1. Background
Breast cancer (BC) is one of the most prevalent malignancies among women, and increasing efforts are being made to better understand its pathophysiology and to develop novel therapeutic strategies with enhanced anticancer efficacy and fewer side effects (1, 2). Conventional therapies are often associated with considerable side effects; therefore, plant-derived polyphenols — among various natural compounds — have gained attention for their diverse biochemical properties, including antioxidant and antiproliferative effects, many of which may prove beneficial in cancer prevention and treatment (3-5).
Nobiletin (NOB), a polymethoxyflavonoid found in various herbs, especially in Citrus depressa, has been reported to exhibit anti-inflammatory and antiviral activities (6-8). It has been shown that the anti-inflammatory effects of NOB could help prevent rheumatoid arthritis through the reduction of Matrix metalloproteinase-9 and Prostaglandin E2 expressions (9). In terms of anticancer activity, NOB is capable of decreasing the viability of certain malignancies, such as prostate, skin, and colon cancers. Furthermore, the herbal polyphenol NOB inhibits the invasion of gastric cancer cells (10-13). According to a previous study by Sp et al. on the MCF-7 cell line, NOB also exhibits anticancer activities against BC (14).
Although the antitumor role of NOB has been established, the exact mechanism has not yet been fully elucidated (15-17). Therefore, estrogen receptors (ERs), particularly estrogen receptor alpha (ERα), are considered important targets in laboratory research. The ERα, due to its central role in estrogen signal transduction, plays a significant role in BC, and its status is commonly used to predict the tumor’s response to hormonal therapy (18-20). Cyclin D1, a well-known CDK4/6-related factor, plays a prominent role in regulating the cell cycle, primarily facilitating the G1/S progression (21). The nucleus-resident protein, due to its connection with various genes and major cellular functions, such as cell proliferation and migration, has been shown to contribute to carcinogenesis, especially in BC (22). It has been shown that the overexpression of Cyclin D1 is commonly observed in approximately 30 - 50% of human malignancies (23). According to the findings of the study by He et al., Cyclin D1 overexpression may result from the abnormal expression of β-catenin, leading to enhanced cancerous characteristics, particularly increased metastasis, in tumor cells (24).
2. Objectives
Many studies have reported the beneficial effects of NOB on various signaling pathways implicated in cancer. This study investigates the influence of NOB on the expression of apoptosis-related genes, including Bax, Bcl-2, and Cyclin D1, and evaluates its effect on the proportion of apoptotic cells in the MCF-7 cell line.
3. Methods
3.1. Cell Line Culture and Processing
This study was conducted using the MCF-7 cell line, which was purchased from the Pasteur Institute (Tehran, Iran). Cell culture was performed in DMEM supplemented with 10% FBS, and cells were incubated at 37˚C with 5% CO2. Nobiletin was obtained from Sigma-Aldrich (USA) and dissolved in culture-grade Dimethyl Sulfoxide (DMSO) immediately before use, with the concentration of DMSO maintained below 1% (V/V) to nullify any possible side effects.
3.2. MTT Assay
Cancer cells were evaluated in terms of proliferation using the MTT assay. Concisely, the 96-well plates containing NOB-treated cells (25, 50, 100, 200, and 300 µM) were incubated for 24 and 48 hours. The drug-containing medium was replaced with fresh medium containing 10% MTT solution for 4 hours. The MTT solution was then replaced with DMSO, and the plates were shaken until the formazan crystals dissolved completely. The optical densities (OD) for the entire plate were read at 570 nm using a BioTek microplate reader (Vermont, USA). Cell viability was calculated using the ODs of treated cells and the untreated control group.
3.3. Experimental Groups
Five experimental groups were established to assess the effects of NOB on MCF-7 cells:
(1) Control group: Untreated MCF-7 cells
(2) Positive control group: Cells treated with 100 nM estrogen (E2)
(3) Experimental group 1: Cells treated with 100 nM E2 and 25 µM NOB
(4) Experimental group 2: Cells treated with 100 nM E2 and 50 µM NOB
(5) Experimental group 3: Cells treated with 100 nM E2 and 100 µM NOB.
