1. Background
Rheumatoid arthritis (RA) is a chronic autoimmune disorder affecting 0.5% to 1% of the global population (1, 2). It primarily targets the joints, causing synovial hyperplasia, immune cell infiltration, cartilage degradation, and bone damage (2-4). Morning stiffness is a common symptom experienced by RA patients. If untreated, the disease can cause small focal necrosis, granulation adhesion, and the development of fibrous tissue on the articular surface. These changes ultimately lead to progressive joint ankylosis, deformities, and disability (5). Oxidative stress plays a key role in RA progression, disrupting immune activity and worsening inflammation (6). Reactive oxygen species (ROS) and derivatives, such as hydroxyl radicals, degrade proteins and DNA, inhibiting chondrocyte proteoglycan production. Peroxynitrite and hypochlorous acid damage cartilage by suppressing metalloprotein inhibitors, while H2O2 and O2 boost osteoclast-driven bone resorption (7). Oxidative stress contributes to RA by overproducing ROS, activating NF-κB and MAPK pathways, and causing fibroblast-like synoviocyte proliferation. This increases inflammatory cytokines, immune cell infiltration, and sustained inflammation. Reactive oxygen species elevate MMP-2 and MMP-9 levels, leading to cartilage breakdown, pannus invasion, deformities, and disability. Reactive oxygen species also trigger mitochondrial dysfunction and lipid peroxidation, worsening chondrocyte apoptosis and bone erosion. Addressing oxidative stress and MMP activity could protect joints (8, 9).
Matrix metalloproteases (MMPs), particularly MMP-2 and MMP-9 (gelatinases), are key enzymes in RA progression. They degrade cartilage by breaking down extracellular matrix components such as type II collagen and proteoglycans. Their activity is driven by pro-inflammatory cytokines and oxidative stress, forming a cycle of inflammation and tissue destruction. Inhibiting MMPs could slow degenerative effects and protect joints of RA (10). Targeting inflammation and oxidative stress shows promise in RA management (2). Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce swelling, inflammation, and pain (11). Corticosteroids, such as glucocorticoids, have anti-inflammatory and immunosuppressive effects by modulating gene expression through glucocorticoid receptors (12). Disease-modifying antirheumatic drugs (DMARDs) prevent RA progression by targeting inflammatory mediators, including IL-6, IL-1β, IL-17, and TNF-α (13, 14). However, these treatments may cause side effects, such as gastrointestinal issues, osteoporosis, diabetes, allergies, and infection risks (12, 13, 15). In this regard, exploring natural products with anti-inflammatory and antioxidant properties and fewer side effects represents a valuable resource for further investigation in this field.
Several botanical extracts, including turmeric (Curcuma longa), ginger (Zingiber officinale), and Boswellia (Boswellia serrata), have shown anti-inflammatory effects in RA management. These work by reducing pro-inflammatory cytokines and oxidative stress but through distinct mechanisms. Turmeric inhibits NF-κB and cytokines with minimal impact on MMP activity and neuroprotection (16, 17). Ginger suppresses prostaglandins and leukotrienes, reducing inflammation and oxidative stress, but shows limited effects on MMPs and neuroprotection (18, 19). Boswellia decreases leukotriene synthesis via 5-lipoxygenase inhibition but has a lesser role in MMP regulation and neuroprotection compared to other extracts (20, 21).
Fraxinus excelsior L. (European ash) is a wind-pollinated tree from the Oleaceae family, native to temperate Asia and Europe (22, 23). Traditionally, it has been used to treat ailments such as arthritis, rheumatism, gout, and neuroinflammation (24-28). Its bark contains hydroxycoumarins, including isofraxidin, fraxetin, fraxinol, and fraxidin, which exhibit anti-inflammatory and antioxidant effects. These compounds combat oxidative stress by scavenging ROS, boosting glutathione (GSH) levels, and reducing lipid peroxidation. They also inhibit NF-κB activation, decreasing pro-inflammatory cytokines and cartilage degradation (25, 29, 30). Moreover, F. excelsior bark contains flavonoids, lignans, phenylethanoids, and secoiridoids, many of which have immunomodulatory effects (25). Flavonoids, including quercetin and rutin, suppress inflammatory cytokine production (TNF-α, IL-6, and IL-1β) and downregulate MMP-2 and MMP-9 activity, thereby protecting cartilage integrity (31, 32). Lignans such as syringaresinol and phenylethanoids like verbascoside have been reported to reduce oxidative stress and inhibit joint inflammation (33, 34). These multifunctional compounds contribute to the protective effects of F. excelsior in RA treatment.
Fraxinus excelsior L. bark hydromethanolic extract (FEE) stands out for its high levels of hydroxycoumarins like isofraxidin-7-O-diglucoside, fraxidin, fraxetin, and fraxinol, which act through diverse mechanisms compared to other botanical extracts. Besides reducing inflammatory mediators, FEE regulates MMP-2 and MMP-9 activity, safeguarding the cartilage extracellular matrix from degradation. Additionally, its neuroprotective effects may address cognitive impairment, a recognized RA comorbidity. These properties suggest FEE could provide broader therapeutic benefits than other botanical extracts used for RA (23, 35).
Our previous liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis identified isofraxidin-7-O-diglucoside as a novel coumarin derivative in this genus, which has shown promising anti-inflammatory and antioxidant properties (23). This compound, along with other hydroxycoumarins, has been reported to modulate MMP-2 and MMP-9 activity, contributing to its therapeutic effects in inflammatory diseases like RA and neurodegenerative conditions (23). Some research studies have demonstrated the anti-inflammatory properties of aqueous/alcoholic extracts derived from F. excelsior (36, 37). Notably, the alcoholic extract from F. excelsior bark exhibits comparable anti-inflammatory activity to diclofenac (38, 39). Due to the presence of a wide range of biologically active phytochemicals, recent pharmacological reports have also highlighted the antirheumatic, anti-inflammatory, antioxidant, analgesic, immunomodulatory, antihypertensive, diuretic, antidiabetic, antihypertriglyceridemic, antimalarial, anti-collagenase, antibacterial, and antiproliferative effects of F. excelsior (25, 31, 35, 40). It has been suggested that this herb can be considered in the treatment of osteoarthritis and arthritis (41).
In our recent study, we showed the presence of coumarins in the FEE in LC-MS/MS analysis. Additionally, FEE exhibited a notable protective effect in a rat model of Alzheimer’s disease. This is due to its ability to act through both antioxidant and anti-inflammatory pathways (23).
