1. Background
Epithelial cell adhesion molecule (EpCAM) is one of the critical targets in immunotherapy for various cancers. The expression of this protein, which is one of the earliest identified biological markers of cancer, is increased in many human cancers, such as colon, ovarian, breast, lung, and epithelial malignancies — especially adenocarcinoma (1, 2). Despite the significant contribution that monoclonal antibodies (mAbs) have made to the diagnosis and treatment of various diseases, particularly cancers, the adverse effects associated with these therapeutic agents have prompted initiatives aimed at developing more efficient compounds with a reduced incidence of side effects.
In this context, engineered antibodies, especially single-chain variable fragments (scFvs), can be considered suitable alternatives. Compared to full-length antibodies, the absence of the Fc region and the small size of this molecule result in distinct pharmacokinetic profiles, including faster clearance, better tissue penetration, and reduced stability under physiological conditions. Industrially, scFvs are preferred for bispecific antibody engineering due to their ease of genetic manipulation. Furthermore, based on the reduced need for post-translational modifications, scFvs can often be expressed in prokaryotic systems with higher efficiency than antibody fragments like Fab, which frequently require proper disulfide bond formation for correct folding (3, 4). Due to their simpler structure, scFvs are more amenable to different expression strategies, including cell-free protein synthesis systems (5). However, compared to Fab fragments, scFvs tend to aggregate more, requiring specialized production strategies such as optimal expression conditions (6).
The unique characteristics of Escherichia coli, including its simple and well-known genetics, high growth rates, and the low cost of its culture medium, have made this microorganism one of the most valuable factories for scFv production (7, 8). Optimization of the culture medium is particularly important for the large-scale production of therapeutic proteins, where expression levels and appropriate folding are often limiting factors. The cell growth rate, final cell density, and protein expression efficiency in microbial cell factories have been found to be significantly affected by the composition of the growth medium. Industrially, even small improvements in volumetric efficiency can lead to significant cost savings and increased production capacity (9). Accordingly, a systematic evaluation of various culture media seems essential to identify conditions that maximize scFv yield and result in the production of biologically active protein. The classification of culture media includes three distinct categories: Defined, complex, and semi-defined. High cell densities are mainly achieved by defined media containing precisely specified nutrient concentrations that can be adjusted during the culture process. However, in some cases, complex or semi-defined culture media are required to improve protein production (10, 11).
Luria bertani (LB) culture medium, which is rich in nutrients containing peptides, water-soluble vitamins, amino acids, and carbohydrates, is the most common culture medium for various bacteria (12). To achieve a higher yield of recombinant proteins, changing the type of culture medium can be considered as one of the strategies (13). For instance, in research conducted by Choi and Geletu, TB and LB media were utilized to assess the impact of culture medium composition on the amount of recombinant flounder growth hormone (fGH), and the findings showed that more protein was expressed in the richer culture medium (14). Furthermore, to enhance the yield, stability, and scalability of recombinant scFv production, recent studies have focused on optimizing growth conditions. For example, antiEpEX-scFv expression in E. coli BW25113 (DE3) was optimized using response surface methodology with central composite design (RSM-CCD). The optimal conditions [induction at OD600 0.8 with 0.8 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for 24 hours at 37°C] were identified by modeling interactions between IPTG concentration, post-induction duration, induction cell density, and temperature, which yielded the production of 197.33 μg/mL of protein (15).
2. Objectives
In this study, the gene encoding the scFv of an antibody derived from the mAb 4D5MOC-B, which binds to the extracellular domain of EpCAM (EpEX), was expressed in a strain of E. coli containing deletions in the pka (acetyl-CoA synthetase) and arcA (dual transcriptional regulator for anoxic redox control) genes — both of which are involved in acetate metabolism (16). It has been demonstrated that inactivation of acetyl-CoA synthetase through deletion of the pka gene, along with deletion of arcA, a known repressor of the tricarboxylic acid (TCA) cycle, leads to coordinated activation of the phosphotransacetylase-acetyl-CoA synthetase (PTA-ACS) pathway and the TCA cycle, thereby reducing acetate excretion. Reducing acetate overflow can enhance the potential for higher recombinant protein yields in bioprocesses (16). Using a comparative approach, the main objective of this study was to evaluate how variations in the nutrient composition of four commonly used culture media — minimal medium (M9), TB, LB, and yeast tryptone 2x (TY2x) — affect cell density, scFv yield, and protein solubility.