3.4. RNA Extraction and Real-time PCR
A Yekta Tajhiz Azma (Tehran, Iran) kit was employed to extract total RNA from cell isolates. The RNA concentration and purity were evaluated using a Nanodrop 2000 device, and 1.5% agarose gel electrophoresis indicated that the isolates were intact. The RNA extracts were reverse-transcribed into cDNA using a Sina Clon (Tehran, Iran) kit, following the producer's protocols. The final products were maintained at -70°C for further use. Through qRT-PCR, using an ABI Quantstudio 3 (Massachusetts, USA) and an Ampliqon SYBR Green kit, the relative expressions of Bax, Cyclin D1, and Bcl-2 genes were obtained, with GAPDH as the internal reference gene. The primer sequences are listed in Table 1.
| Gene | Primers Sequence | Size of PCR Product (bp) |
|---|---|---|
| Bax | F. 5′-TGGACACCCAGAAGACAG-3′ | 182 |
| R. 5′-GTCTGTTTCGGTACTGTCAT-3′ | ||
| Cyclin D1 | F. 5′-GATGCTGGAGGTCTGCGAG-3′ | 193 |
| R. 5′-GGCGGCTCTTTTTCACGGC-3′ | ||
| Bcl-2 | F. 5′-CAAGAGTAACATGTTGAAG-3′ | 186 |
| R. 5′-CCAGGCAAGTCTCCTCATTG-3′ | ||
| GAPDH | F. 5′-TCGCAGTCAACGCATTTGGT-3′ | 189 |
| R. 5′-ATGGCGTTCTCACGCTTTGAC-3′ |
3.5. Caspase-3 and Caspase-9 Activity
The cells were harvested and suspended in a lysis buffer, followed by centrifugation to obtain the cell lysates. The protein concentration of each sample was measured, and equal protein amounts from each extract were used for subsequent analyses. To measure the activities of Caspase-3 and Caspase-9, the samples were incubated with specific colorimetric substrates for one hour. Absorbance was recorded at 405 nm to quantify substrate cleavage, which reflects the enzymatic activity of these caspases.
3.6. Flow Cytometry
Apoptosis was evaluated using flow cytometry with an Annexin V-FITC/PI kit (IQ Products, Groningen, Netherlands), following the manufacturers protocol. After NOB treatment, trypsinized cells were collected, washed with calcium buffer, and centrifuged to obtain a pellet. The cells were resuspended in the same buffer with Annexin V-FITC and incubated at 4°C for 20 minutes. The buffer was then replaced with one containing PI, and incubation continued for another 10 minutes. Samples were analyzed using a flow cytometer (Becton, Dickinson, San Jose, CA, USA), and apoptosis was determined based on the Annexin V-FITC/PI staining patterns. Data were processed using FlowJo software version 10.
3.7. Western Blot Analysis
The cells were lysed in a buffer containing HEPES, NaCl, EDTA, and Triton X-100, and then centrifuged to collect the supernatant. Protein levels were measured using the BCA method. Equal amounts of protein were separated by SDS-PAGE and transferred to PVDF membranes. After blocking with skim milk, the membranes were incubated with anti-p53 antibodies overnight at 4°C. They were then treated with HRP-conjugated secondary antibodies for 1 hour. Protein bands were visualized using ECL, and their intensity was quantified with ImageJ. The protein levels were normalized to GAPDH, which was used as a loading control to ensure equal protein loading across all samples.
3.8. Statistical Assessment
Each experiment was repeated a minimum of three times, and the average values were used for subsequent analysis. GraphPad Prism was utilized for data analysis. Through one-way ANOVA, the comparison of experimental groups was performed, and the statistical significance level was considered at < 0.05.
