1. Background
Ethanol (ETH) has the ability to breach the blood-brain barrier, placenta, and various biological membranes (1). While excessive consumption of ETH impacts both the peripheral and central nervous systems (CNS) in adults, its primary target remains the brain (2). Overindulgence in ETH affects both the developing and mature brain, resulting in immediate and long-term consequences. Furthermore, ETH consumption during pregnancy can detrimentally affect the developing brain of the fetus (3). Studies on humans and animals have demonstrated that ETH triggers apoptosis in fibroblasts, the CNS (particularly in the hypothalamus and hippocampus), the prostate, liver, and generally in epithelial cells of most body tissues and endothelial cells of blood vessels (4). Some research indicates that the apoptotic effects of ETH can be mitigated by various antioxidants (5).
The restoration of damage to the nervous system, especially the central nerves, has long been a central focus of regenerative and medical sciences. Many of these injuries are considered irreversible, some arising endogenously through wear and tear or immune system attacks. Previously, it was widely believed that compensating for these damages was unattainable (6). However, advancements in therapeutic and restorative techniques have enhanced the prospects of addressing such injuries. Notably, the identification of sources of cellular compensation, such as neural progenitor cells (NPCs) in internal brain regions like the Subventricular Zone (SVZ) and the hippocampus, holds significant importance (7). It is now recognized that NPCs exist in the brains of adult mammals and contribute to brain dynamics throughout their lifespan (8). Two primary neurogenic zones in the brains of adult mammals house NPCs: The SVZ adjacent to the ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus (7).
Research conducted by Chen et al. has sparked optimism regarding the potential use of neurogenesis of NPCs in brain repair (9). Subsequently, a multitude of discoveries in this realm have broadened our comprehension of adult stem cells/neuronal progenitors and their inherent processes (10, 11). The identification of these cells in the CNS of mammals, previously thought to be incapable of repair and regeneration, has instilled hope for treating degenerative diseases such as MS, Parkinson’s, Alzheimer’s, strokes, and spinal cord injuries (12). Nevertheless, the process of regeneration and repair in the CNS remains one of the most intricate challenges in modern medical science, as well as in fields like cell therapy and tissue engineering (13). Rosa damascena, part of the Rosaceae family, is a shrub recognized for its small or curved thorns, large and intensely fragrant flowers, and compound leaves with numerous leaflets (14). Flourishing in various regions of Iran, this plant yields an extract known as Otto of Rose, a pale-yellow liquid with a slightly sweet taste and a potent, pervasive aroma (15). Studies reveal that R. damascena is rich in vitamin C, carboxylic acid, tannins, and notably flavonoid and polyphenolic compounds. These constituents render it effective against free radicals and inflammatory cells, endowing it with anti-cancer properties, the ability to prevent gene mutations, and antioxidant capabilities, among other advantages. Consequently, this plant is esteemed as a crucial medicinal resource for averting and treating ailments triggered by free radical activity (16). In the investigation conducted by Homayoun et al., all three doses of the hydroalcoholic extract of R. damascena, while exhibiting convulsive effects, markedly hindered the formation of dark neurons in diverse regions of the hippocampus in the utilized animal model (17).
2. Objectives
Leveraging the properties of the flavonoid compounds present in R. damascena, this study seeks to delve into the neuroprotective and neurodegenerative impacts of essential oil (ESO) on NPCs in vitro.
3. Methods
3.1. Isolation, Culture, and Differentiation of Neural Progenitor Cells
This experimental research was carried out with the authorization of the ethics review board at the Islamic Azad University, Science and Research Branch (IR.IAU.SRB.REC.1401.111). The study adhered to the ethical guidelines for working with laboratory animals as specified by the Ministry of Health. Experiments were performed on baby Wistar rats (1 - 2 weeks old) to isolate NPCs.
3.1.1. Tissue Dissection and Preparation
Following deep anesthesia, the hippocampi were carefully excised from both hemispheres of the brain. The tissue was mechanically minced to approximately twice its original volume. Collagenase enzymes were applied to the tissue for enzymatic digestion, incubating at 37°C for 30 minutes. Fetal bovine serum (FBS) was added to neutralize the enzymes.