3. Methods
3.1. Bacterial Strains, Growth Media, and Reagents
The previously constructed pET22b(+) harboring the gene encoding Scfv against EpEX was transformed into the E. coli strain DH5α (Dr. Karamati at Pasteur Institute, Tehran) for propagation (17). The E. coli strain K-12 BW25113 Δpka ΔarcA (University of Tartu, Estonia) was also used as an expression host (16). By reducing the accumulation of acetate — a byproduct that inhibits growth and recombinant protein expression in high-density cultures — deletions in the pka and arcA genes make E. coli K-12 BW25113 Δpka ΔarcA particularly suitable for industrial-scale production (16). The expression of the protein was investigated in four different culture media.
1. Luria bertani (yeast extract 5 g/L, tryptone 10 g/L, and NaCl 10 g/L);
2. Yeast Tryptone 2× (yeast extract 10 g/L, tryptone 16 g/L, and NaCl 5 g/L);
3. Terrific broth (TB) (tryptone 12 g/L, glycerol 4 mL/L, yeast extract 24 g/L, K2HPO4 12.54 g/L, and KH2PO4 2.31 g/L);
4. The M9 [Na2HPO4 0.6% (w/v), glucose 0.2% (w/v), MgSO4 0.024% (w/v), NH4Cl2 0.1% (w/v), NaCl 0.05% (w/v), and KH2PO4 0.3% (w/v)].
All chemical compounds used in this study were purchased from standard commercial sources.
3.2. Growth Analysis
To monitor bacterial growth, we utilized a UV-visible spectrophotometer (SHIMADZU, UV-2550) to measure optical density (OD) at 600 nm. The maximal cell density was determined by plotting OD600 against time.
3.3. The AntiEpEX-Single-Chain Variable Fragment Expression
To express the recombinant protein, an overnight culture derived from a single colony of the expression host harboring the recombinant plasmid was grown in ampicillin-containing broth (100 µg/mL) at 37°C for 18 hours. Then, 10% (v/v) of the overnight culture was inoculated into 50 milliliters (mL) of fresh medium. After the culture reached an OD600 of 0.6 - 0.8 at 37°C, 1 mM IPTG was added to the medium. The use of 1 mM IPTG ensures precise control of recombinant protein expression under the T7 promoter system, providing strong induction while minimizing potential metabolic stress on E. coli cells. After 24 hours, cells were harvested by centrifugation (10,000 × g, 10 minutes at 4°C) and stored at -20°C. The resulting cell pellets were resuspended in 5 mL of lysis buffer (50 mM Tris, 50% glycerol, and 50 mM NaCl), designed to enhance protein solubility and stability during extraction. In this buffer: Tris acts as a pH stabilizer, glycerol functions as a chemical chaperone to prevent aggregation and denaturation, and NaCl stabilizes the protein by mimicking physiological ionic strength and reducing electrostatic interactions that can lead to aggregation (18, 19). Cells were incubated on ice for 40 minutes and then lysed by sonication using three cycles for a total of 18 minutes (7 seconds ON, 8 seconds OFF) at 400 W. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was employed to analyze the expression of the recombinant protein.
3.4. Solubility Analysis of Recombinant Single-Chain Variable Fragment
To assess the solubility of the expressed protein, the cell pellets were subjected to centrifugation at 8,000 × g for 15 minutes, followed by resuspension in 5 mL of lysis buffer. The resuspended cells were vortexed, and sonication was performed for 18 minutes at 400 W (7 seconds ON, 8 seconds OFF) to ensure complete cell lysis. After sonication, the lysate was centrifuged at 10,000 × g for 25 minutes at 4°C. The resulting insoluble debris was pelleted, and the supernatant — representing the soluble protein fraction — was collected. Both soluble and insoluble fractions were analyzed by SDS-PAGE. Protein bands corresponding to the expressed scFv were visualized following staining with Coomassie brilliant blue R-250.
