1. Background
Chronic obstructive pulmonary disease (COPD), affecting over 380 million people and causing 3.5 million deaths annually (WHO, 2024), is characterized by oxidative stress and a protease–antiprotease imbalance, which collectively contribute to lung damage. These conditions drive inflammation, apoptosis, and impaired cell regeneration, all of which play a crucial role in disease progression (1). When the balance between oxidation and the antioxidant defense system is disrupted, it plays a crucial role in developing lung damage. The increased reactive oxygen species (ROS) levels, including superoxide, hydrogen peroxide, and hydroxyl radicals, can damage lipids, proteins, and DNA, disrupting cellular function and structure. While natural antioxidant defenses such as catalase (CAT) help mitigate ROS, these systems often become overwhelmed in diseases like COPD (2, 3).
The NRF2/Keap1 pathway is pivotal in regulating the cellular antioxidant response. Under normal conditions, NRF2 is bound to Keap1, keeping it inactive. Upon exposure to oxidative stress, ROS modify Keap1, leading to the release and activation of NRF2. This allows NRF2 to translocate to the nucleus and induce the expression of antioxidant genes that protect the cell from further oxidative damage (4). Elastase, an enzyme that disrupts the protease-antiprotease balance through excessive elastin degradation, plays a significant role in COPD. It contributes to lung damage by not only degrading elastin but also by promoting the production of ROS through the activation of inflammatory pathways (5). The increased ROS further exacerbates oxidative stress, leading to tissue damage and inflammation in the lungs (6).
Therefore, selegiline has emerged as a promising candidate. Selegiline, originally introduced as an antidepressant and later widely used for Parkinson’s disease as a selective monoamine oxidase-B (MAO-B) inhibitor, exhibits antioxidant properties, reducing ROS levels and inhibiting oxidative stress-induced apoptosis (3). Given the critical role of the NRF2/Keap1 pathway in oxidative stress response and the limited exploration of selegiline’s effects on human lung cells, we hypothesized that treatment with selegiline would significantly reduce elevated ROS levels induced by elastase exposure. Specifically, we anticipated that selegiline at optimal concentrations would reduce ROS levels by at least 25%, while higher concentrations might exert pro-oxidant or toxic effects. Moreover, we hypothesized that selegiline would increase NRF2 mRNA expression, downregulate Keap1, and reduce ROS levels, thereby mitigating elastase-induced oxidative damage in lung cells.
2. Methods
Selegiline (≥ 98% purity, storage at -20°C, CAS No. 14611-51-9) and porcine pancreatic elastase (PPE) (≥ 98% purity, CAS No. 39445-21-1) were purchased from Sigma-Aldrich (Germany). RNA extraction kit, cDNA synthesis kit (Cat. No. A101162), SYBR Green Master Mix, and primers (housekeeping and target genes) were obtained from Pars Tous (Iran) and Ampliqon (Denmark). Dulbecco’s Modified Eagle Medium (DMEM) (Cat. No. BI-1003), FBS (Cat. No. BI-1201), Penicillin/Streptomycin (Cat. No. BI-1203), and MTT assay kit (Cat. No. BI-2004) were purchased from Ideh Zist Co. (Iran). Catalase and malondialdehyde (MDA) kits were purchased from Kiazist Co. (Iran).
2.1. Cell Culture and Treatment
The human pulmonary epithelial cell line (A549) was purchased from the Pasteur Institute, Tehran, Iran. The cells were cultured in DMEM with 10% FBS and 1% antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin) at 37°C in a humidified incubator with 5% CO₂. The cells were then divided into six treatment groups (passage 3). All experiments were performed in triplicate.
2.2. Experimental Groups
(1) Control: Conditioned medium (untreated cells).
(2) H2O2: 100 μM for 24 hours.
(3) E group: 60 mU/mL for 24 hours (7)
(4) E+S20 group: 60 mU/mL elastase + 20 μM selegiline for 24 hours.
(5) E+S30 group: 60 mU/mL elastase + 30 μM selegiline for 24 hours.