4. Results
4.1. The Effects of Nobiletin on Cancer Cell Proliferation
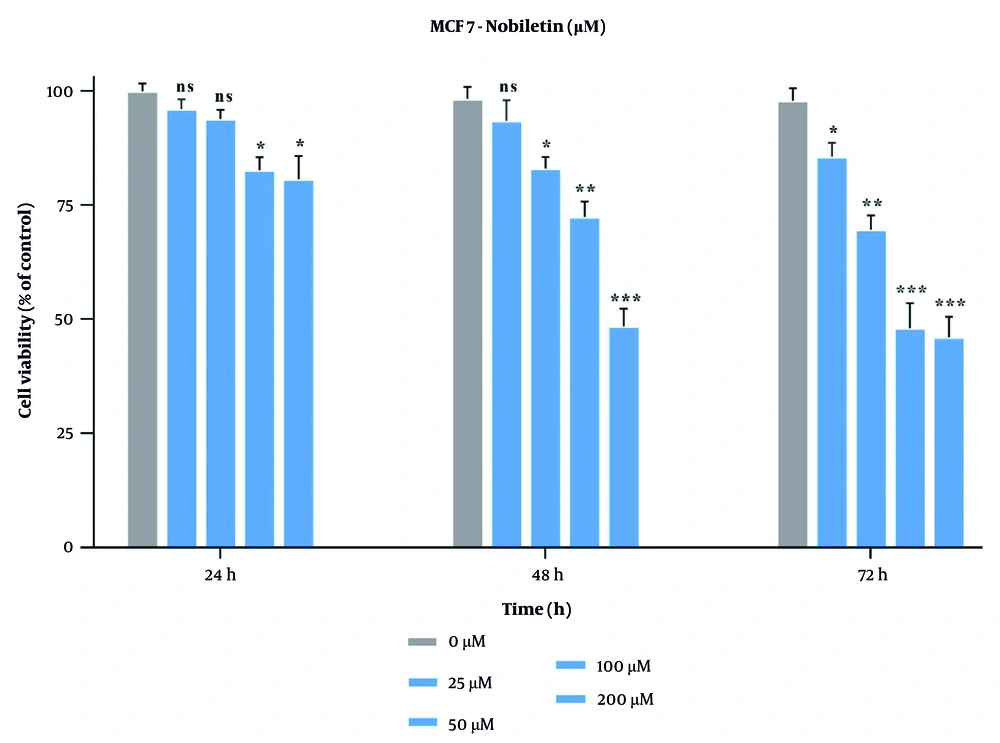
Through the MTT assay, we investigated whether NOB impacts the viability of MCF-7 cells. The least effective concentration of NOB (50 µM) reduced MCF-7 cell proliferation to 74.3% after 24 hours, while at 48 hours, 25 µM of NOB reduced cell viability to 67.0% (P < 0.05), both of which were statistically significant (Figure 1). All other tested concentrations of NOB also reduced MCF-7 cell viability. The IC50 of NOB on MCF-7 cells after 24-hour treatment was 124.5 µM. As the effects of NOB increased with higher doses, this suggests a significant dose-dependent, but not time-dependent, effect on the cancer cell line. Based on these findings, three concentrations of NOB with low to moderate cytotoxicity — 25, 50, and 100 µM — under a 24-hour treatment course were selected for further experiments (Figure 1).
The effects of nobiletin (NOB) on the cell viability of MCF-7 cell line – (a) NOB exhibited a significant dose-dependent reduction in MCF-7 cell viability after 24, 48 and 72 hours. ** P < 0.01, *** P < 0.001 significant compared to the untreated control group; ns denotes "not significant".