3.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis, Western Blotting, and Protein Quantification
To quantitatively evaluate the total protein concentration, the bicinchoninic acid (BCA) assay (Takara, Japan) was used, with bovine serum albumin (BSA) employed as the standard. A standard curve was generated using known concentrations of BSA standard samples, and the total protein concentration in each sample was determined based on this standard curve. Protein samples were separated by SDS-PAGE using a 5% (w/v) stacking gel and a 12% (w/v) running gel, operated at 80 V and 150 V, respectively. The density of polyacrylamide gel bands was analyzed using TotalLab TL120 software (Nonlinear Inc., Durham, NC, USA). Based on the estimated band intensity, TL120 calculated the quantity of recombinant protein as a percentage of the total protein. Using the total protein concentrations obtained from the BCA assay and the percentage of recombinant protein calculated from TL120 analysis, the concentration of recombinant protein was determined (17).
To confirm protein expression, the separated bands from SDS-PAGE were electrophoretically transferred to a polyvinylidene fluoride (PVDF) membrane. Blocking was performed using 5% skim milk diluted in phosphate-buffered saline (PBS) containing 0.1% Tween® 20. The membrane was then incubated with a 1:10,000 dilution of anti-his-tag polyclonal antibody (Sigma, UK) for 1 hour, followed by washing and incubation with a secondary antibody (HRP-conjugated anti-mouse immunoglobulin, Sigma, UK). Detection was performed using 3, 3'-diaminobenzidine (DAB) substrate staining. A pre-stained protein ladder (SinaClon, I.R. Iran) was used as a molecular mass marker.
3.6. Statistical Analysis
Data are reported as mean ± standard deviation (SD) from independent experiments, analyzed using GraphPad Prism 6.0 for Windows (GraphPad Prism, USA). Two biological replicates and four technical replicates were performed in this study. One-way analysis of variance (ANOVA), followed by the Tukey post-test, was used for statistical comparisons. P-values less than 0.05 were considered statistically significant.
4. Results
4.1. Single-Chain Variable Fragment Antibody Expression and Detection
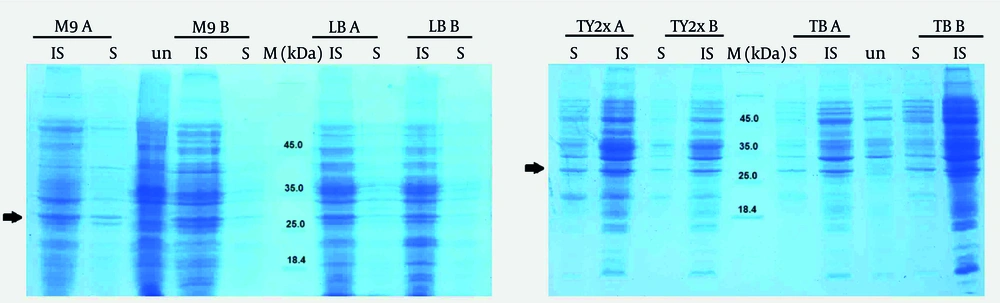
Following the transformation of competent cells with the pET22b(+)-antiEpEX-scFv expression vector using the conventional heat shock method, expression of the recombinant protein was induced with 1 mM IPTG at 37°C for 24 hours. After cell lysis, recombinant protein expression was evaluated by SDS-PAGE analysis. A protein band of approximately 30 kDa was observed in the induced samples, in contrast to the non-induced samples (negative control) (Figure 1A). The presence of a histidine tag on the recombinant protein enabled its specific detection by western blotting using an anti-his antibody (Figure 1B).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting analyses of the antiEpEX-single-chain variable fragment (scFv) recombinant protein. After separation on a 12% SDS-PAGE gel, coomassie brilliant blue R250 staining was visualized protein bands. Total protein derived from the Escherichia coli strain K-12 BW25113 Δpka ΔarcA harboring pET22b-antiEpEX-scFv vector before induction (lane un) and after induction with isopropyl-β-D-thiogalactopyranoside (IPTG), (1 mM) for 24 h at 37°C in different media. The experiments were performed in duplicates for each medium and the replicates were indicated as A and B. A, lane M, unstained protein marker (14.4-116 kDa). Western blotting analysis of the recombinant scFv antibody produced in luria bertani (LB) medium. B, Bacterial lysate of the E. coli strain K-12 BW25113 Δpka ΔarcA before (lane un) and after (lane 1, 2, and 3 which are three technical replicates) induction was subjected to the anti His monoclonal antibody (mAb) [M: Pre-stained protein molecular weight standards (10 - 250 kDa)]. The expressed antibody with the expected size of ~ 30 kDa was indicated by an arrow.