3.1.2. Cell Isolation and Processing
The resulting suspension was filtered through a 70-micron nylon mesh to remove debris. The cell suspension was then subjected to centrifugation at 3000 rpm for 10 minutes. The pellet obtained was resuspended and cultured in DMEM/F12 medium, supplemented with Epidermal Growth Factor (EGF), Human recombinant basic Fibroblast Growth Factor (bFGF) (Millipore), B-27 supplement (GIBCO), Growth factors (Sigma), 1% Penicillin-Streptomycin (Sigma), and 3% FBS serum.
3.1.3. Cell Culture and Maintenance
The cell suspension was incubated at 37°C with 5% CO2. The culture medium was replaced every 24 hours. The NPCs were passaged when they reached 70 - 80% confluence, using trypsin to detach the adherent cells. Cells were passaged at a 1:2 ratio, and the third passage was used for further experiments. The NPCs were differentiated into neural cells (NCs) by withdrawing growth factors (EGF, bFGF) from the culture medium, following standard protocols (18).
3.2. Evaluation of Cell Viability with the MTT Method
Within this research, NPCs and NCs were exposed to ETH solutions spanning concentrations from 0.1% to 10% (v/v) to explore cellular activities and behaviors. While all cells were eradicated after a one-minute exposure to 15 - 40% ETH, concentrations as modest as 0.1 - 10% led to a full response within 24 - 72 hours of exposure. Hence, the cytotoxic effects were influenced by both the exposure duration and the concentration of ETH. The cell viability was assessed using the MTT cell proliferation assay kit provided by Sigma Company, following previously established research protocols. The experimental procedure was as follows.
1. Cell culture: Cells were cultured in a 96-well plate at a density of 10,000 cells per well, with a total volume of 200 microliters per well. The cells were maintained in an incubator with 5% CO2 and 95% humidity at a temperature of 37°C for 24, 48, and 72 hours.
2. Exposure to ETH and ESO: The cells were exposed to varying concentrations of ETH [0.1, 0.5, 1, 2, 4, 6, 8, and 10% (v/v)] and ESO (25, 50, 100, 200, 300, 400, and 500 μM) obtained from Nico Chemical Company in Iran.
3. MTT reagent addition: After incubating the cells with the different concentrations of ETH and ESO for 24, 48, and 72 hours, 20 microliters of the MTT reagent was added to each well.
4. Mixing: The mixture was gently mixed for one minute on an orbital shaker.
5. Incubation: The cells were then incubated for 3 hours, resulting in the formation of dark-colored crystals at the bottom of the wells.
6. Medium removal: The culture medium was carefully removed from each well without disturbing the cell monolayer.
7. Crystal solvent addition: A crystal solvent solution (50 - 70 microliters) was then added to each well, producing a violet-colored solution.
8. Absorbance measurement: The absorbance of each sample was measured at a wavelength of 570 nanometers using a microplate reader.
Following the initial MTT assay, statistical analysis was conducted on the data to ascertain significance across various concentrations and time points. Consequently, doses were determined through a preliminary dose-response experiment, selecting 2% ETH and 200 µM ESO as effective concentrations.
3.3. Treatment Groups
Following the determination of effective doses, their influence on NPCs and NCs was evaluated. We divided the cells into three distinct groups in each stage (NPC or NC stage).
1. Control group: No treatment (NPC/NC).
2. The ETH exposure group (NPC/NC + ETH): Cells exposed to 2% ETH.
3. Combined treatment group (NPC/NC + ETH + ESO): Cells treated with both 2% ETH and 200 μM ESO.
All samples were obtained from three independent biological replicates.
3.4. Evaluation of TAU Protein Expression by Immunocytochemistry Technique
In this procedure, NCs underwent three washes with a PBS solution initially. Subsequently, they were immobilized with 4% paraformaldehyde for a duration of 1 hour at room temperature, and then underwent an overnight exposure to a 30% sucrose solution at 4°C. Following this, the cells were permeabilized with 0.2% Triton X-100 for 20 minutes and subsequently subjected to blocking with 10% BSA in PBS for 30 minutes at room temperature. The cells were subsequently treated with a Mouse TAU monoclonal antibody T46 (Thermo Scientific) at a 1:500 dilution in blocking buffer overnight at 4°C. Following this, they were exposed to an Alexa Fluor 488-labeled donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at a 1:1000 dilution and DAPI (blue; 1:2500) in blocking solution at room temperature for 1 hour. Finally, examination was carried out using a fluorescence microscope (19).