4.2. The Effects of Nobiletin on Gene Expression
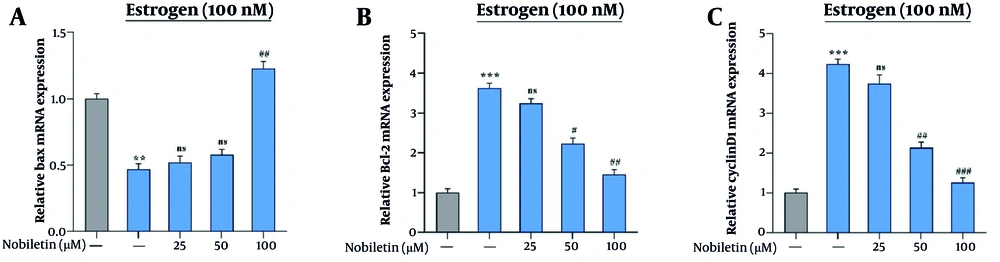
Our qRT-PCR results demonstrated that NOB significantly downregulated the expression of Bcl-2 and Cyclin D1, while upregulating the pro-apoptotic gene Bax. Estrogen-treated (E+) cells (100 nM) were used as a positive control to assess whether NOB’s effects are mediated through ERα. Other experimental groups received either 50 or 100 µM of NOB for 24 hours, alongside an untreated control group. In MCF-7 cells, treatment with 50 and 100 µM of NOB resulted in a significant reduction in Cyclin D1 expression to 1.33-fold (P < 0.001) and Bcl-2 to 1.20-fold (P < 0.01) (Figure 2B and C). In the E⁺ MCF-7 cells, Bax expression decreased to 0.48-fold (P < 0.01), whereas in the NOB-treated E+ group (100 µM), Bax expression was restored and significantly upregulated to 1.36-fold (P < 0.01) (Figure 2A).
The effects of nobiletin (NOB) on the expression of Bax, CyclinD1 and Bcl-2 in the MCF-7 breast cancer (BC) cell line – the estrogen (100 nM)-treated (E+) groups consist of an untreated group and a NOB (50, and 100 µM)-treated group: A, 100 µM of NOB significantly increased the gene expression of Bax in MCF-7 cells; B, fifty and 100 µM of NOB inhibit the gene expression of Bcl-2 in MCF-7 cells, significantly; C, fifty 50 and 100 µM of NOB inhibit the gene expression of CyclinD1 in MCF-7 cells, significantly. ** P < 0.01, *** P < 0.001 significant compared to the control; # P < 0.05, ## P < 0.01, ### P < 0.001 significant compared to the E+ group; ns denotes "not significant".
4.3. The Effect of Nobiletin on Caspase Activity
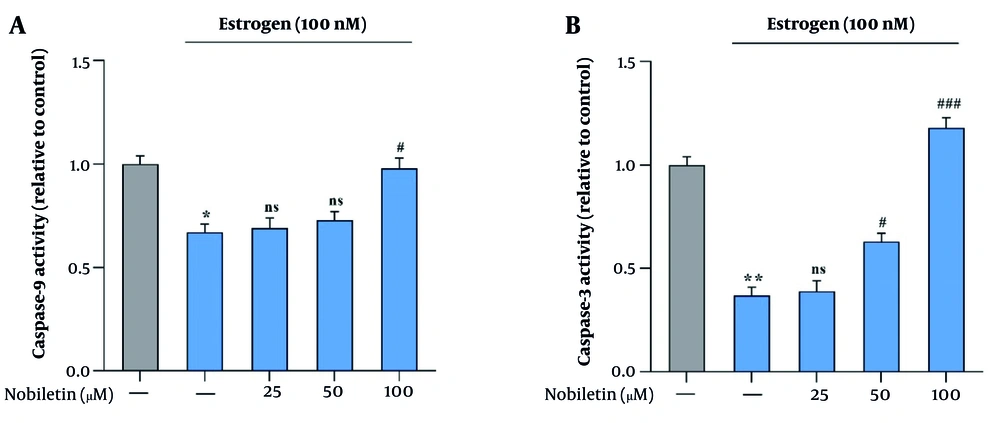
The potential effect of NOB on the activity of caspase-9 and caspase-3 in MCF-7 cells was assessed by measuring the enzymatic activity of these key apoptotic markers. The results showed a marked increase in the activity of both caspase-9 and caspase-3 in response to treatment with NOB, suggesting that it may induce apoptosis through the intrinsic pathway. Specifically, treatment with 100 µM of NOB led to a 0.92-fold increase in caspase-9 activity (P < 0.05) and a 1.23-fold increase in caspase-3 activity (P < 0.001), further supporting the pro-apoptotic role of NOB in MCF-7 cells (Figure 3A and B).