4.2. Effect of Different Media on Cell Density
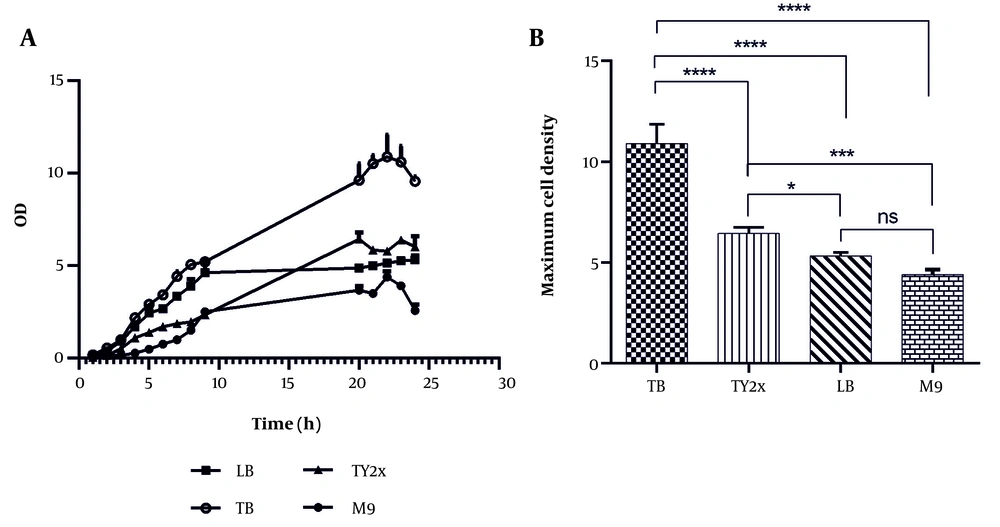
In this study, various culture media (LB, TB, M9, and TY2x) were evaluated to determine which medium promotes the highest cell growth for E. coli K-12 BW25113. Significant differences in lag phase duration across the media suggest that nutrient availability strongly influences the adaptability of E. coli (Figure 2A). In TB, LB, and TY2x media, the lag phase lasted approximately 2 hours, indicating that these nutrient-rich media provide sufficient resources to allow E. coli to adapt quickly and initiate growth. The shorter physiological adaptation time is attributed to the presence of high concentrations of nutrients and amino acids, which enhance cellular metabolism.
Conversely, the M9 medium, which lacks many of the nutrients found in TB, LB, and TY2x, resulted in a prolonged lag phase of about 4 hours. In minimal media such as M9, E. coli must synthesize many cellular components from limited primary sources, leading to an extended adaptation period. This longer lag phase reflects the additional metabolic time required for the synthesis of essential amino acids and nutrients (20, 21). As shown in Figure 2B, the highest cell density (10.89 ± 0.84) was achieved in TB medium during the stationary phase (after 22 hours), followed by TY2x (6.44 ± 0.26; P < 0.0001), LB (5.32 ± 0.17; P < 0.0001), and M9 (4.4 ± 0.22; P < 0.0001). However, extended cultivation times may further improve yields by prolonging the stationary phase. For instance, fed-batch strategies can be employed to continuously supply nutrients, prevent nutrient depletion, and maintain optimal growth conditions over extended periods (22).