3.5. Analysis of Gene Expression, Real-time PCR Method
The real-time PCR method was employed to assess the expression levels of neuroepithelial stem cell protein (Nestin), Sex determining region Y-box 2 (Sox2), and Paired Box 6 (Pax6) genes in the NPCs phase, and III β-tubulin (Tuj1) and microtubule associated protein 2 (Map2) genes in the NCs phase, utilizing the GAPDH gene as an internal control across all experimental groups. Cells were subjected to total RNA extraction utilizing the RiboEX kit (GeneAll Biotechnology, South Korea) according to the manufacturer's guidelines, and subsequently transcribed into cDNA employing a reverse transcription enzyme.
Five hundred ng of cDNA were employed to measure the mRNA levels of Nestin, Sox2, Pax6, Tuj1, and Map2. The primers utilized are detailed in Table 1. PCR reactions were carried out over 40 cycles in a 25-microliter volume with Syber Green dye on the Applied Biosystem cycler device. Subsequently, a relative mRNA analysis was conducted using the ΔΔCT method (20).
| Primer Name | Prime Sequence | Primer Length |
|---|---|---|
| Nestin | Forward: AGCACTCCCATCCCACCTAT; reverse: GGGTTGTGGCTAAGGAGGTC | 220 |
| Sox2 | Forward: CCCACCTACAGCATGTCCTAC; reverse: CTGGAGTGGGAGGAAGAGGTA | 125 |
| Pax6 | Forward: CCGAATTCTGCAGGTGTCCA; reverse: TGGCTTACTGCCTCCGATTG | 100 |
| Tuj1 | Forward: GCAACTATGTGGGGGACTCG; reverse: CAGCACCACTCTGACCGAAGA | 192 |
| Map2 | Forward: ACTGGAAGAAGCCTCGAAGATG; reverse: TACTTGTGTCCGGCTGAGGA | 158 |
| GAPDH | Forward: GAAGCTGGTCATCAACGGGA; reverse: GAAGGGGCGGAGATGATGAC | 180 |
Abbreviations: Nestin, neuroepithelial stem cell protein; Sox2, Sex determining region Y-box 2; Pax6, Paired Box 6; Tuj1, III β-tubulin; Map2, microtubule associated protein 2.
3.6. Statistical Analysis
Statistical analysis was conducted utilizing SPSS 22 software, and all data were expressed as mean ± standard error of mean (SEM). Group comparisons were made through Tukey's post-hoc test, with significance set at P < 0.05.
4. Results
4.1. Ethanol Toxicity on Neural Progenitor Cells and Neural Cells
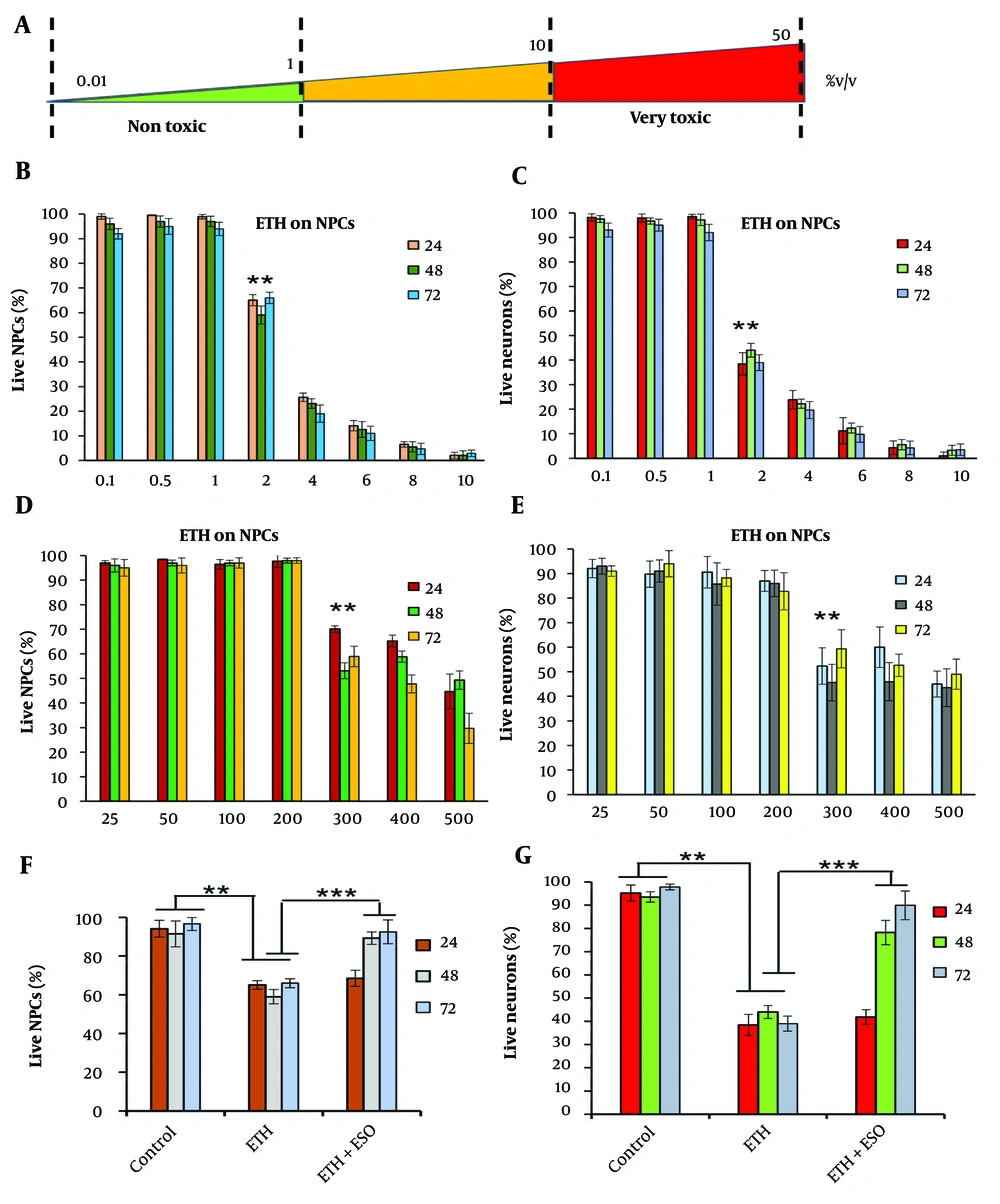