(6) E+S40 group: 60 mU/mL elastase + 40 μM selegiline for 24 hours (8)
2.3. Cell Viability Assay
The MTT assay was performed 24, 48, and 72 hours post-treatment to assess cell viability. For the assay, 5,000 cells/well were seeded in each well of a 96-well microplate (triplicate wells per group) in 200 µL of complete culture medium. On the experimental day, 10 μL of MTT solution was added to each well, and the plates were incubated for 4 hours at 37°C. After incubation, the medium was carefully removed, and 100 μL of DMSO was added to solubilize the formazan crystals. The absorbance was measured at 570 nm using a microplate reader (Bio-Rad, USA; bandwidth 10 nm) (9).
2.4. Flow Cytometric Assay for Reactive Oxygen Species Detection
The A549 cells were trypsinized and centrifuged (8 minutes, 1200 rpm); the supernatant was discarded. Following a 24-hour treatment, ROS levels were measured using DCFH-DA and flow cytometry. After washing the cells, the ROS content was analyzed using flow cytometry. Intracellular ROS was measured using DCFH-DA (Sigma-Aldrich, Cat. No. D6883). After 24-hour treatment, A549 cells were harvested, washed twice with PBS, and incubated with 10 μM DCFH-DA for 30 minutes at 37°C in the dark. After incubation, cells were washed and resuspended in PBS for flow cytometry analysis. A standard gating strategy was applied during flow cytometric analysis. Forward scatter (FSC) and side scatter (SSC) parameters were used to exclude debris, and a singlet gate was applied to eliminate doublets based on FSC-H vs. FSC-A. ROS-positive cells were then identified based on fluorescence intensity in the appropriate detection channel (FITC for DCF fluorescence). A minimum of 10,000 events were collected per sample for analysis.
2.5. Oxidative Stress Assessment in A549 Cells
As a consequence, the medium was collected from each group and centrifuged. Then, MDA and CAT levels were quantified in cell supernatants using spectrophotometric assays (Bio-Rad, USA; bandwidth 10 nm) (10).
2.6. Gene Expression Assessment in A549 Cells
The cell lysates were collected and immediately stored at −80 °C until further analysis. RNA was extracted and reverse-transcribed, and NRF2/Keap1 expression was analyzed via qRT-PCR. RNA purity and concentration were assessed using a Nanodrop spectrophotometer. cDNA synthesis was performed using a cDNA synthesis kit. qRT-PCR reactions contained 1 µL of cDNA and 0.5 μM of each forward and reverse primer. The primer sequences used were as follows:
- NRF2: Forward 5′-CACATCCAGTCAGAAACCAGTGG-3′, reverse 5′-GGAATGTCTGCGCCAAAAGCTG-3′ (112 bp, NM_006164)
- Keap1: Forward 5′-CAACTTCGCTGAGCAGATTGGC-3′, reverse 5′-TGATGAGGGTCACCAGTTGGCA-3′ (108 bp, NM_203500)
- GAPDH: Forward 5′-CAGCCTCAAGATCATCAGCAATG-3′, reverse 5′-CATGAGTCCTTCCACGATACCA-3′ (100 bp, NM_002046.7)
Thermal cycling conditions were: Initial denaturation at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. All reactions were run in triplicate (10).
2.7. Statistical Analysis
In this experiment, GraphPad Prism 8 was used. Results were reported as mean ± SEM (n = 3). The normality of data distribution was assessed using the Shapiro–Wilk test. The difference between the experimental groups was determined using a one-way ANOVA with repeated measures, followed by a post-hoc Tukey's test. A P-value of < 0.05 was considered statistically significant.