The complete Freund’s adjuvant (CFA)-induced RA model was used in this study as it replicates key features of human RA, such as chronic joint inflammation, immune activation, and cartilage destruction. The CFA, containing inactivated mycobacteria in mineral oil, provokes a prolonged inflammatory response, making it a widely applied tool in preclinical RA research (42). This model enables the evaluation of inflammation- and oxidative stress-related markers, making it ideal for assessing the therapeutic potential of F. excelsior bark extract in RA. A hydromethanolic (85% methanol-water) extraction method was chosen for this study due to its superior efficiency in extracting both polar and semi-polar bioactive compounds, including phenolic compounds and flavonoids. Methanol enhances the solubility of phenolic and lipophilic compounds, while water facilitates the extraction of polar molecules (43). This combination optimizes the yield of antioxidant and anti-inflammatory agents while preserving their bioactivity. Compared to other solvents, hydromethanolic extraction ensures higher recovery of phytochemicals with therapeutic potential for RA management. Furthermore, methanol prevents oxidation during the extraction process, thus protecting the integrity of phenolic compounds.
2. Objectives
The present study introduces FEE as a promising adjuvant treatment in managing RA by addressing the inflammatory and oxidative stress pathogenes of the disease.
3. Methods
3.1. Plant Material Collection, Preparation, and Extraction Technique
Fraxinus excelsior L. bark was collected in March 2021 from Kermanshah province, Iran (34.392267° N, 47.104332° E). The plant name was verified using the World Flora Online (WFO) database. The plant specimens were authenticated at the Herbarium Biology Department (HBRK), Razi University, Kermanshah, Iran, and assigned voucher number 4202. Samples were air-dried in the shade, powdered with a blender (Moulinex DPA144, France), and macerated (70 g per sample) using 200 mL of 85% aqueous methanol in a separatory funnel. The 85% methanol solvent enhances the extraction of bioactive compounds by optimizing solubility and diffusion. Methanol efficiently extracts phenolics, flavonoids, and coumarins, which contribute to the anti-inflammatory and antioxidant effects of F. excelsior. Water in the mixture aids in extracting hydrophilic compounds while recovering moderately lipophilic ones, ensuring high phytochemical extraction efficiency.
The extracts were collected three times at 24-hour intervals. The collected extracts were filtered using Whatman No. 1 filter paper and concentrated under reduced pressure in a rotary evaporator (Heidolph Instruments GmbH & Co., Germany) at 40°C, then stored at 2 - 8°C until further use. The LC-MS/MS analysis was also performed on the obtained extract in an earlier study (23). The final yield of the dried hydromethanolic extract after rotary evaporation was calculated as X% (w/w) relative to the initial dried plant material weight.
The bark of F. excelsior L. was obtained in March 2021 from the Kermanshah province of Iran (34.392267° N, 47.104332° E). The plant was identified at the Herbarium Biology Department, Razi University (No. 4202). A voucher specimen was deposited for future reference. The collected bark samples were air-dried under shade and powdered using a mechanical blender (Moulinex DPA144, France). For extraction, 70 g of powdered bark was macerated in 200 mL of 85% methanol-water (v/v) in a separatory funnel for 24 hours at room temperature. This process was repeated three times at 24-hour intervals to maximize the extraction yield. The combined extracts were filtered using Whatman No. 1 filter paper, concentrated under reduced pressure at 40°C using a rotary evaporator (Heidolph Instruments GmbH & Co., Germany), and stored at 2 - 8°C for further analysis.
The 85% methanol-water solvent is highly effective for extracting both polar and semi-polar bioactives, enhancing antioxidant and anti-inflammatory compound yields while preserving bioactivity. It provides better phytochemical recovery than ethanol or water alone (43). Methanol prevents oxidation, safeguarding phenolic compound integrity. Although this study did not optimize parameters like solvent ratio, extraction time, or temperature, prior research supports 85% methanol as a reliable solvent for extracting F. excelsior bark bioactive. Future research could refine this method to further improve bioactive yields.
The extracts were collected three times at 24-hour intervals. The collected extracts were filtered using Whatman No. 1 filter paper and concentrated under reduced pressure in a rotary evaporator (Heidolph Instruments GmbH & Co., Germany) at 40°C, then stored at 2 - 8°C until further use. The LC-MS/MS analysis was also performed on the obtained extract in an earlier study (23).
3.2. Animals and Ethical Considerations
Thirty adult male Wistar rats (180 - 200 g) were sourced from the Animal House Unit, School of Pharmacy, Kermanshah University of Medical Sciences. They were kept under standard laboratory conditions: Free access to food and water, a temperature of 25 ± 2°C, and a 12:12 light/dark cycle. The Ethics Committee of Kermanshah University of Medical Sciences approved all procedures (IR.KUMS.REC.1399.519). The study followed Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines and national/institutional regulations for animal care and use.
3.3. Rheumatoid Arthritis Induction
On day 0, RA was induced by injecting 0.1 mL of CFA subcutaneously into the subplantar region of the animal’s right ankle. On days 0, 7, 14, 21, 28, and 35, joint swelling was measured using a plethysmometer, which was calibrated before each measurement session using a standardized water displacement method to ensure accuracy and consistency (44). Swelling in the joints within 24 hours post-injection confirmed the successful induction of RA.
On the 14th day, RA-confirmed animals were randomly assigned to six groups (n = 5 per group) using a simple randomization method and were treated via gastric gavage for three weeks. Each rat was assigned a unique number and allocated to one of the groups using a random number generator to ensure unbiased distribution.
- Group I [normal control (NC) group]: Healthy rats received distilled water (10 mL/kg/day, orally) for three weeks without RA induction.
- Group II [RA group (negative control)]: Rheumatoid arthritis rats were given distilled water (10 mL/kg/day, orally) for three weeks.
- Group III [prednisolone group (positive control)]: Rheumatoid arthritis animals were treated with prednisolone (10 mg/kg/day, orally) for three weeks, from days 14 to 35 (RA + P). Prednisolone was selected for its rapid anti-inflammatory effects, unlike DMARDs, which act over the long term. It quickly suppresses cytokines, leukocyte infiltration, and oxidative stress, making it a validated standard for short-term RA studies (23). This dosage was chosen based on previous studies demonstrating its efficacy in reducing joint inflammation, oxidative stress, and pain-related symptoms in RA models (45-47).
