considered (14). In fact, contact dermatitis is noted as the main adverse effect associated with KA in topical formulations (13). The KA and its derivatives also exhibit cytotoxic effects on certain cancer cell lines, including melanoma, hepatocellular carcinoma, ovarian cancer, breast cancer, and colon cancer (15). Although KA is not approved by the U.S. FDA as an OTC treatment for hyperpigmentation, it is approved for cosmetic use in Canada at concentrations ranging from 0.1% to 30% (w/w). It has been stated by the SCCP that the use of KA over 1% in topical formulations can lead to skin sensitization and harmful effects on the thyroid due to systemic absorption (16). Additionally, according to the cosmeceutical ingredient review (CIR), this component can be safely employed at a concentration not exceeding 1% because of its cytotoxic properties (14).
In addition to active ingredients, topical cosmetic formulations typically contain preservatives to prevent microbial degradation (including triclosan, methyl isothiazolinone, and parabens) and oxidative damage [such as AA, butylated hydroxytoluene (BHT), and butylated hydroxyanisole (BHA)] (17). Although the actual effects of parabens as preservatives [including methyl paraben, ethyl paraben, propyl paraben (PP), and butyl paraben] on human health are in doubt despite safety reviews conducted by the FDA, emerging evidence warns about the potential hazards of these compounds. Parabens are absorbed through the skin, with estimated daily exposure levels reaching around 50 mg. It has been demonstrated that their dermal application containing 0.2% methyl paraben and PP for three months can cause skin irritation, erythema, and edema, with more evident effects observed at higher doses (18). The buildup of parabens in the human body after repeated dermal application of cosmetic products is significant. From a safety perspective, parabens commonly used in cosmetics generally do not induce allergic reactions, with most sensitization cases occurring when these products are applied to damaged skin. However, despite their low allergenic potential, increasing attention has been directed toward their estrogenic and endocrine-disrupting effects. Although in vivo and in vitro studies suggest that their estrogenic activity is relatively weak, prolonged exposure may contribute to endocrine disruption and potential carcinogenesis (19). The maximum permissible concentrations of parabens in cosmetic products are regulated at 0.14% for individual compounds and 0.8% for mixtures by European Commission Regulation (EU) No. 1004/2014 (20).
The AA, a hydrophilic compound, is typically employed at a concentration of about 10% to provide UV protection and inhibit photoaging (21). Vitamin C is additionally utilized for the treatment of hyperpigmented skin spots and is applied topically, intravenously, and transdermally (22). The BHA and BHT are used in cosmetic formulations as synthetic hydrophobic antioxidants in a range of 0.0002 - 0.5% (w/w). The amount of 0 - 0.125 mg/kg has been established for the acceptable daily intake (ADI) of BHA by the WHO. While limited toxicological data are available regarding topical application, some research claims that higher doses may induce pulmonary toxicity in mice and rats (23).
2. Objectives
Considering the daily application of skin lighteners in OTC cosmetic products in Iran, and since there are some international standards and regulations for the active ingredients and certain preservatives, accurate quantification of these compounds is of importance (6). Since no standards have been established for these active ingredients in whitening creams in Iran, one of the goals of this study is to highlight the importance of controlling and monitoring these ingredients in whitening creams and the establishment of standards.
Various analytical methods have been documented for the quantification of skin-whitening agents in cosmetic formulations. High-performance liquid chromatography (HPLC) with ultraviolet detection (HPLC-UV) has been employed for the simultaneous analysis of p-benzoquinone, HQ, and arbutin (24), as well as arbutin, niacinamide, and 3-O-ethyl AA (25). Additionally, separation and detection of these compounds have been achieved using HPLC coupled with diode-array detection (HPLC-DAD) (26), gas chromatography-mass spectrometry (GC-MS) (27), UV-VIS spectrophotometry (28), ultra-high-performance liquid chromatography (UHPLC) (29), and electroanalytical techniques (30). While these methods exhibit high sensitivity, a major analytical challenge remains the simultaneous determination of HQ, arbutin, KA, AA, PP, and BHA (31). Given that previous studies have investigated skin-lightening products for HQ, mercury, clobetasol propionate, arbutin, KA, and L-AA content using HPLC as an accurate and reliable analytical method (6, 9, 32, 33), the current study was developed based on HPLC methodology. In the current study, various active ingredients (including HQ, KA, arbutin, AA, PP, and BHA) in commercial skin-lightening creams are determined and quantified using HPLC.