3. Results
3.1. The Protective Influence of Selegiline on the Viability of A549 Cells
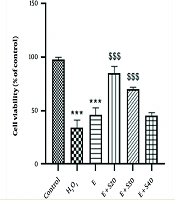
Cell viability was assessed through the MTT assay, and the findings demonstrated a significant reduction in cell viability after treatment with H2O2 and elastase compared to the control cells (P < 0.001). As illustrated in Figure 1, 24-hour treatment with 20 and 30 µM selegiline led to a notable increase in viable cells relative to the elastase group (P < 0.001). In contrast, increasing the concentration of selegiline to 40 µM did not yield a statistically significant difference in cell viability relative to the elastase group (P > 0.05). Indeed, cell viability was significantly reduced in cells treated with H2O2 alone, showing an average viability of 35.13% relative to the control. Treatment with elastase alone resulted in a moderate recovery, with cell viability increasing to 47.36%. Co-treatment with elastase and increasing concentrations of selegiline (E + S20, E + S30, and E + S40) led to a dose-dependent change in viability. The E + S20 group exhibited the highest protection (87.18%), followed by E + S30 (71.81%) and E + S40 (46.63%). These results suggest a concentration-dependent effect of selegiline when combined with elastase in mitigating oxidative stress-induced cell damage.
Cell viability in A549 cells after 24-hour treatment with elastase and selegiline (mean ± SEM, n = 3). Negative control, positive control (H2O2), E (60 mU/mL), E + S20 (E 60 mU/mL+ S 20 µM), E + S30 (E 60 mU/mL+ S 30 µM), E+S40 (E 60 mU/mL+ S 40 µM). *** P < 0.001 vs. control group; $$$ P < 0.001 vs. E group
3.2. The Impact of Selegiline on the Measurement of Reactive Oxygen Species
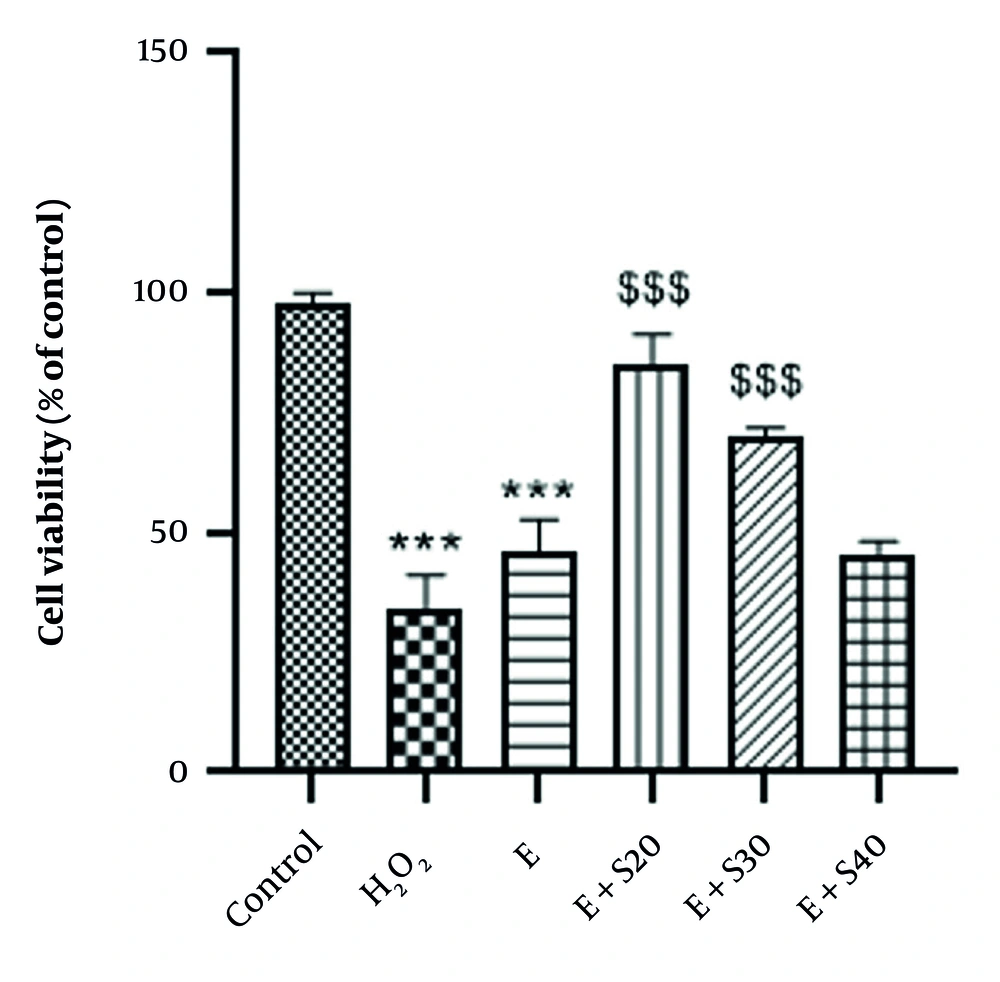
The results illustrated in Figure 2 indicate a significant increase in ROS following treatment with H2O2 and elastase relative to the control cells (P < 0.001). Furthermore, a marked disparity in ROS levels was found between the H2O2 and elastase groups (P < 0.001). A 24-hour treatment with 20 and 30 μM of selegiline substantially reduced ROS levels relative to