- Group IV (The FEE-treated group): Rheumatoid arthritis rats were treated with FEE (20 mg/kg/day, orally) for three weeks, from days 14 to 35 (RA + FEE20).
- Group V (the FEE-treated group): Rheumatoid arthritis rats were treated with FEE (40 mg/kg/day, orally) for three weeks, from days 14 to 35 (RA + FEE40).
- Group VI (the FEE-treated group): Rheumatoid arthritis rats were treated with FEE (80 mg/kg/day, orally) for three weeks, from days 14 to 35 (RA + FEE80).
A behavioral hot plate test was performed at the conclusion of the trial. On day 35, blood samples were collected to assess GSH, malondialdehyde (MDA), and the activity of MMP-2 and -9. On the thirty-fifth day, rats were euthanized with an intraperitoneal injection of ketamine and xylazine (100 and 20 mg/kg, respectively), and the foot joints were removed for histological examination.
3.4. Measurement of Weight Change
Weight changes were recorded for each group every four days, from day 0 to 35.
3.5. Hot Plate Test
The hot plate test was used to assess the animals’ response to thermal stimuli. The hot plate was maintained at 52°C, and each rat was placed on it to measure reaction time, recorded in seconds, defined as the duration until the rat licked its paws or jumped off due to pain. To prevent tissue damage, the test was capped at 60 seconds. The experiment was repeated three times with a 5-minute gap between each iteration. The average reaction time was reported as withdrawal latency. To reduce stress and acclimate the rats to the apparatus, they were exposed to the unheated hot plate for 3 minutes daily for three days prior to testing (48, 49). The FEE-treated groups showed increased withdrawal latency, reflecting enhanced pain tolerance likely due to its anti-inflammatory effects. By modulating pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6, FEE reduces nociceptive neuron sensitization, improving pain response (50). Its antioxidant properties may further prevent oxidative stress-related neuronal damage, aiding pain relief.
3.6. Measurement of Malondialdehyde and Glutathione
On the last day of treatment, after the hot plate test and foot volume measurement were completed, animals were euthanized via intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) (25). Blood samples were collected from the caudal vein. Serum samples were isolated by centrifugation at 3000 rpm for 15 minutes. Malondialdehyde and GSH enzyme activities in serum were measured using American Cayman Chemical kits and an enzymatic colorimetric method. Glutathione reacts with DTNB to produce a yellow TNB compound quantified at 412 nm, while MDA levels are assessed via the TBA reaction, forming a pink MDA-TBA complex detectable at 532 nm. Measurements were done in triplicate to ensure accuracy (51, 52). The FEE-treated groups showed reduced MDA levels, correlating with histopathological improvements such as decreased synovial hyperplasia, lower inflammatory cell infiltration, and better cartilage integrity. As MDA indicates oxidative stress-induced joint damage, these findings highlight FEE’s antioxidant role in preserving joint tissue structure in RA.
3.7. Zymography Gelatin
Gelatin-substrate zymography was employed to quantify the gelatinolytic activity of MMP-2 and MMP-9 in serum samples. This technique detects gelatinases that digest the gelatin matrix, producing visible bands on the gel. Total protein content was determined using the Bradford assay. For the procedure, 20 μL of serum was loaded onto SDS-polyacrylamide gels (0.1% SDS, 8% acrylamide, 1 mg/mL gelatin). Following electrophoresis, gels were incubated in renaturation buffer (2.5% Triton X-100 in 50 mM Tris-HCl) for 1 hour with shaking, then transferred to development buffer (0.02% NaN3, 10 mM CaCl2, 0.15 M NaCl in 50 mM Tris-HCl) and incubated at 37°C for 18 hours in an orbital shaker. Gels were stained with Coomassie Brilliant Blue G-250, destained, and analyzed for translucent bands indicative of gelatinolytic activity (10, 23). Band intensity was quantified using ImageJ® software (NIH). To ensure accuracy, each sample was assayed in duplicate using two serial dilutions.
3.8. Histopathological Studies
The animals’ right ankle joints were quickly removed and fixed in 10% formalin for histological assessments. After fixation, the samples were decalcified in 10% EDTA at 4°C for 30 days to soften bone tissue for sectioning. The decalcified samples were then processed using a paraffin-embedding technique, followed by rinsing with xylene and dehydration through a graded ethanol series. Thin sections of 5 μm thickness were prepared and stained using hematoxylin and eosin (H&E) for histological evaluation (53). A semi-quantitative histological scoring system was employed to evaluate RA-induced joint damage using the following criteria (54).
- Synovial hyperplasia (0 - 3): Ranges from normal synovium (0) to severe hyperplasia with pannus formation (3).
- Inflammatory cell infiltration (0 - 3): Spans from no inflammatory cells (0) to dense inflammatory infiltrates (3).
- Cartilage degradation (0 - 3): Assessed from intact cartilage (0) to severe cartilage loss exposing subchondral bone (3).
- Bone erosion (0 - 3): Rated from no damage (0) to severe destruction and deformities (3).
- Pannus formation (0 - 3): Evaluated from no pannus (0) to severe formation with joint destruction (3).
Each parameter was scored independently, with a maximum total score of 15 indicating greater joint damage and inflammation. Assessments were conducted by a blinded experimenter to ensure unbiased evaluation.
3.9. Statistical Analysis
Group differences were assessed using one-way analysis of variance (ANOVA) with repeated measures, followed by Tukey’s post-hoc test. Data were expressed as mean ± SEM, with statistical significance set at P < 0.05.
4. Results
4.1. Liquid Chromatography-Tandem Mass Spectrometry Analysis of Fraxinus excelsior Extract
The chemical composition of FEE was analyzed using LC-MS/MS. The results confirmed the presence of hydroxycoumarins, including isofraxidin-7-O-diglucoside, fraxidin, fraxetin, fraxinol, and their glycosylated derivatives. Isofraxidin-7-O-diglucoside was identified as a novel derivative in this genus, demonstrating significant antioxidant and anti-inflammatory properties (23). The presence of these coumarins supports the potential role of FEE in regulating oxidative stress and inflammatory pathways, particularly through the modulation of MMP-2 and MMP-9 activity, which is critical in the progression of RA and neurodegenerative diseases.