Effects of nobiletin (NOB) on caspase-9 and caspase-3 activities in MCF-7 cells – treatment with 100 µM of NOB significantly increased the activities of caspases (A, B) in MCF-7 cells (3 and 9). Statistical significance is indicated as follows: * P < 0.05 and ** P < 0.01 compared to the untreated group; # P < 0.05 and ### P < 0.001 compared to Estrogen-treated (E+) group; ns denotes "not significant". This indicates that NOB effectively enhances apoptotic activity in MCF-7 cells, with a more pronounced effect compared to estrogen treatment alone.
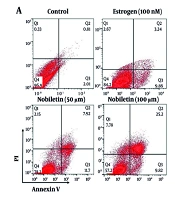
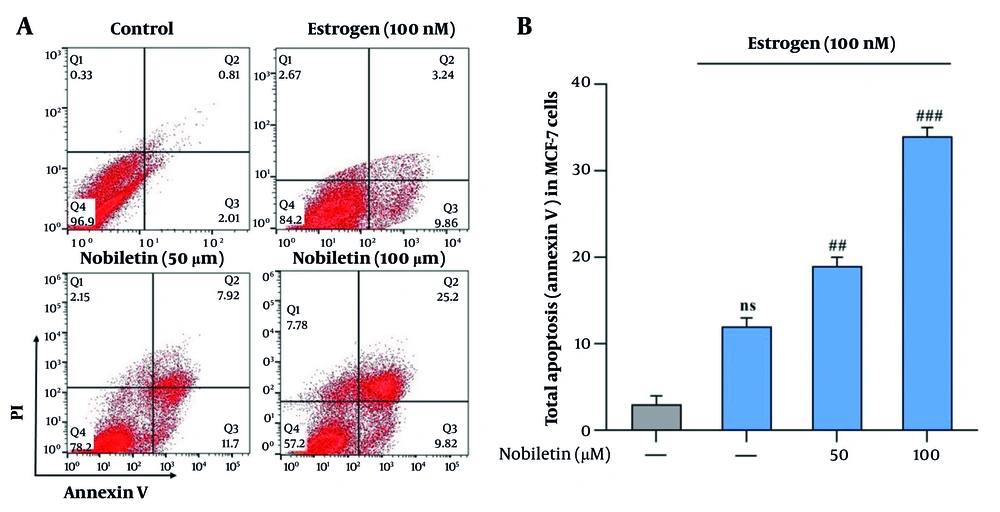
4.4. Potential Effect of Nobiletin on Apoptosis
The flow cytometry analysis of apoptosis in MCF-7 cells treated with NOB revealed several key findings. Treatment with NOB led to a significant increase in the apoptotic cell percentage compared to the control group. Specifically, there was a notable rise in the early and late apoptotic cell populations. The 100 µM concentration of NOB resulted in the highest level of apoptosis, showing a substantial increase in the annexin V-positive and propidium iodide-positive cells (2.4-fold increase, P < 0.001). In contrast, lower concentrations (e.g., 50 µM) induced a moderate increase in apoptosis (1.7-fold increase, P < 0.01). These results indicate that NOB effectively promotes apoptosis in MCF-7 cells, with a concentration-dependent response observed. Flow cytometry provided clear evidence of NOB's potential to enhance apoptotic cell death, supporting its role as a pro-apoptotic agent in BC cells (Figure 4A and B).