NSCs and NCs were exposed to ETH concentrations ranging from 0% to 50% (v/v) for exposure times of 1 minute to 72 hours. The concentrations tested included 0%, 5%, 10%, 15%, 20%, 25%, 30%, 40%, and 50%. As shown in Figure 1A, all cells were destroyed after a one-minute exposure to ETH concentrations between 15% and 50%. However, ETH concentrations as low as 0.1% - 10% led to significant cellular responses after 24 - 72 hours of exposure. The cytotoxic effects of ETH were found to be dependent on both the exposure duration and the ETH concentration.
Comparison of the effects of exposure to different concentrations of ethanol (ETH) and Rosa damascena essential oil (ESO) on cell viability in neural progenitor cells (NPCs) and neural cells (NCs): A, the figure shows the schematic diagram of cytotoxic impact of different ETH concentrations; B, illustrates the effects of various ETH concentrations on cell viability in NPCs; and C, NCs after 24, 48 and 72 hours of exposure; D, depicts the effects of different concentrations of ESO on cell viability in NPCs after 24, 48, and 72 hours of exposure; (E) demonstrates the effects of various concentrations of ESO on cell viability in NCs after 24, 48, and 72 hours of exposure; F, shows the effects of simultaneous exposure to ESO + ETH on cell viability in NPCs after 24, 48, and 72 hours of exposure; G, illustrates the effects of simultaneous exposure to ESO + ETH on cell viability in NCs after 24, 48, and 72 hours of exposure (asterisks show significant differences among control and treated samples in each column; **P < 0.01 and ***P < 0.001).
The toxicity of ETH on NPCs (Figure 1B) and NCs (Figure 1C) was assessed following various exposure times (24, 48, and 72 hours) to ETH concentrations of 0.1%, 0.5%, 1%, 2%, 4%, 6%, 8%, and 10% (v/v). A decrease in cell viability was observed in all ETH-treated groups, with the most significant reductions noted in groups exposed to higher ETH concentrations (P < 0.01). The lowest cell viability was observed in the 10% ETH group compared to the control group. Based on these results, the 2% ETH concentration, which induced a significant mortality rate (~50%), was selected for subsequent experiments.