the elastase group (P < 0.001). Conversely, an increase in selegiline concentration to 40 μM posed a substantial rise in ROS levels relative to the elastase group (P < 0.001), suggesting a potential toxic effect of selegiline at high doses. A 24-hour treatment with 20 μM selegiline reduced ROS levels by approximately 45.5% compared to the elastase group. Treatment with 30 μM selegiline reduced ROS levels by approximately 24.4%. Interestingly, 40 μM selegiline led to an 11.5% increase in ROS levels, suggesting a possible biphasic effect or reduced efficacy at higher concentrations.
Effect of selegiline on measurement of reactive oxygen species (ROS) in all groups consisting of negative control, positive control (H2O2), E (60 mU/mL), E+S20 (E 60 mU/mL+ S 20 µM), E + S30 (E 60 mU/mL+ S 30 µM), E+S40 (E 60 mU/mL+ S 40 µM). *** P < 0.001 relative to the control group; ### P < 0.001 vs. H2O2 group; $$$ P < 0.001 vs. E group
3.3. The Impact of Selegiline on Oxidative Stress Parameters
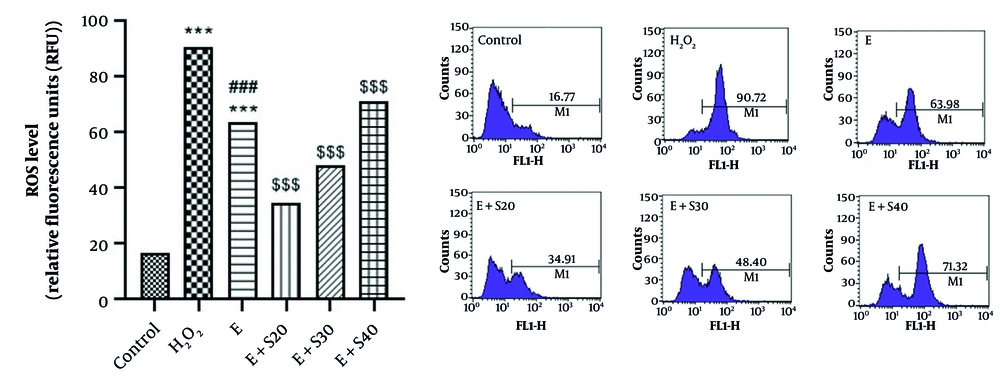
As depicted in Figure 3, elastase significantly increased MDA levels approximately 3-fold and reduced CAT activity by about 50% compared to the control group (P < 0.001). H2O2 also elevated MDA and decreased CAT activity significantly (P < 0.001), with notable differences between the H2O2 and elastase groups for both parameters (P < 0.001 and P < 0.01, respectively). Selegiline treatment at 20 and 30 μM markedly reduced MDA levels and restored CAT activity in a dose-dependent manner, with significant effects observed at 20 μM (P < 0.001) and 30 μM (MDA: P < 0.001; CAT: P < 0.01). Treatment with 20 μM selegiline reduced oxidative stress by approximately 26.5%. Treatment with 30 μM selegiline resulted in a 16.3% reduction. Treatment with 40 μM selegiline led to a slight increase (~3.9%) in oxidative stress levels, suggesting a possible loss of antioxidant efficacy at this higher dose. Moreover, 20 μM selegiline increased catalase activity by approximately 61.8%. Treatment with 30 μM selegiline led to a 42.8% increase, while 40 μM selegiline showed a marginal increase of 4.0%.