4.2. The Effect of Fraxinus excelsior Extract on the Body Weight of Rats
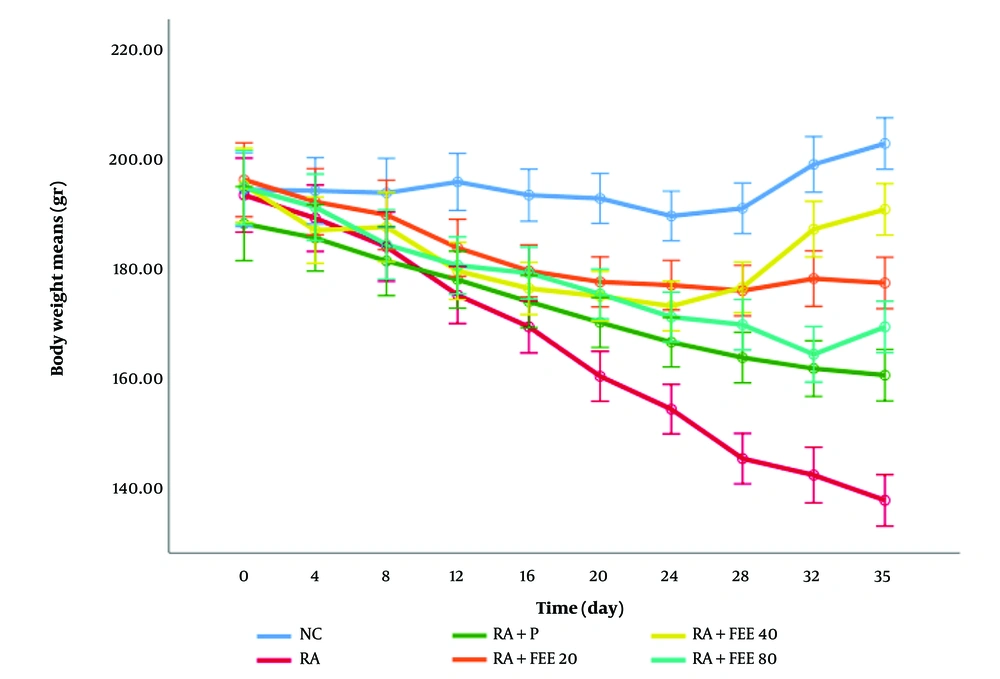
As shown in Figure 1, the body weight of rats in the NC group remained stable, while in the RA group, a significant reduction in body weight was observed throughout the study (P < 0.001 compared to NC), reflecting the systemic impact of RA-induced inflammation and oxidative stress. This decline was partially mitigated in all treatment groups, with significant weight retention observed in the RA + FEE40 and RA + FEE80 groups (P < 0.01 and P < 0.001, respectively). The RA + FEE20 group exhibited a mild improvement in body weight, but the change was not statistically significant (P > 0.05). The RA + FEE80 group demonstrated the most notable recovery, closely resembling the prednisolone-treated group, suggesting a dose-dependent effect of FEE on weight preservation. The observed body weight retention correlates with reduced inflammation, as indicated by the lower paw volume measurements (Figure 2).
The effect of Fraxinus excelsior L. bark hydromethanolic extract (FEE; treatment group) dosages of 20, 40, and 80 mg/kg against prednisolone on rheumatoid arthritis (negative control) caused by complete Freund’s adjuvant (CFA) in the plethysmometer test. The results are presented as mean ± SD (n = 5). NC, normal control; P, prednisolone (positive control).
The RA-induced weight loss is associated with systemic inflammation, increased pro-inflammatory cytokines (TNF-α, IL-6), and oxidative stress, all of which contribute to muscle wasting and metabolic imbalance. The ability of FEE to mitigate weight loss, particularly at higher doses, highlights its potential role in modulating these inflammatory responses. Furthermore, body weight preservation in FEE-treated groups serves as a key marker of improved overall health in the RA model. Chronic inflammation in RA often leads to cachexia, where metabolic dysfunction and oxidative stress accelerate weight loss. The significant weight retention observed in the RA + FEE40 and RA + FEE80 groups suggests that the anti-inflammatory and antioxidant effects of FEE helped restore metabolic stability and enhance general well-being. The correlation between weight maintenance and reduced paw volume further supports the systemic benefits of FEE in alleviating both local joint inflammation and broader disease symptoms.
4.3. The Effect of Fraxinus excelsior Extract on Paw Volume
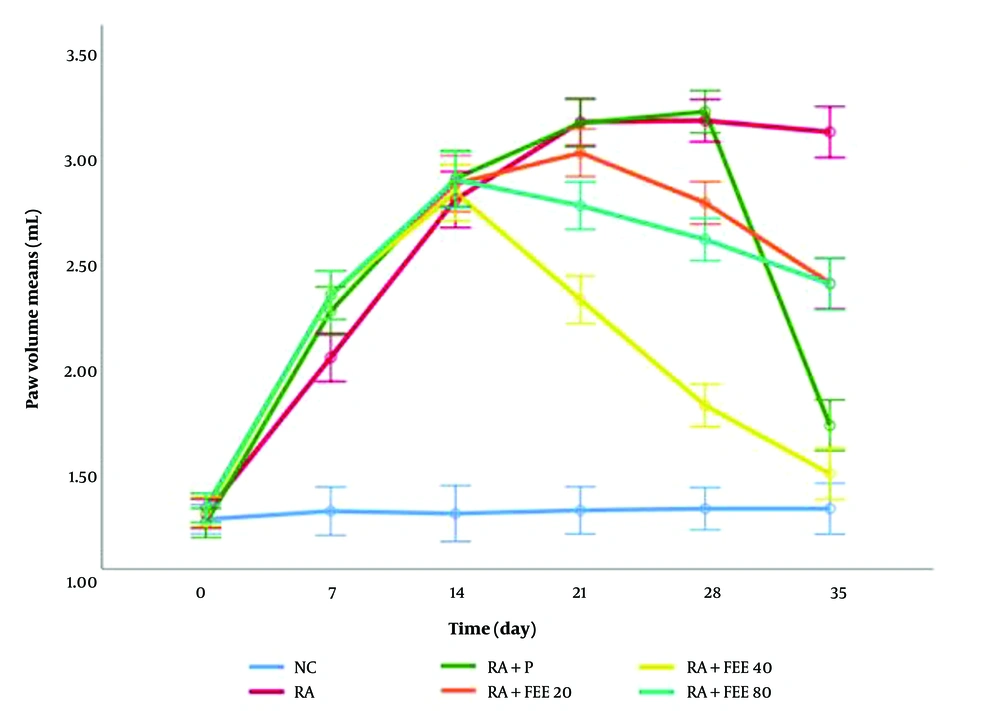
As depicted in Figure 2, paw volume in the NC group remained stable, whereas in the RA group, paw volume increased significantly, reaching its peak on day 21 and remaining elevated (P < 0.001 vs NC). After treatment initiation, all FEE-treated groups showed a reduction in paw volume, with the RA + FEE40 and prednisolone groups exhibiting the most significant decrease by day 35 (P < 0.001 vs RA control). The RA + FEE80 group also displayed a significant reduction (P < 0.01), whereas the RA + FEE20 group had a mild but non-significant reduction (P > 0.05). All statistical analyses were performed using one-way ANOVA followed by Tukey’s post hoc test, with P < 0.05 considered statistically significant.