Effects of nobiletin (NOB) on apoptosis in MCF-7 cells measured by flow cytometry –treatment with 100 µM of NOB significantly increased the percentage of apoptotic cells in MCF-7 cells, as determined by flow cytometry (A and B). Statistical significance is indicated as follows: ## P < 0.01 and ### P < 0.001 compared to the Estrogen+ (E+) group; ns denotes "not significant". These results demonstrate that NOB effectively induces apoptosis in MCF-7 cells, with a more pronounced effect compared to estrogen treatment alone.
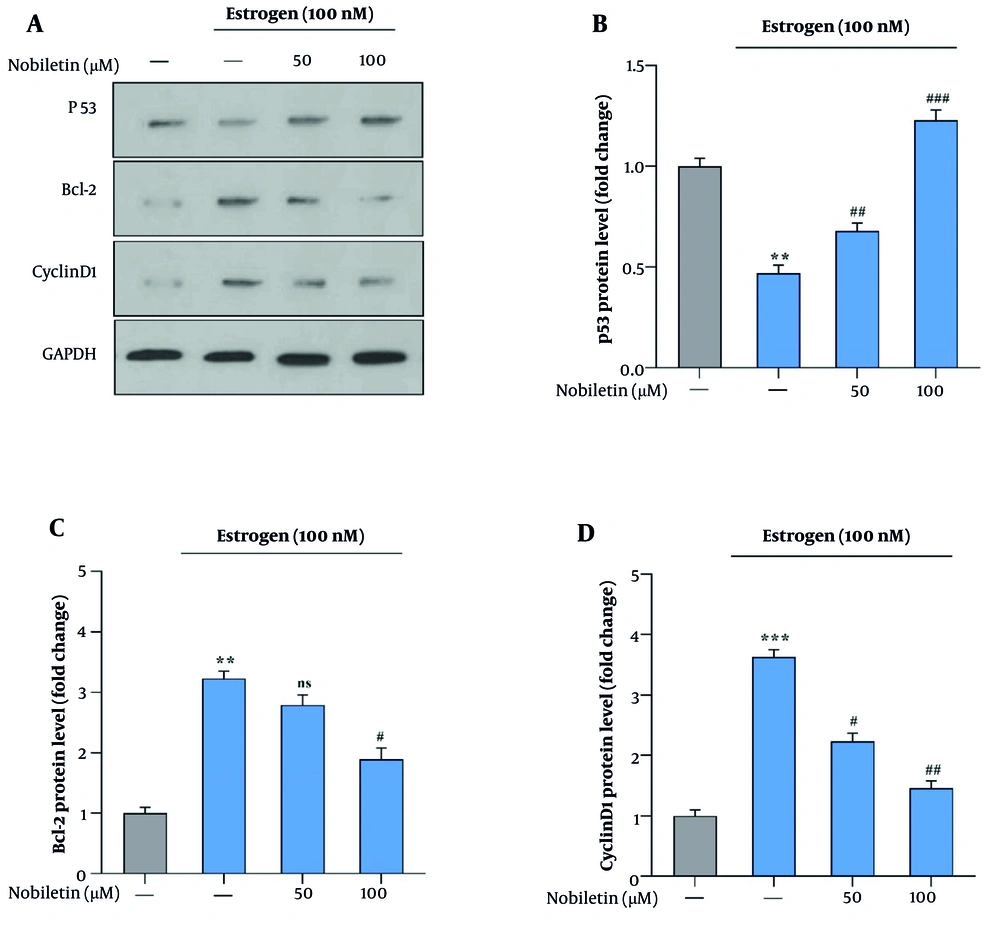
4.5. Effect of Nobiletin on Protein Levels
The effect of NOB on the protein levels of p53, Cyclin D1, and Bcl-2 was investigated. Nobiletin treatment caused a significant rise in p53 protein levels (P < 0.001), indicating enhanced activation of this tumor suppressor (Figure 5A and B). Conversely, NOB treatment resulted in a marked decrease in Cyclin D1 and Bcl-2 protein levels (P < 0.01 for Cyclin D1 and P < 0.05 for Bcl-2). Specifically, Cyclin D1 levels were reduced to 1.32-fold, and Bcl-2 levels decreased to 1.70-fold compared to the E+ group (Figure 5C and D). Additionally, the 50 µM dose of NOB significantly increased p53 protein levels (P < 0.01) and significantly decreased Cyclin D1 levels (P < 0.05) compared to the E+ group, but it had no effect on Bcl-2 protein levels (Figure 5).