Effect of selegiline on A: MDA, B: CAT in all groups consisting of Negative control, positive control (H2O2), E (60 mU/mL), E+S20 (E 60 mU/mL+ S 20 µM), E+S30 (E 60 mU/mL + S 30 µM), E + S40 (E 60 mU/mL+ S 40 µM). *** P < 0.001 vs. control group. ## P < 0.01 and ### P < 0.001 vs. H2O2 group; $$ P < 0.01 and $$$ P < 0.001 vs. E group
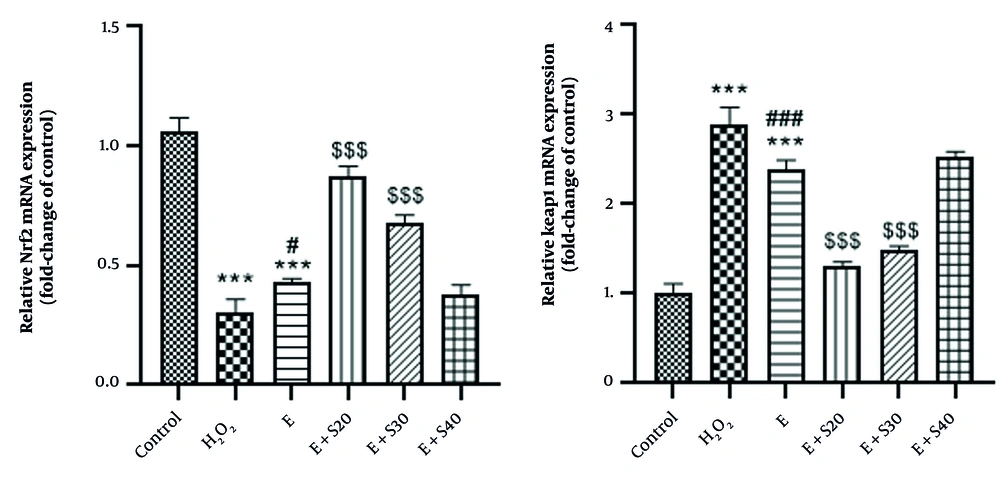
3.4. The Impact of Selegiline on the NRF2 and Keap1 mRNA Expression in A549 Cells
As depicted in Figure 4, NRF2 mRNA levels decreased significantly in the H2O2 and elastase groups compared to the control (P < 0.001). Treatment with selegiline increased NRF2 mRNA expression approximately 2.5-fold at 20 μM and 3.5-fold at 30 μM in the E + S20 and E + S30 groups, respectively, relative to the elastase group (P < 0.001). Conversely, Keap1 gene expression was significantly elevated by about 2.8-fold in the H2O2 and elastase groups compared to the control (P < 0.001), with a notable difference between the elastase and H2O2 groups (P < 0.001). Selegiline treatment at 20 and 30 μM markedly reduced Keap1 expression by approximately 60% and 75%, respectively, in the E + S20 and E + S30 groups relative to the elastase group (P < 0.001).
Quantitative analysis showed that NRF2 expression was markedly reduced following H2O2 treatment, with expression levels averaging 28.88% of control. Elastase-treated cells showed a slight improvement (40.71%). Co-treatment with elastase and selegiline at 20 µM significantly restored NRF2 levels to 82.28%. Moderate expression was observed with E+Selegiline30 (64.41%), while E+Selegiline40 led to a reduction (35.52%), suggesting a potential concentration-dependent effect where higher concentrations may reduce NRF2 induction.
In this context, Keap1 expression was markedly upregulated in response to H2O2, reaching an average of 285.71% relative to control. Elastase treatment also elevated Keap1 levels (235.66%). Co-treatment with elastase and selegiline showed a concentration-dependent modulation. E+Selegiline20 and E+Selegiline30 moderately increased Keap1 expression to 129.50% and 147.52%, respectively, while E+Selegiline40 induced a stronger elevation to 249.64%. These findings suggest partial reversal of H2O2-induced Keap1 overexpression at lower selegiline concentrations, with re-elevation at the highest dose.