4.4. The Effect of Fraxinus excelsior Extract on Pain During the Hot Plate Test
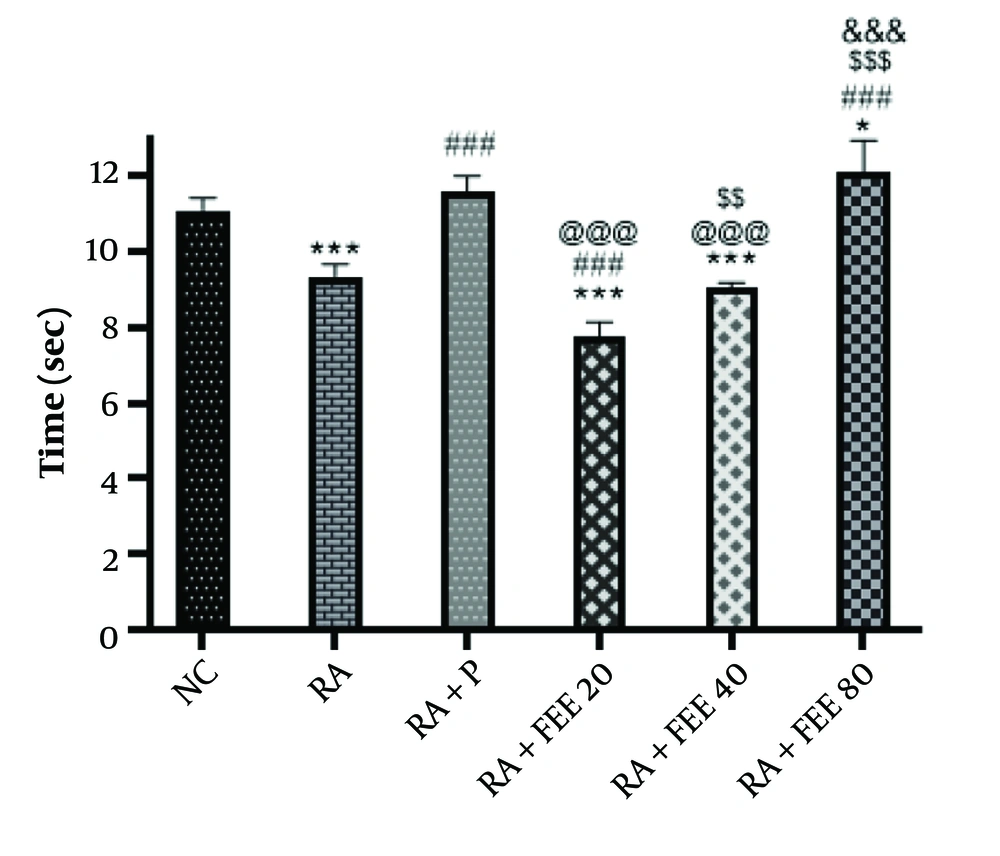
The hot plate test results (Figure 3) revealed significantly reduced withdrawal latency in the RA group compared to the NC group (P < 0.001). However, the RA + P and RA + FEE80 groups showed a notable increase in latency (P < 0.001). The FEE at 80 mg/kg significantly elevated the pain threshold, bringing it to levels similar to healthy rats. Both FEE 40 mg/kg and 80 mg/kg doses significantly improved reaction times compared to the RA group (P < 0.001), with the 80 mg/kg dose displaying the strongest analgesic effect, comparable to prednisolone, indicating a dose-dependent response.
The impact of Fraxinus excelsior L. bark hydromethanolic extract (FEE; treatment group) dosages of 20, 40, and 80 mg/kg in the hot plate test in comparison to prednisolone for complete Freund’s adjuvant (CFA)-induced rheumatoid arthritis (RA) (negative control). Treatment with FEE80 and prednisolone reduced the heat hyperalgesia caused by CFA. NC, normal control; P, prednisolone (positive control). The results are presented as mean ± SEM (n = 5). Statistical significance: ### P < 0.001 vs. RA group, @@@ P < 0.001 vs. RA + P group, * P < 0.05, *** P < 0.001 vs. NC group, $$ P < 0.01, $$$ P < 0.001 vs. RA + FEE20 group, and &&& P < 0.001 vs. RA + FEE40 group, demonstrating the superior efficacy of FEE80 in reducing heat hyperalgesia.
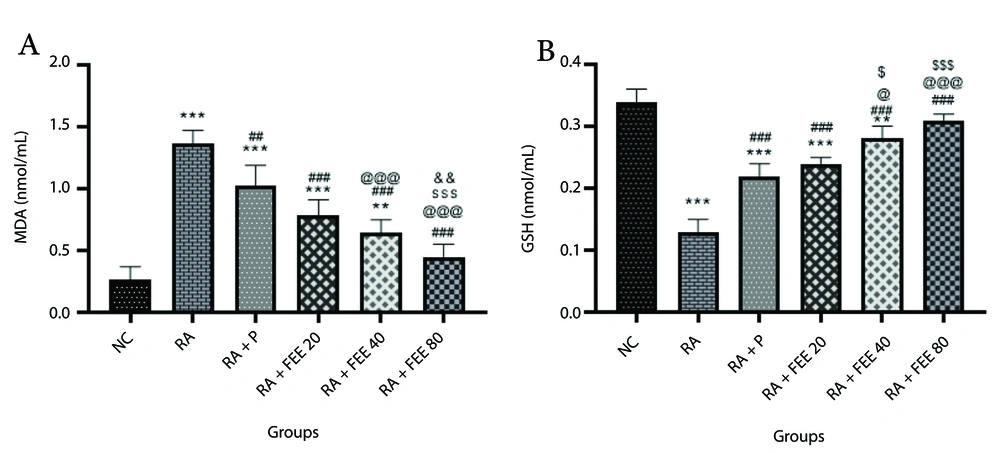
4.5. The Effect of Fraxinus excelsior Extract on Oxidative Stress Parameters
Analysis of MDA levels (Figure 4A) revealed a significant increase in the RA group compared to the NC group (P < 0.001), which FEE treatment markedly reduced. Notably, FEE at 80 mg/kg most effectively lowered MDA levels across all treatment groups. Glutathione, a key antioxidant, was assessed (Figure 4B), showing a significant decrease in the RA group relative to the NC group (P < 0.001). Both FEE- and prednisolone-treated groups exhibited substantial GSH restoration compared to the RA group, with the 80 mg/kg FEE group showing the greatest increase in GSH levels (P < 0.001), indicative of robust antioxidant activity.