The nutrient-rich composition of TB supports enhanced bacterial growth. Glycerol, used as a carbon source in TB, helps minimize acetate accumulation and results in higher cell density compared to other media. Furthermore, the metabolic burden of scFv production differs across the tested media. Rich media such as TB and TY2x provide abundant nutrients that support high metabolic activity without imposing significant stress on the cells. In contrast, the limited nutrient availability in minimal media like M9 induces greater metabolic stress, leading to lower growth rates (23, 24).
Effect of different media on A, growth profile and B, cell density of Escherichia coli K-12 BW25113. Data were presented as mean ± SD. Statistical analysis was conducted using one-way analysis of variance (ANOVA) and Tukey’s post-test (* P < 0.05, *** P < 0.001, and **** P < 0.0001). Abbreviation; ns, not significant.
4.3. Effect of Various Media on Antibody Expression and Solubility
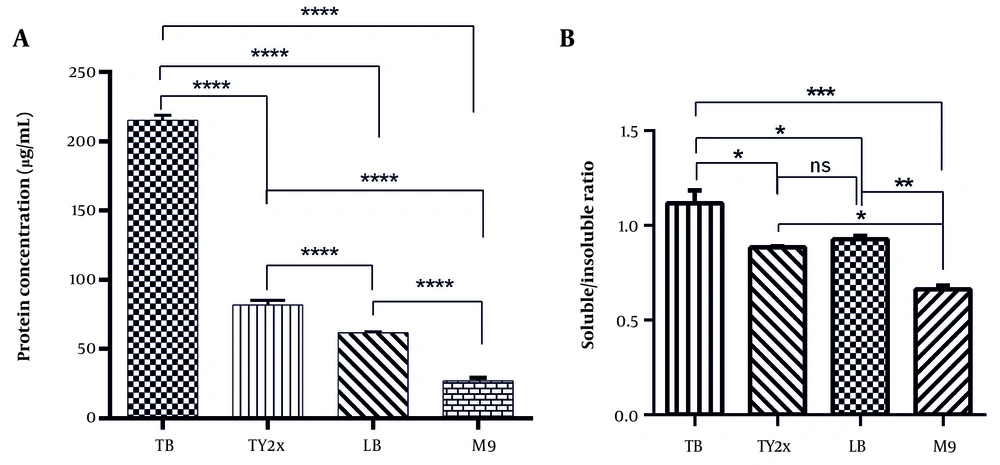
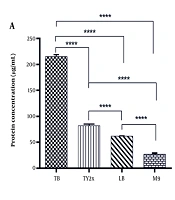
Since culture media influence both the growth profile and performance of microbial processes, they can also regulate the synthesis of bioactive compounds, including recombinant proteins. Therefore, four distinct culture media were used to assess their impact on the expression levels of mAbs (Figure 1A). Densitometry analysis of SDS-PAGE bands, combined with BCA assay results, revealed that the expression levels of antiEpEX-scFv in TB, TY2x, and LB media were 215.19 ± 3.27 µg/mL, 81.89 ± 2.57 µg/mL, and 61.98 ± 0.637 µg/mL, respectively (Figure 3A).
Effect of various media on A, antibody production and B, protein solubility. The cells were induced with isopropyl-β-D-thiogalactopyranoside (IPTG) (1 mM) for 24 hours at 37°C in different media. Data were presented as mean ± SD. Statistical analysis was conducted using one-way analysis of variance (ANOVA) and Tukey’s post-test (* P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001). Abbreviation; ns, not significant.
The E. coli cultured in M9 medium produced the lowest expression level (26.83 ± 1.38 µg/mL), possibly due to the lack of yeast extract, tryptone, and other carbon/nitrogen sources in this medium. TB medium had the highest quantity of antiEpEX-scFv, nearly eight times greater than M9. Moreover, the solubility of scFv protein in four culture media, including LB, TB, M9, and TY2x, was compared. According to the results presented in Figure 4, the antiEpEX-scFv was detectable in both the soluble and insoluble fractions in all media. The highest ratio of soluble to insoluble portions of recombinant protein was observed in TY2x, with a ratio of 1.117 ± 0.048, followed by TB and LB with ratios of 0.926 ± 0.013 and 0.883 ± 0.004, respectively. Finally, the lowest protein solubility was observed in M9, with a ratio of 0.662 ± 0.013 (Figure 3B). The higher proportion of soluble protein in TY2x suggests improved protein folding, indicating reduced aggregation into insoluble bodies. This may be due to minimized stress on host cells and a balanced expression rate that allows proteins to more efficiently achieve native conformations during synthesis (25). The impact of media composition on cell growth, protein yield, and protein solubility is summarized in Table 1.