The toxicity of ESO was investigated in NPCs (Figure 1D) and NCs (Figure 1E) exposed to varying concentrations (25, 50, 100, 200, 300, 400, and 500 μM) for 24, 48, and 72 hours. At all three exposure times, cell viability was higher in groups exposed to lower concentrations of the ESO compared to the control group. As the concentration of ESO increased, cell viability decreased significantly (P < 0.01). The effective dose of 200 μM was determined to be non-toxic to cells and was therefore selected for further experiments.
The effect of a selective dose of ESO (200 μM) and ETH (2%) on NPCs and NCs was evaluated at 24, 48, and 72 hours (Figure 1F and G). A significant increase in cell viability was observed in both NPCs (Figure 1F) and NCs (Figure 1G) after 48 and 72 hours when compared to the ETH group (P < 0.001). No significant differences in cell viability were noted at the 24-hour time point (Figure 1F and G).
4.2. Results of Gene Expression in Neural Progenitor Cells and Neural Cells
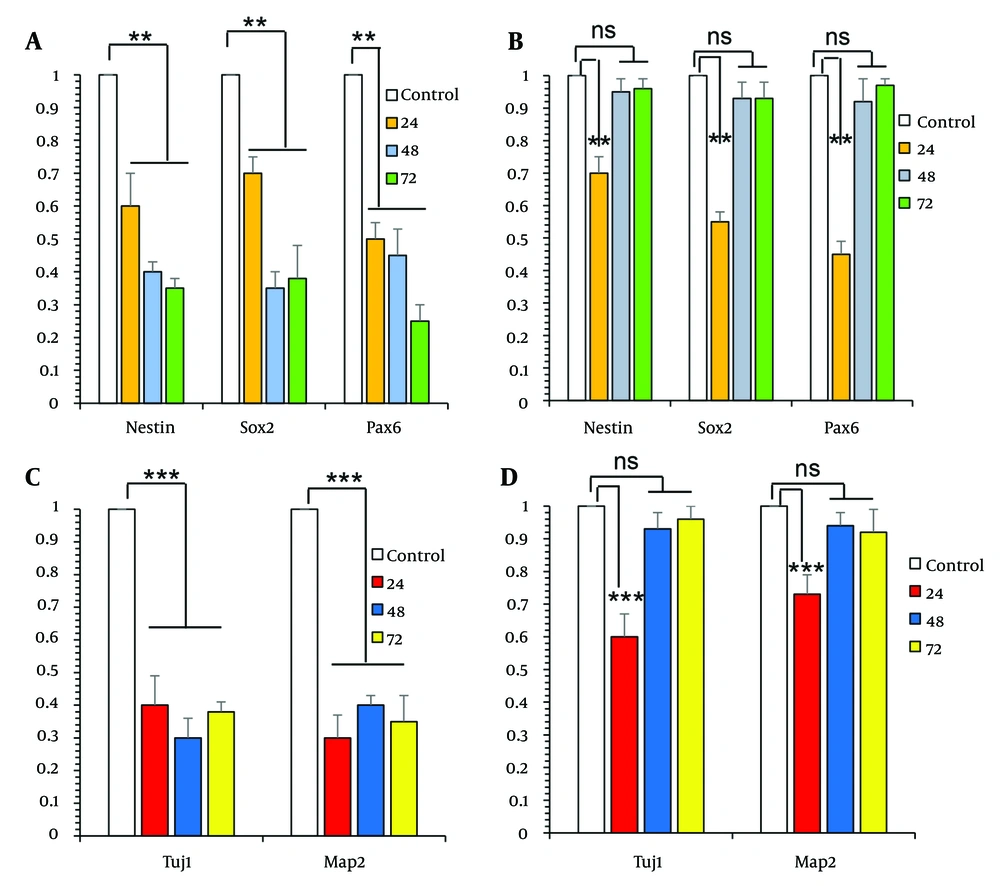
The analysis of the gene expression levels of Nestin, Sox2, and Pax6 showed a significant reduction in the groups treated with ETH for 24, 48, and 72 hours compared to the control group (Figure 2A P < 0.01). This decline was not observed in the groups that received both ETH and ESO for 48 and 72 hours (Figure 2B). In contrast, the expression levels of Nestin, Sox2, and Pax6 significantly decreased in the groups treated with both ESO and ETH for 24 hours when compared to the control group (Figure 2B P < 0.01).