3. Methods
3.1. Standards and Chemicals
The HQ, KA, arbutin, AA, PP, and BHA (HPLC-grade) were purchased from Sigma-Aldrich (Sigma, St. Louis). Additionally, phosphoric acid, acetic acid, monosodium phosphate (NaH2PO4), and acetonitrile (HPLC-grade) were acquired from Merck (Darmstadt, Germany), while HPLC-grade methanol was purchased from Duksan Co., South Korea. Fifty-eight facial skin-lightening cream samples, representing different batches from 25 commercial brands available in Tehran markets, were collected. The expiry dates of analyzed batches ranged from June 2022 to May 2025.
3.2. Standard Preparation
Stock standard solutions (1000 μg/mL) of HQ, KA, arbutin, AA, PP, and BHA were prepared in a 50:50 (v/v) methanol: Water solvent system. Working standard solutions were then prepared with concentrations of 10, 30, 50, 70, and 100 µg/mL through serial dilution of the stock solutions. To boost analytical precision and minimize matrix effects, an internal standard was applied in the current study (34). For this purpose, resorcinol (50 µg/mL) was used as the internal standard. Each calibration curve was obtained by plotting chromatographic peak areas versus the concentration of the standard. All calibration curves exhibited linearity within the specified range (R2 > 0.99).
3.3. Sample Preparation
The extraction solvent consisted of 20 mM NaH2PO4 buffer (2.4 g NaH2PO4 in 1 L of 10% methanol). The pH was adjusted to 2.3 using diluted phosphoric acid. For each sample, 500 mg were accurately weighed and mixed with 8 mL of extraction solvent. Following 30 minutes of sonication, samples were centrifuged at 4000 rpm for 30 minutes. The supernatant was transferred to a 10 mL volumetric flask. The residual pellet was re-extracted with 2 mL of extraction solvent, repeating the sonication and centrifugation steps. The combined supernatants were brought to a final volume of 10 mL with extraction solvent. All extracts were filtered through a 0.45 μm PTFE filter prior to HPLC analysis. Some concentrated samples were further diluted (2 - 200 times) to fit within the linear ranges of the calibration curves (33). Resorcinol (50 μg/mL) was added to all samples as an internal standard.
3.4. Apparatus and Chromatographic Condition
The chromatographic system was an Agilent HPLC (Agilent, USA), equipped with a quaternary pump and a UV/visible detector, coupled to a C18 reversed-phase column (5 μm particle size, 4.6 mm internal diameter × 250 mm length). The mobile phase consisted of 0.01% acetic acid in deionized water (pH = 3.2, phase A) and 0.01% acetic acid in methanol (pH = 4.8, phase B) with a flow rate of 1 mL/min. The gradient program was as follows: 12% B (0 - 14 min), linear increase to 100% B (14 - 22 min), linear decrease to 80% B (22 - 25 min), linear decrease to 60% B (25 - 28 min), linear decrease to 50% B (28 - 30 min), and linear return to 12% B (30 - 36 min), followed by a 5-min re-equilibration at 12% B. The injection volume was 20 µL, and the column temperature was maintained at 25°C. Detection was performed at 280 nm.
3.5. Method Validation
After plotting the calibration curves for HQ, arbutin, KA, AA, PP, and BHA over the specified concentration range, the limits of detection (LoD) and quantification (LoQ) were calculated using the formulae LoD = 3.3 × Sy/S and LoQ = 10×Sy/S, where Sy and S represent the intercept standard deviation and slope of the calibration curve, respectively. These parameters were derived from the calibration curve data. Method accuracy was evaluated through recovery studies by spiking samples with 50 μg/mL standard solutions. Precision was assessed by analyzing each sample in triplicate both on the same day (intraday precision) and over three consecutive days (interday precision), with results expressed as relative standard deviation (RSD) (35).