A, Effects of nobiletin (NOB) on Protein Levels of p53, Bcl-2, and Cyclin D1 in MCF-7 cells measured by Western blot; B, treatment with 100 µM of NOB significantly increased the protein levels of p53; and C and D, decreased the levels of Bcl-2 and Cyclin D1 in MCF-7 cells. Statistical significance is indicated as follows: ** P < 0.01 and *** P < 0.001 compared to the untreated control; # P < 0.05, ## P < 0.01, and ### P < 0.001 compared to the Estrogen+ group. ns denotes "not significant." These findings highlight that NOB modulates key apoptotic and cell cycle proteins, promoting apoptosis and disrupting cell cycle regulation in MCF-7 cells.
5. Discussion
Breast cancer is a common malignancy in women and necessitates the development of novel therapies with higher efficacy and fewer side effects. Conventional treatments often result in side effects, which stimulates research into natural compounds such as plant polyphenols, which exhibit antioxidant and antiproliferative properties that have beneficial effects on malignancies (25, 26). Nobiletin, a polymethoxy flavonoid found in various herbs, particularly Citrus depressa, has shown anti-inflammatory and antiviral effects. Its anti-inflammatory effects are reported to prevent rheumatoid arthritis by reducing the expression of matrix metalloproteinase 9 and prostaglandin E2. Nobiletin also exhibits anti-cancer properties by reducing the viability of prostate, skin, and colon cancers and inhibiting the invasion of stomach cancer cells (27, 28).
Despite the proven antitumor effect of NOB, the exact mechanism remains unclear. The ERα is critical in BC research due to its central role in estrogen signaling and its utility in predicting tumor response to hormone therapy. Cyclin D1, a key regulator of the cell cycle, facilitates G1/S progression and is overexpressed in 30 - 50% of human malignancies, contributing to carcinogenesis and metastasis through its association with β-catenin. The potential of NOB as an anticancer agent and its interaction with key cellular signaling pathways require further investigation, particularly in the context of BC (29, 30).
Our study demonstrated that NOB significantly reduces MCF-7 cell viability, aligning with findings that its metabolism by CYP1A1 enzymes enhances cytotoxicity (31). Nobiletin at 50 µM reduced cell proliferation to 74.3% after 24 hours, and 25 µM NOB reduced viability to 67.0% after 48 hours, supporting the role of metabolic activation in its anti-proliferative activity. The study identified NM1 as a significant metabolite and highlighted CYP1A1's role in NOB's metabolism, providing a mechanistic basis for our results. The lower IC50 value (44 µM) reported when CYP1A1 is active, compared to our IC50 of 124.5 µM, suggests that metabolic activation by CYP1A1 significantly boosts NOB's cytotoxicity. This implies that the anti-proliferative effects observed in our study may be partially attributed to the metabolic activation of NOB by CYP1A1 enzymes. The induction of CYP1A1 enzymes by NOB could also explain the dose-dependent effects observed, where higher doses led to greater reductions in cell viability. These findings underscore the importance of considering metabolic activation when evaluating the anti-cancer potential of NOB and other flavonoids. Our findings that NOB significantly downregulates Bcl-2 and Cyclin D1 while upregulating Bax in MCF-7 cells are consistent with the antitumor activity observed in the study by Hatami et al. In that study, quercetin-loaded solid lipid nanoparticles (QC-SLN) were used to inhibit the proliferation of MDA-MB-231 and MCF-7 cells (32).The study demonstrated that QC-SLN significantly decreased cell viability and colony formation and increased apoptosis through the modulation of Bax and Bcl-2 genes. Similarly, our results show that NOB treatment leads to a significant reduction in Bcl-2 and Cyclin D1 expression, which are key regulators of cell survival and proliferation, respectively. The upregulation of Bax by NOB, a pro-apoptotic gene, further supports its role in promoting apoptosis, aligning with the increased