Solubility assessment of recombinant antibody in different media. The antiEpEX-single-chain variable fragment (scFv) was detectable in both soluble (S) and insoluble (IS) fractions in all media when assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The experiments were conducted in duplicates for each medium and the replicates were indicated as A and B. Lane M, unstained protein molecular weight standards (14.4-116 kDa), and lane un, the Escherichia coli strain K-12 BW25113 Δpka ΔarcA containing pET22b-antiEpEX-scFv plasmid before induction. The expressed antibody with the expected size of ~ 30 kDa was indicated by an arrow.
| Parameters | TB | TY2X | LB | M9 |
|---|---|---|---|---|
| Media components | High yeast extract (24 g/L), tryptone (12 g/L), glycerol (4 mL/L), phosphate buffer | Yeast extract (10 g/L), high tryptone (16 g/L), low NaCl (5 g/L) | Yeast extract (5 g/L), tryptone (10 g/L), NaCl (10 g/L) | Defined salts, glucose (0.2% w/v), no amino acids |
| Cell growth OD | 10.89 ± 0.84 | 6.44 ± 0.26 | 5.32 ± 0.17 | 4.4 ± 0.22 |
| Protein yield (µg/mL) | 215.19 ± 3.27 | 81.89 ± 2.57 | 61.98 ± 0.637 | 26.83 ± 1.38 |
| Solubility ratio | 0.926 ± 0.013 | 1.117 ± 0.048 | 0.883 ± 0.004 | 0.662 ± 0.013 |
Media Composition Impact on Cell Growth, Protein Yield, and Protein Solubility
5. Discussion
Optimization of culture media for antibody production, as demonstrated in the current study, holds significant industrial relevance by markedly improving protein yield and solubility — two critical factors for scalable biopharmaceutical manufacturing (26). Strategies such as utilizing TB as a nutrient-rich medium and incorporating glycerol as a solubility-enhancing additive help reduce dependence on costly protein refolding processes, simplify production workflows, and improve batch-to-batch consistency (18).
Moreover, carefully optimized induction parameters — such as temperature and IPTG concentration — help minimize metabolic stress in microbial cell factories, thereby enhancing the efficiency and reliability of expression systems. These advancements not only streamline downstream processing and lower production costs, but also support compliance with stringent quality standards, making them applicable across a broad range of recombinant protein production platforms (27).
In this study, we examined the effects of four different media — TB, LB, TY2x, and M9 — on growth and scFvs production in engineered E. coli K-12 BW25113. According to our findings, compared to TB, LB, and TY2x, which exhibited lag phases of approximately two hours, a prolonged lag phase of four hours was observed in the M9 medium, emphasizing E. coli’s dependence on nutritional richness for rapid adaptation and development (21, 28).