4. Results
Beyond cosmetic applications, skin lighteners are employed in the management of dermatological conditions such as melasma, skin darkening, and acne to brighten the skin and increase the uniformity of the skin. In the present study, three depigmenting agents (HQ, KA, and arbutin) along with three preservative compounds (PP, AA, and BHA) were simultaneously evaluated using the HPLC method (1, 2, 6). The validation parameters of the HPLC method for the analyzed active ingredients in skin-lightening creams are presented in Table 1.
| Quantified Sample | Concentration Range (µg/mL) | Calibration Equation | Linearity (R2) | LoD (µg/mL) | LoQ (µg/mL) | Recovery (%) | Spiked Concentration (µg/mL) | RSD (Standard) | RSD (Samples) |
|---|---|---|---|---|---|---|---|---|---|
| Arbutin | 10 - 100 | y = 0.0016 x | 0.999 | 3.070 | 9.315 | 105.350 | 50 | 0.615 | 0.845 |
| KA | 10 - 100 | y = 0.022 x | 0.999 | 3.050 | 9.244 | 102.512 | 50 | 1.410 | 0.266 |
| HQ | 10 - 100 | y = 0.0098 x | 0.999 | 3.256 | 9.848 | 95.361 | 50 | 1.878 | 1.068 |
| AA | 10 - 100 | y = 0.0028 x + 0.0057 | 0.999 | 3.097 | 9.367 | 96.298 | 50 | 2.560 | 0.232 |
| PP | 10 - 100 | y = 0.0132 x | 0.999 | 0.852 | 2.582 | 95.296 | 50 | 1.080 | 1.281 |
| Butylated hydroxyl anisole | 10 - 100 | y = 0.0081 x | 0.999 | 1.389 | 4.211 | 95.034 | 50 | 1.415 | 1.863 |
Abbreviations: LoD, limits of detection; LoQ, limits of quantification; RSD, relative standard deviation; KA, kojic acid; PP, propyl paraben; AA, ascorbic acid; HQ, hydroquinone.
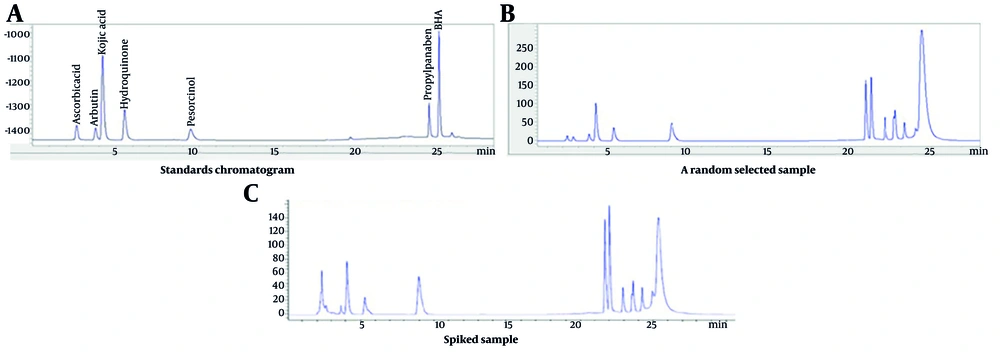
The chromatogram of the assessed standards, a randomly selected sample, and a spiked sample are shown in Figure 1.
In the present study, six target active ingredients were simultaneously detected with high resolution using the HPLC method. Among the 58 analyzed samples in this study, five samples contained HQ in the range of 0.026 - 2.482% (w/w) with a mean concentration of 1.174% (w/w). Regulatory agencies, including the U.S. FDA and the WHO, have established a maximum allowable concentration of 2% for HQ in cosmetic formulations. In contrast, Pakistan’s national standard (PS3228/2006) permits a higher threshold of 5% (36). The current analysis identified two samples exceeding regulatory limits for HQ content. The HQ has been associated with adverse health effects, including respiratory disturbances, cutaneous irritation, and potential carcinogenic activity. Due to these safety concerns, its use in cosmetics has been prohibited in numerous countries, such as Australia, as well as several nations across Europe, Asia, and Africa (36). Additionally, the U.S. FDA currently prohibits OTC sales of HQ-containing products. Nevertheless, when administered at approved concentrations under proper medical oversight, HQ demonstrates both safety and therapeutic efficacy (7).