The impact of Fraxinus excelsior L. bark hydromethanolic extract (FEE) on A, serum malondialdehyde (MDA) and B, glutathione (GSH) levels. Treatment with FEE (80 mg/kg) led to a significant increase in serum GSH levels and a decrease in MDA levels compared to the RA group. The results are presented as mean ± SEM (n = 5). NC, normal control; RA, rheumatoid arthritis; P, Prednisolone. Statistical significance: ** P < 0.01, *** P < 0.001 vs. NC group, ## P < 0.01, ### P < 0.001 vs. RA group, @ P < 0.05, @@@ P < 0.001 vs. RA + P group, $ P < 0.05, $$$ P < 0.001 vs. RA + FEE20 group, and && P < 0.01 vs. RA + FEE40 group.
The antioxidant effects of FEE likely stem from its hydroxycoumarin content, including isofraxidin, fraxetin, and fraxinol (23), which potently scavenge ROS, neutralizing free radicals and mitigating oxidative damage (55). Additionally, these coumarins may enhance GSH synthesis by upregulating glutamate-cysteine ligase (GCL) and modulating NRF2 signaling (56), a mechanism supported by the pronounced GSH elevation in the 80 mg/kg FEE group, highlighting FEE’s potential in ameliorating oxidative stress in RA.
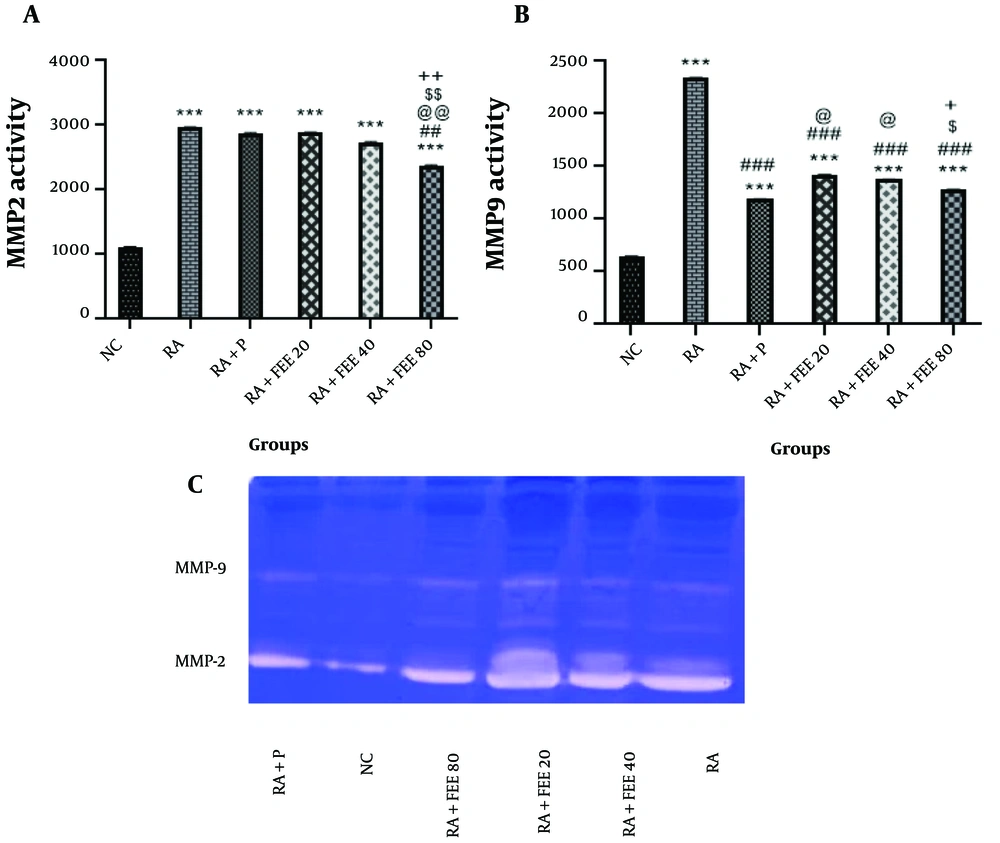
4.6. The Effect of Fraxinus excelsior Extract on MMP-2 and MMP-9 Enzymatic Activity
The MMP-2 activity increased significantly in all groups compared to the NC group (P < 0.001). However, the RA + FEE80 group showed significantly lower MMP-2 activity than the RA group (P < 0.01). In high doses, the therapeutic activity of FEE was more pronounced, modulating MMP-2 activity in serum (Figure 5A). Regarding MMP-9, the RA group showed a significant increase compared to the NC group (P < 0.001). Moreover, FEE significantly reduced MMP-9 gelatinase activity (P < 0.001 vs. RA group). The RA + P and RA + FEE80 groups exhibited the strongest anti-inflammatory activity during the study (P < 0.001) (Figure 5B).
Effects of Fraxinus excelsior L. bark hydromethanolic extract (FEE) on A, MMP-2 and B, MMP-9 gelatinase activities. C, displays the gelatin zymograms of MMP-2 and MMP-9. The results are presented as mean ± SEM (n = 5). NC, normal nontrol; RA, rheumatoid arthritis; P, prednisolone. Statistical significance: *** P < 0.001 vs. NC group, ## P < 0.01, ### P < 0.001 vs. RA group, @ P < 0.05, @@ P < 0.01 vs. RA + P group, $ P < 0.05, $$ P < 0.01 vs. RA + FEE20 group, and + P < 0.05, ++ P < 0.01 vs. RA + FEE40 group.