In terms of cell density, the highest OD (10.89 ± 0.84) was achieved in TB medium, followed by TY2x (OD = 6.44 ± 0.26), LB (OD = 5.32 ± 0.17), and M9 (OD = 4.4 ± 0.22). Thus, TB proved to be the most effective medium for promoting bacterial growth, reaching its peak OD during the stationary phase after 22 hours. Additionally, TB supported the highest level of scFvs antibody production (215.19 ± 3.27 µg/mL), representing an approximately 3.5-fold increase compared to LB (61.98 ± 0.637 µg/mL). A similar pattern was observed in a study by Latifah et al., where TB yielded higher levels of recombinant rhEGF (503.48 μg/mL) and cell densities nearly five times greater than those in LB (29). It is well established that media composition directly affects bacterial growth rates and the metabolic pathways involved in protein biosynthesis (11, 30). TB and LB are among the most commonly used media for E. coli cultivation, particularly in recombinant protein production (21). Compared to LB, which contains 0.5% yeast extract and 1% tryptone, TB contains significantly higher concentrations — 2.4% yeast extract and 1.2% tryptone. Tryptone serves as a rich source of amino acids, nitrogen, and peptides, which are essential for both bacterial growth and protein synthesis. Meanwhile, yeast extract provides a wide array of vitamins, growth factors, and minerals that facilitate enzymatic reactions supporting various metabolic pathways, improving cell viability, and accelerating growth. The increased concentration of yeast extract in TB (2.4% vs. 0.5% in LB) enhances nutrient availability, promoting enzymatic activity and reducing cellular stress responses — such as protease production — that can impair recombinant protein yield and stability (31). The abundance of amino acids, carbon sources, vitamins, and peptides in nutrient-rich media boosts bacterial metabolism and ATP production. This energy surplus supports more efficient transcription and translation processes, reducing stress on host cells during protein synthesis (32). Additionally, glycerol in TB functions as a chemical chaperone, reducing protein aggregation during synthesis and promoting proper protein folding. Glycerol also contributes to increased ATP generation by enhancing carbon availability for cellular respiration (18). Because cells can use glycerol not only as an energy source but also as a precursor for biosynthetic pathways, its presence in TB further supports biomass accumulation and elevated metabolic activity.
Since amino acids are the primary carbon source in LB medium, bacterial growth tends to cease once these amino acids are depleted, leading to lower overall recombinant protein production. In contrast, TB includes phosphate buffering agents such as KH2PO4 and K2HPO4, which help maintain a stable pH during fermentation. This buffering capacity prevents pH fluctuations that could otherwise induce cellular stress or death, thereby negatively impacting both cell growth and protein yield. In LB medium, the pH can increase significantly (up to pH 9) due to ammonium excretion resulting from amino acid catabolism. Elevated pH levels can inhibit enzyme activities essential for protein synthesis. Moreover, low phosphate concentrations in LB can limit cell density, as phosphate is an essential nutrient that may become depleted under intensive culture conditions (11, 33).
According to our results, LB — a commonly used bacterial culture medium — led to lower antiEpEX-scFv production compared to TY2x (1.32-fold increase) and TB (3.5-fold increase). Similar findings have been reported in previous studies where alternative complex media outperformed LB in supporting recombinant protein production. For example, nutrient broth was shown to enhance cell growth and lipase production by approximately 1.3-fold compared to LB broth (34). Additionally, Tripathi et al. compared different media compositions — including LB, super broth (SB), TB, M9, TY, 5× LB, and GE (glucose-enriched) — for their effectiveness in achieving high cell density of E. coli and high yield of recombinant dengue epitopes. Among these, LB resulted in the lowest protein content (10.37 mg/L) and cell density (1.12 g/L) (10).
Similarly, Hanapiah et al. investigated the impact of different culture conditions — including media type [LB, TB, super optimal broth (SOB), M9, and TY2×] and nitrogen source (tryptone and yeast extract) — on E. coli BL21 (DE3) growth and xylonic acid production. Their findings indicated that SOB medium yielded significantly higher biomass and xylonic acid production (up to 8.69 g/L) compared to LB (35). In our study, a substantial 8-fold reduction in protein expression was observed in M9 medium compared to TB, reinforcing conclusions from other studies that minimal media often lack critical nutrients required for effective recombinant protein production (12). The markedly reduced scFv yield in M9 underscores the importance of using nutrient-rich media to achieve high levels of recombinant protein expression.
The data indicate that in TY2x medium, most of the expressed scFv protein is soluble and potentially functional, which is critical for efficient downstream processing. Differences in protein solubility are likely influenced by the specific nutrient composition of each medium (11). The presence of particular nutrients in TY2x may promote proper protein folding by supporting the biosynthesis of molecular chaperones. In contrast, the absence of essential amino acids and growth factors in the M9 medium may contribute to the formation of insoluble protein due to incomplete folding or aggregation.