Arbutin occupies a unique position among skin depigmenting agents, balancing demonstrated efficacy against potential adverse effects. In fact, a comprehensive understanding of the formulation, regulation, and proper application of arbutin-containing cosmetic products becomes essential (12). An evaluation of patch test cases involving arbutin at varying concentrations (3%, 5%, and 7%) revealed that treated patients exhibited multiple contact dermatitis manifestations. These included edematous pruritic erythema, infiltrated small erythematous lesions, depigmented spots, pruritic erythema, and oedematous erythema. These adverse reactions were observed across distinct facial regions, such as the cheeks, eyelids, and forehead (10).
The number of 23 samples contained arbutin in the range of 0.036 - 2.992% (w/w) with an average level of 0.81% (w/w). The SCCS has established 0.5% and 2% as effective and safe levels of arbutin for body lotions and facial creams, respectively. Four samples exceeded these regulatory limits. The KA was detected in three samples at concentrations between 0.014 and 0.339% (w/w), with a mean concentration of 0.127%. The SCCP has reported some adverse effects from using KA over 1% in topical formulations. All analyzed samples complied with this safety threshold. A significant limitation of KA is its propensity to induce adverse dermatological reactions, including erythema and irritant contact dermatitis. These effects underscore the necessity for comprehensive safety assessments and toxicological evaluations (15).
The potential health implications of paraben exposure remain a subject of ongoing international scientific debate (37). The EU No. 1004/2014 determined a maximum amount of 0.14% for a single paraben and 0.8% for a mixture of parabens in cosmetic products. Thirty-three samples comprising PP in the range of 0.023 - 0.084% (w/w) had an average of 0.038% (w/w). All PP-containing samples complied with the mentioned limit. The BHA and BHT, synthetic hydrophobic antioxidants, are used in the range of 0.0002 - 0.5% (w/w) in cosmetic products. All fourteen BHA-containing samples complied with regulatory limits, exhibiting concentrations between 0.022 and 0.155% (w/w) with a mean concentration of 0.08%.
Regarding AA content, there were seven samples containing AA in the range of 0.004 - 3.967% (w/w) with a mean concentration of 0.511%. Gbetoh and Amyot employed HPLC to quantify mercury, HQ, and clobetasol propionate in skin-lightening creams and soaps, revealing concentrations exceeding FDA safety limits (6). In another study in Nigeria, Olatunbosun Oyedeji and Obaroakpo-Akemu analyzed cosmetic emulsions for HQ, KA, and L-AA content using HPLC. Their findings identified HQ as the predominant skin-lightening agent in Nigerian cosmetic products (32). Amponsah et al. conducted HPLC analysis of 50 skin-lightening creams to quantify HQ levels in comparison with the maximum acceptable amount (2%) established by WHO using HPLC (9). An HPLC method was developed by Wang et al. for the simultaneous determination of α-arbutin, β-arbutin, KA, nicotinamide, HQ, resorcinol, 4-methoxyphenol, 4-ethoxyphenol, and AA in skin-whitening products (33). In another research, Al-Saleh et al. analyzed 33 lightening creams for mercury, titanium oxide, HQ, and corticosteroids content as toxic compounds using HPLC and atomic absorption spectrophotometric methods (38).
5. Discussion
This study developed an HPLC method with high precision, accuracy, and analytical efficiency for the simultaneous determination of HQ, arbutin, KA, AA, PP, and BHA in skin-whitening creams. These active ingredients were detected at concentrations exceeding international safety standards in some analyzed samples. Given the frequent non-compliance with labeling and the presence of regulated compounds above permissible limits, it is recommended that strict regulations should be applied in countries. Considering that the Iranian standard organization does not specify an acceptable limit for these compounds and the side effects reported in various studies, it is suggested to define a standard for these cases by conducting relevant clinical studies and paying attention to international references.