Comparison of the effects of exposure to ethanol (ETH) and essential oil (ESO) on gene expression in neural progenitor cells (NPCs) and neural cells (NCs): A, the figure showed a significant reduction of neuroepithelial stem cell protein (Nestin), Sex determining region Y-box 2 (Sox2), and Paired Box 6 (Pax6) gene expression in the groups treated with ETH for 24, 48, and 72 hours; B, this decline was not observed in the groups that received both ETH and ESO for 48 and 72 hours; C, showed a significant decrease in the expression levels of III β-tubulin (Tuj1) and microtubule associated protein 2 (Map2) genes in the groups treated with ETH; D, in contrast, in the groups treated simultaneously with ETH and ESO for 48 and 72 hours, this decrease in gene expression was not observed (asterisks show significant differences among control and treated samples in each column; **P < 0.01 and ***P < 0.001; Abbreviation: NS, not significant).
The examination of Tuj1 and Map2 gene expression in NCs showed a significant decrease in the expression levels of these genes in the groups treated with ETH (Figure 2C P < 0.001) and those treated with ETH along with ESO for 24 hours (Figure 2D P < 0.001), when compared to the control group. In contrast, in the groups treated simultaneously with ETH and ESO for 48 and 72 hours, this decrease in gene expression was not observed (Figure 2D).
4.3. Results of TAU Protein Expression in Neural Progenitor Cells and Neural Cells
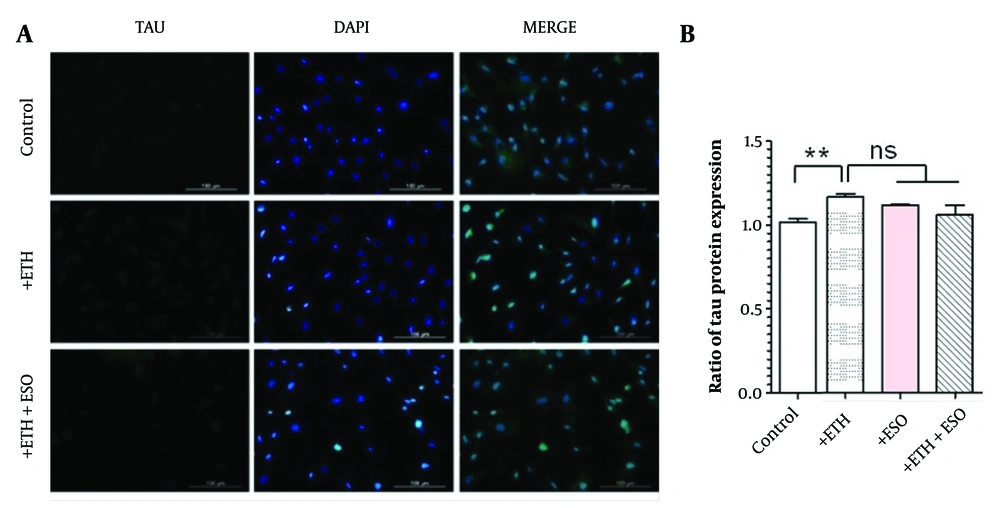
The assessment of the non-physiologic form of TAU protein expression through immunocytochemistry (ICC) indicated that ETH treatment increased TAU protein levels in comparison to the control group (Figure 3 P < 0.01). Furthermore, the groups receiving a combination of ETH and ESO exhibited lower TAU protein expression levels than the ETH-only group. However, these alterations were not statistically significant (Figure 3B).
Assessment of TAU protein expression (non-physiologic form) in neural progenitor cells (NPCs): A, the figure depicts the TAU protein expression levels in NPCs, observed using the immunocytochemistry (ICC) technique at 10X magnification; B, illustrates the TAU protein expression results across various groups of NPCs (**P < 0.01; Abbreviation: NS, not significant).