In gelatin zymography, band intensities were normalized to total protein content for accurate enzymatic activity quantification. Protein concentrations were measured using the Bradford assay, and equal amounts of protein were loaded onto gels (Figure 5C). After electrophoresis and incubation, densitometry software (e.g., ImageJ) quantified band intensities relative to total protein content. This approach minimized loading variations, ensuring precise comparison of MMP-2 and MMP-9 activity across treatment groups (57).
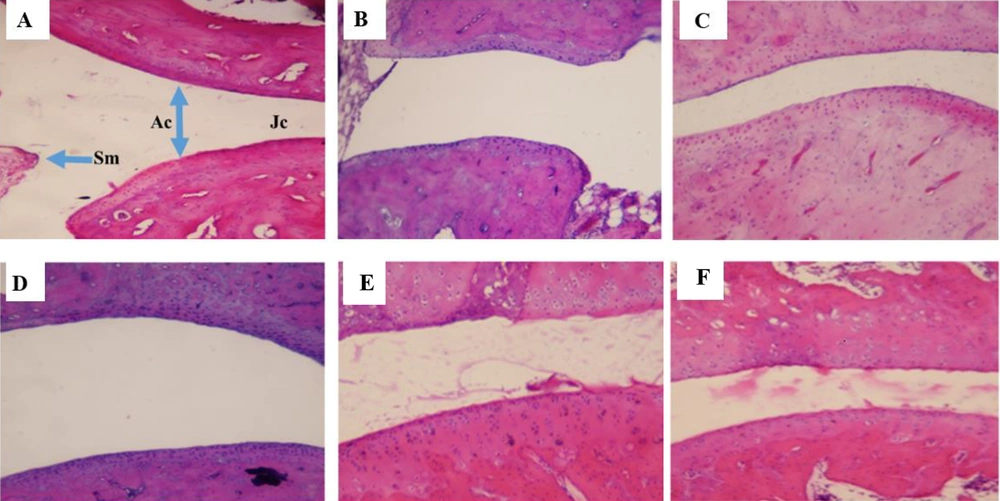
4.7. The Effect of Fraxinus excelsior Extract on Histopathological Changes
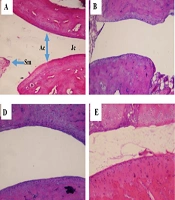
Histopathological analysis of knee and ankle joint tissues was conducted to evaluate FEE’s therapeutic effects at the microscopic level (Figure 6). In the NC group (A), the joint cavity, synovial membrane (SM), joint capsule (JC), and articular cartilage (AC) exhibited normal morphology. In contrast, the RA group (B) displayed an enlarged joint cavity, a distorted joint capsule, and irregular, occasionally fragmented cartilage. The RA + P group (C) showed moderate improvement, whereas the RA + FEE20 group (D) exhibited no notable recovery. The RA + FEE40 group (E) retained some structural abnormalities, but the RA + FEE80 group (F) demonstrated a substantial reduction in tissue damage, closely resembling the RA + P group (C). These findings confirm that FEE at 80 mg/kg exerted the most pronounced therapeutic effect, comparable to prednisolone.
Hematoxylin and eosin staining at magnification ×100. The effects of Fraxinus excelsior L. bark hydromethanolic extract (FEE) on histopathological changes in joint tissues of experimental groups: A, normal control (NC): Intact joint architecture with no signs of inflammation; B, rheumatoid arthritis (RA) group: Severe synovial hyperplasia, inflammatory cell infiltration, and cartilage erosion; C, RA group treated with prednisolone (RA + P, 10 mg/kg, orally, 21 days): Reduced inflammation and improved cartilage structure; D, RA group treated with FEE (RA + FEE20, 20 mg/kg, orally, 21 days): Moderate reduction in inflammatory cell infiltration; E, RA group treated with FEE (RA + FEE40, 40 mg/kg, orally, 21 days): Further improvement with reduced hyperplasia and preserved cartilage; F, RA group treated with FEE (RA + FEE80, 80 mg/kg, orally, 21 days): Most significant anti-inflammatory effect with near-normal joint morphology.
5. Discussion
Rheumatoid arthritis is a chronic, inflammatory autoimmune disease affecting the joints to varying degrees among individuals. This autoimmune and inflammatory disease usually affects many joints in the body. Numerous complications may arise, such as rheumatoid vasculitis, permanent joint damage necessitating arthroplasty, and Felty syndrome, which may require splenectomy if left unattended (12, 58). Rheumatoid arthritis has been linked to oxidative stress, a condition where the accumulation of ROS rises over time, either due to increased production, decreased antioxidant defenses, or a combination of both. Activated macrophages and T-cells in the synovium have been identified as contributors to synovitis, highlighting the relationship between proinflammatory cells and oxidative stress mediators. The activation and proliferation of synoviocytes may lead to the secretion of pro-inflammatory cytokines, such as TNF-α, MMPs, IL-1, IL-6, IL-8, prostaglandins, and leukotrienes. The invasion of the synovium into nearby articular structures causes damage to the cartilage and bone, resulting in joint swelling (59-61). Additionally, there is already recognition of a positive feedback loop between oxidative stress and inflammation, through which both elements amplify the detrimental effects of each other (60). Increased MDA and decreased GSH levels in RA reflect oxidative stress-induced joint damage. Malondialdehyde, a marker of lipid peroxidation, promotes synovial inflammation and cartilage degradation, while reduced GSH weakens antioxidant defenses, making joint tissues more vulnerable to oxidative damage. This imbalance accelerates inflammation and RA progression, reinforcing the critical role of oxidative stress in disease pathology (59, 62, 63).
So far, various alternatives have been employed in the treatment of RA. Nevertheless, none of the presently available therapies have demonstrated full efficacy while also being associated with a multitude of adverse effects (12). Hence, drug discovery for RA requires multi-faceted approaches that affect various interactive mechanisms like inflammation, oxidative stress, etc., with lower side effects. Natural products have become popular in treating many diseases, especially autoimmune disorders linked to oxidative stress and chronic inflammation, due to their efficacy and reduced side effects (64, 65). The clinical potential of FEE as a complementary RA therapy is notable (25). With its potent antioxidant and anti-inflammatory effects, FEE may support conventional treatments by reducing oxidative stress, synovial inflammation, and cartilage degradation. Its regulation of MMP-2 and MMP-9 levels could help preserve joint integrity, slowing disease progression. Additionally, the anti-inflammatory properties of FEE might alleviate symptoms, possibly reducing the reliance on corticosteroids and NSAIDs and their associated side effects. However, further clinical studies are essential to validate its efficacy and safety in human RA patients.