Although TB and LB also demonstrated acceptable levels of protein solubility, these were lower than those observed in TY2x. This suggests that even among nutrient-rich media, specific formulations can significantly impact protein folding and solubility, likely by modulating intracellular stress responses and folding efficiency.
In conclusion, our findings demonstrate that the metabolic rate of E. coli is directly influenced by the composition of TB, which supplies essential substrates and maintains optimal growth conditions. Among the four tested media, complex media such as TB resulted in the highest yields of viable cells and recombinant proteins — showing an approximately 8-fold increase in scFv expression compared to M9. While TB maximized overall yield, the highest soluble-to-insoluble protein ratio (1.117 ± 0.048) was observed in TY2x, suggesting more favorable conditions for proper protein folding in this medium. These results highlight a critical trade-off in industrial scFv production: Terrific broth provides higher yield, whereas TY2x supports greater solubility and potentially higher functional activity. Future process optimization should aim to enhance TB’s productivity through strategies such as continuous culture or fed-batch fermentation, while also improving solubility using genetic engineering tools. These may include co-expression of molecular chaperones and the incorporation of solubility-enhancing fusion tags to increase the proportion of biologically active scFv. However, several limitations should be acknowledged. These include the use of shake flasks, which may not accurately replicate bioreactor conditions; the use of a semi-quantitative method for protein quantification; the limited number of media tested; the absence of induction condition optimization; and the restricted generalizability of the results, given the focus on a single scFv construct and a specific E. coli strain. These constraints underscore the need for further research to fully optimize scFv production and validate the robustness of these findings. Nevertheless, the study suggests broader implications for the production of recombinant proteins.
![Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting analyses of the antiEpEX-single-chain variable fragment (scFv) recombinant protein. After separation on a 12% SDS-PAGE gel, coomassie brilliant blue R250 staining was visualized protein bands. Total protein derived from the <i>Escherichia coli</i> strain K-12 BW25113 Δpka ΔarcA harboring pET22b-antiEpEX-scFv vector before induction (lane un) and after induction with isopropyl-β-D-thiogalactopyranoside (IPTG), (1 mM) for 24 h at 37°C in different media. The experiments were performed in duplicates for each medium and the replicates were indicated as A and B. A, lane M, unstained protein marker (14.4-116 kDa). Western blotting analysis of the recombinant scFv antibody produced in luria bertani (LB) medium. B, Bacterial lysate of the <i>E. coli</i> strain K-12 BW25113 Δpka ΔarcA before (lane un) and after (lane 1, 2, and 3 which are three technical replicates) induction was subjected to the anti His monoclonal antibody (mAb) [M: Pre-stained protein molecular weight standards (10 - 250 kDa)]. The expressed antibody with the expected size of ~ 30 kDa was indicated by an arrow. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting analyses of the antiEpEX-single-chain variable fragment (scFv) recombinant protein. After separation on a 12% SDS-PAGE gel, coomassie brilliant blue R250 staining was visualized protein bands. Total protein derived from the <i>Escherichia coli</i> strain K-12 BW25113 Δpka ΔarcA harboring pET22b-antiEpEX-scFv vector before induction (lane un) and after induction with isopropyl-β-D-thiogalactopyranoside (IPTG), (1 mM) for 24 h at 37°C in different media. The experiments were performed in duplicates for each medium and the replicates were indicated as A and B. A, lane M, unstained protein marker (14.4-116 kDa). Western blotting analysis of the recombinant scFv antibody produced in luria bertani (LB) medium. B, Bacterial lysate of the <i>E. coli</i> strain K-12 BW25113 Δpka ΔarcA before (lane un) and after (lane 1, 2, and 3 which are three technical replicates) induction was subjected to the anti His monoclonal antibody (mAb) [M: Pre-stained protein molecular weight standards (10 - 250 kDa)]. The expressed antibody with the expected size of ~ 30 kDa was indicated by an arrow.](https://services.brieflands.com/cdn/serve/3170b/8c2bef1f005225fb7eeb95269ffed85309c104d0/jjnpp-160690-i001-F1-preview.webp)