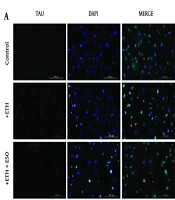
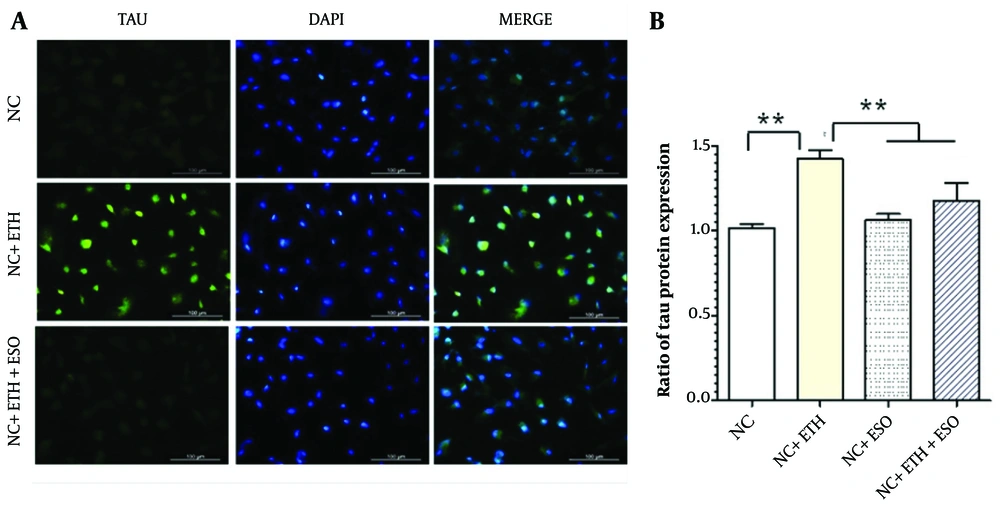
The results of examining TAU protein expression in NCs using ICC indicate that ETH treatment increased the level of TAU protein expression compared to the control group (Figure 4A P < 0.01). Additionally, the level of TAU protein expression significantly decreased in the group treated with both ETH and ESO (Figure 4B P < 0.01).
Assessment of TAU protein expression in neural cells (NCs): A, the figure illustrates the level of TAU protein expression in NCs, observed using the immunocytochemistry (ICC) technique at 10X magnification; B, presents the results of TAU protein expression in various groups of NCs (asterisks show significant differences among control and treated samples in each column; **P < 0.01).
5. Discussion
Oxidative stress, a key contributor to a range of diseases, including neurodegenerative disorders, is caused by the oxidation of biological molecules. This process produces free radicals, which can cause cellular damage. When the levels of natural antioxidants decline or reactive oxygen species (ROS) increase, cell degeneration takes place, especially in the brain, leading to complications for affected individuals (21). In recent years, plants have garnered significant interest from researchers because of their pharmacological properties, low toxicity, minimal side effects, widespread acceptability, and cost-effectiveness. Compounds derived from plants, particularly antioxidants, show potential for treating a variety of diseases (22).
This study aimed to assess the neuroprotective effects of ESO against ETH-induced neurodegeneration in NPCs derived from baby Wistar rats. This study showed that 200 µM ESO improved cell viability in ETH-treated NPCs and NCs compared to ETH alone, though higher ESO concentrations alone (e.g., 400 - 500 µM) reduced viability, clarifying that the protective effect is specific to the 200 µM dose. It has been firmly established that ETH has both immediate and long-term effects on the human body, significantly affecting the brain by disrupting its normal functions (23). Ethanol affects the body by disrupting cell death mechanisms, impairing the immune system, and altering the settings of neuronal transmission networks associated with GABA, glutamate, and serotonergic systems (24). Furthermore, ETH modifies the permeability of the blood-brain barrier by impacting the gap junctions between the epithelial cells in the brain’s capillaries (25). Recent research has identified a familial connection between alcohol consumption and carcinogenesis, highlighting ETH’s influence on growth factors, especially vascular endothelial growth factor (VEGF). The VEGF is crucial for angiogenesis, the formation of new blood vessels, and has been found to be produced in greater quantities in response to alcohol exposure, particularly in tumors (26). Alcohol consumption can have toxic effects on our bodies, leading to significant cellular changes. Shelford’s law, established in 1911, illustrates this fragile balance by indicating that the intensity of an ecological factor can restrict the growth of living organisms. Many substances are harmless within specific thresholds but can become detrimental if their concentrations surpass these limits. Even low doses of alcohol can produce side effects. The introduction of ETH through food can lead to a range of effects, and similarly, other substances entering the body at elevated levels may cause complications (27).
Researchers, motivated by a commendable goal, aim to discover drugs that activate brain regions responsible for spontaneous repair, cell replacement, and healing (28). The use of ESO has been linked to an increase in neuronal numbers, indicating a potential for stimulating neurogenesis (29). Some natural plant extracts contain compounds similar to neurotrophic factors found in the body, which support nerve cell survival and may aid neurogenesis (30).