The present study utilized a CFA-induced rat model of RA to find the potential therapeutic efficacy of FEE. Upon the demonstration of swelling in the tibiotarsal joint of rats, the administration of treatment to the affected rats commenced. To probe the process of healing, different tests, including the hot plate test, biochemical analyses, and the measurement of rats’ weights, were employed. The results indicated that the group receiving treatment with FEE 80 mg/kg was able to significantly alleviate the principal symptoms of CFA-induced RA. Notably, the hot plate test demonstrated that FEE exhibited a dose-dependent analgesic effect, with the highest dose (80 mg/kg) significantly increasing withdrawal latency compared to the RA group (P < 0.001). This analgesic effect was comparable to prednisolone, suggesting that FEE effectively modulates pain perception.
Bark extract from F. excelsior L. has demonstrated strong antioxidant and anti-inflammatory qualities (23, 35, 66), potentially relieving oxidative stress and inflammation — two important contributors to RA development (59, 60, 67). The therapeutic effects of FEE can be attributed to its hydroxycoumarin-rich composition, including isofraxidin, fraxetin, fraxinol, fraxidin, and their glycosylated derivatives, alongside lignans, flavonoids, polyphenols, and secoiridoids isolated from various Fraxinus species (23, 31, 68). We confirmed the presence of isofraxidin 7-O-diglucoside in FEE via LC-MS/MS analysis (23). These coumarins inhibit NF-κB activation, reducing expression of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β), central mediators of synovial inflammation and cartilage destruction in RA (35), while their antioxidant properties scavenge ROS, enhance GSH levels, and reduce MDA, mitigating oxidative stress-induced damage (69). The neuroprotective effects of FEE are also noteworthy, given emerging links between RA-associated systemic inflammation and neuroinflammatory disorders (23). Numerous pharmacological properties, such as antirheumatic, immunomodulatory, skin-regenerating, and antibacterial activities, further support its therapeutic potential (23, 31, 68).
Although FEE showed promising effects, potential long-term toxicity remains unknown. While coumarin-containing extracts are generally well-tolerated, some coumarins are linked to hepatotoxicity at high doses (70). Future studies should assess chronic administration to determine safety, particularly regarding liver and kidney function and drug interactions.
Regarding the role of MMP-2 and MMP-9 in RA, these gelatinases, typically expressed at low levels, increase in pathological conditions, driving synovial fibroblast-mediated inflammation and cartilage degradation by enhancing production of IL-1β, IL-6, IL-8, and TNF-α via activation of NF-κB, extracellular signal-regulated kinase, and c-Jun NH2-terminal kinases (71, 72). MMP-9 levels rise more significantly than MMP-2 during RA progression (73), and no effective gelatinase inhibitors have been introduced (74). Our study showed that FEE reduced MMP-2 and MMP-9 activity, possibly via NF-κB inhibition, a key regulator of cytokine and MMP expression, limiting cartilage degradation (75, 76). This suppression preserves joint integrity, as MMPs degrade extracellular matrix components (77, 78). Further mechanistic studies are needed to confirm this NF-κB-mediated effect.
Both FEE and prednisolone significantly reduced inflammatory markers and oxidative stress in the RA model. However, FEE uniquely suppressed MMP-2 and MMP-9 activity, suggesting superior cartilage protection compared to prednisolone, which, while rapidly alleviating symptoms, is linked to adverse effects such as osteoporosis and immunosuppression. The dose-dependent improvement in reaction times during the hot plate test further indicates that FEE effectively modulates pain via its anti-inflammatory and antioxidant properties, reinforcing its potential as a natural analgesic agent for RA management. Beyond pain and inflammation reduction, FEE showed antioxidant effects — evidenced by increased GSH and decreased MDA levels — promoting joint health and possibly slowing RA progression. Moreover, neuroprotective benefits of FEE may address RA-related neuroinflammation, an area prednisolone does not target. While prednisolone remains vital for acute RA management, FEE, particularly at high doses (80 mg/kg), achieved results comparable or superior to prednisolone in some parameters, suggesting its potential as a complementary therapy to reduce corticosteroid reliance. Overall, FEE mitigates RA by modulating MMP-2 and MMP-9 levels, decreasing inflammatory factors, and preserving joint health.
The study also revealed a generally dose-dependent effect of FEE (20, 40, and 80 mg/kg), with the 80 mg/kg dose consistently yielding the strongest outcomes across most measures, including pain relief, oxidative stress modulation, MMP inhibition, and histopathological improvements. However, the dose-response was not uniformly linear; the 40 mg/kg dose slightly outperformed 80 mg/kg in reducing paw volume (P < 0.001 vs. P < 0.01), suggesting an optimal effect for edema reduction. This may reflect pharmacokinetic saturation or target-specific mechanisms, where edema suppression plateaus earlier than MMP inhibition. These findings suggest that while higher doses enhance efficacy, optimal dosing may vary by outcome, necessitating further pharmacokinetic and mechanistic studies.
5.1. Conclusions
The present study found that treating RA mice with FEE reduced RA symptoms by lowering oxidative stress and inflammatory markers (GSH, MDA, and MMPs). Histopathological tests corroborated these findings. The FEE at a high dose (RA + FEE80 group) demonstrated the strongest therapeutic results, and in certain circumstances, it outperformed the standard treatment prednisolone (RA + P group). The observed functional improvements, together with its antioxidant and anti-inflammatory properties, highlight its potential as a treatment for RA. Given the significant therapeutic effects of FEE, particularly at high doses, it may serve as a complementary or alternative option to corticosteroids, potentially reducing the long-term dependency on these drugs and minimizing their associated adverse effects. Future research should prioritize clinical trials to expand on these findings and examine their application in treatment. Additionally, mechanistic studies are needed to elucidate the molecular pathways through which FEE exerts its effects, particularly its role in NF-κB signaling and MMP inhibition. Long-term safety evaluations should also be conducted to assess potential toxicity and establish a safe therapeutic dosage.