apoptosis observed in the QC-SLN study. These parallel findings highlight the potential of flavonoids like NOB and quercetin in modulating key genes and pathways involved in cell survival, proliferation, and apoptosis, underscoring their potential as therapeutic agents in cancer treatment. Our results that NOB significantly increases the activity of caspase-9 and caspase-3 in MCF-7 cells, suggesting the induction of apoptosis through the intrinsic pathway, are consistent with the observations made by Hatami et al. in their study using QC-SLN on MDA-MB-231 and MCF-7 cells (32). The study demonstrated that QC-SLN led to a marked increase in the expression of PARP, caspases 9, and 3, along with an increase in Bax and a decrease in Bcl-2 expression, without significantly altering caspase 8 levels. This pattern of protein expression changes indicates that QC-SLN induces apoptosis through the intrinsic pathway, similar to the effects of NOB observed in our study. The activation of caspase-9 followed by caspase-3 in response to NOB treatment supports the notion that NOB triggers the intrinsic apoptotic pathway, which is further corroborated by the increased expression of these proteins in the QC-SLN study.
Our findings that NOB significantly increases apoptosis in MCF-7 cells, as evidenced by flow cytometry analysis, are consistent with the observations made by Afarin et al. in their study using a combination of etoposide (ETO) and QC-SLN on MDA-MB-231 cells (33). The study demonstrated that both QC-SLN and ETO significantly promote programmed cell death, with the combination leading to a 36.2% increase in apoptosis. Similarly, our results show that NOB treatment leads to a concentration-dependent increase in the percentage of apoptotic cells, with the highest level of apoptosis observed at 100 µM. The increase in both early and late apoptotic cell populations in response to NOB treatment suggests that it effectively promotes apoptosis in MCF-7 cells, aligning with the pro-apoptotic effects observed with QC-SLN and ETO. The apoptosis-inducing properties of flavonoids, such as quercetin and NOB, are highlighted by these findings, underscoring their potential as therapeutic agents in combating cancer.
Our findings that NOB significantly increases p53 protein levels while decreasing Cyclin D1 and Bcl-2 in MCF-7 cells are consistent with Afarin et al.’s observations in MDA-MB-231 cells (33). The study revealed that ETO alone significantly increased p53 and p21 levels, which QC-SLN further enhanced to a 3.42-fold elevation. Similarly, our results show that NOB treatment activates p53 and reduces Cyclin D1 and Bcl-2, promoting pro-apoptotic signaling. This is supported by evidence that both QC-SLN and ETO independently elevated Bax expression (up to 4.8-fold) and decreased Bcl-2 to 0.36-fold, akin to effects seen with flavonoids like quercetin. This suggests flavonoids may have practical applications as cancer therapeutic agents.
5.1. Conclusions
Our study provides compelling evidence that NOB exhibits significant anti-cancer properties in MCF-7 BC cells. The dose-dependent reduction in cell viability, modulation of key apoptotic and cell cycle regulatory proteins, and induction of apoptosis via the intrinsic pathway are consistent with findings that the metabolism of NOB by CYP1A1 enzymes enhances its cytotoxicity. The upregulation of p53, downregulation of Cyclin D1, and activation of caspase-9 and caspase-3 highlight NOB's potential as a pro-apoptotic agent. These results, along with the observed increase in apoptotic cells using flow cytometry, emphasize the therapeutic potential of NOB and other flavonoids in cancer treatment.
5.2. Limitations
This study has the following limitations: (1) It was conducted entirely in vitro and may not fully reflect in vivo tumor biology; (2) it utilized only a single ERα-positive cell line, limiting generalizability. Future research should: (3) Include in vivo studies to validate therapeutic potential; and (4) investigate combination strategies with standard chemotherapeutics to assess potential synergistic effects.