In the CNS, nerve growth factor (NGF) is particularly important, especially within the cholinergic system. The NGF promotes the survival of damaged cholinergic neurons in laboratory settings and helps regulate their phenotype. Furthermore, NGF shows neurotransmitter-like effects, influencing cholinergic neurotransmission and neuronal excitability (31). Considering the vulnerability of cholinergic neurons in Alzheimer’s disease, drugs or compounds that enhance NGF production in the CNS may be advantageous for treatment. It is important to acknowledge that previous studies have also emphasized NGF’s influence on neuronal branching (32). The current findings provide strong support for the positive effects of ESO on neuronal density. Thus, it can be concluded that its beneficial impact on neuronal density likely stems from its recognized role in promoting NGF production (33).
Moreover, diets high in flavonoids have been shown to have beneficial effects on brain health and can help reduce the risk of degenerative diseases such as Alzheimer’s and Parkinson’s disease (34). Research involving adult female Syrian mice has indicated that treatment with blueberries (35), red grape extract (36), and green tea leads to improvements in memory (37). Flavonoids seem to protect neurons from damage inflicted by neurotoxins and inflammatory agents in the nervous system (38). Additionally, they show potential for enhancing memory, learning, and cognitive functions (39). These compounds initially promote angiogenesis and neurogenesis, especially in the hippocampus, while also inhibiting apoptosis caused by neurotoxic agents (40).
Flavonoids influence protein kinases, lipid kinases (PI3/Akt), and the mitogen activated protein (MAP) kinase pathway, which enhances neuronal connections in the dentate gyrus and CA3 regions of the hippocampus, ultimately leading to improved long-term potentiation (LTP) (41). The R. damascena plant is known to contain over 50 phenolic compounds, which fall into various categories, including phenolic acids and their derivatives (42). Research suggests that flavonoids within these biological compounds can regulate specific enzymes and deactivate reactive molecules such as nitrite peroxide and hydroxide radicals. The R. damascena plant also contains other beneficial compounds, including vitamin C, carboxylic acid, tannins, and notably, flavonoids and polyphenols with strong antioxidant properties, such as kaempferol and quercetin. These compounds are effective at neutralizing free radicals and controlling the activity of enzymes and reactive species like nitrite peroxide (43). While many previous studies have demonstrated the anti-inflammatory and antioxidant benefits of ESO, the current research highlights a novel aspect by revealing its positive effects on the proliferation and survival of NPCs under oxidative stress conditions in a laboratory setting. This further supports the potential of R. damascena in the nerve repair process (44).
Additionally, the findings indicate that treating NPCs with a 300 μM concentration significantly promotes their growth and proliferation. Thus, it seems that the memory-enhancing and neurogenic effects of ESO are likely due to the polyphenol and flavonoid compounds present in the extract. Upon analysis, it was found that the expression levels of the Nestin, Sox2, and Pax6 genes were significantly lower in the ETH exposure groups compared to the control group (P < 0.01). The treatment with a combination of ETH and ESO resulted in a notable increase in the expression of these genes compared to treatment with ETH alone (P < 0.01). Additionally, the findings of the current study indicated that the application of ESO significantly boosted the expression of the Tuj1 and Map2 genes in comparison to the ETH treatment (P < 0.01).
The reduction in TAU protein expression (non-physiologic form of TAU) in NCs treated with ETH + ESO suggests a potential role in mitigating neurodegeneration, as elevated TAU is linked to disorders like Alzheimer’s. In this study, limitations include the lack of long-term data beyond 72 hours, absence of sex-specific analysis (cells were pooled from both sexes), and no assessment of rapid gene expression changes (e.g., at 12 hours). Future studies should explore these, including in vivo models and molecular mechanisms like PI3K/Akt or MAPK pathways. While ETH is known to induce oxidative stress, we did not measure ROS or oxidative markers, so claims about ESO’s antioxidant effects are speculative and require further validation.
5.1. Conclusions
The ESO mitigates the adverse effects of ETH on the viability of NPCs and NCs. Furthermore, it may contribute to preventing ETH-induced cell death in NPCs, suggesting possible clinical applications for neurodegenerative diseases and conditions associated with alcohol use.