Impact of selegiline on A: Nrf2, B: Keap1 mRNA expression in all groups consisting of negative control, positive control (H2O2), E (60 mU/mL), E + S20 (E 60 mU/mL+ S 20 µM), E + S30 (E 60 mU/mL+ S 30 µM), E + S40 (E 60 mU/mL+ S 40 µM). *** P < 0.001 vs. control group; # P < 0.05 and ### P < 0.001 vs. H2O2 group; $$$ P < 0.001 vs. E group.
4. Discussion
Elastase drives oxidative stress and emphysema by disrupting the protease-antiprotease balance and increasing ROS, effectively mirroring key pathological features of COPD. Previous studies have demonstrated that elastase exposure leads to increased oxidative stress and apoptosis in lung and intestinal epithelial cells, closely reflecting the clinical progression of COPD (11, 12). Our findings revealed that PPE increased ROS production, which was associated with elevated MDA levels. Recent studies have established oxidative stress as a major pathophysiological element in respiratory system dysfunction caused by free radical imbalance. ROS, including oxygen, build up when adverse exposures like particulate matter or poisonous chemicals weaken the antioxidant defense system. High ROS levels in cells can peroxidize lipids, damage proteins, and DNA (13).
In this regard, a study by Hou et al. showed that elastase-induced lung epithelial cell apoptosis and emphysema through the JNK and p38 MAPK pathway and oxidative stress. This study documented that the increase in growth factors-induced JNK and p38 MAPK pathways contributes to porcine pancreatic elastase-induced lung epithelial cell apoptosis and emphysema. Regulatory control of PlGF and agents against its downstream signals may be potential therapeutic targets for COPD (7). Also, Bayati et al. revealed that exposure to elastase caused dose-dependent oxidative stress damage, which was linked to A549 cells' decreased expression of genes for the antioxidant defense system (10).
Our research indicated that selegiline’s antioxidant effects likely stem from MAO-B inhibition and direct ROS scavenging, enhancing cellular defenses. Selegiline, chemically known as N-methyl-1-phenyl-N-prop-2-ynylpropan-2-amine, is a naturally occurring secondary metabolite. This compound acts as a selective MAO-B inhibitor and is widely used in treating Parkinson’s disease (14). Beyond its role as an MAO-B inhibitor, selegiline is believed to provide neuroprotective benefits through mechanisms that do not solely rely on this inhibition. It has been shown to prevent neuronal cell death in various types of neurons, such as dopaminergic, cortical, and facial motoneurons, following different forms of injury. One proposed mechanism for its protective effects involves enhancing cellular antioxidant systems. Numerous studies have demonstrated that selegiline elevates the activities of SOD and CAT in various organs (15).
The findings from the present study confirm that all tested concentrations of selegiline effectively mitigate oxidative stress in alveolar epithelial cells exposed to PPE, which correlates with increased levels of CAT in the cell supernatants. For instance, Qin et al. (2003) demonstrated that selegiline reduced cardiac ROS levels by approximately 30%, leading to decreased oxidative stress and apoptosis in heart failure, correlated with improved cardiac function. This supports the potential of selegiline to mitigate oxidative damage, consistent with our findings (16). Furthermore, Tian et al.'s in vivo and in vitro investigation demonstrated that selegiline displayed antioxidative effects in the context of high-fat diet (HFD) and palmitic acid (PA) exposure in mouse liver and AML-12 cells. This was evidenced by a reduction in the levels of ROS and MDA, alongside an enhancement in antioxidant enzyme activity (17) corroborating the findings of our current study.
Emphysema constitutes a significant aspect of COPD. It is marked by the destruction and expansion of the alveolar region, driven by chronic inflammation, oxidative stress, and an imbalance between proteases and antiproteases. The progression of airway inflammation and obstruction can occur gradually, making early diagnosis and intervention challenging due to the absence of distinctive clinical symptoms. The search for effective biomarkers for early detection and suitable therapeutic agents for COPD continues to be a complex endeavor (18). Cigarette smoke, recognized as the primary contributor to COPD, has been shown to elevate the expression of NRF2/Keap1 (19).
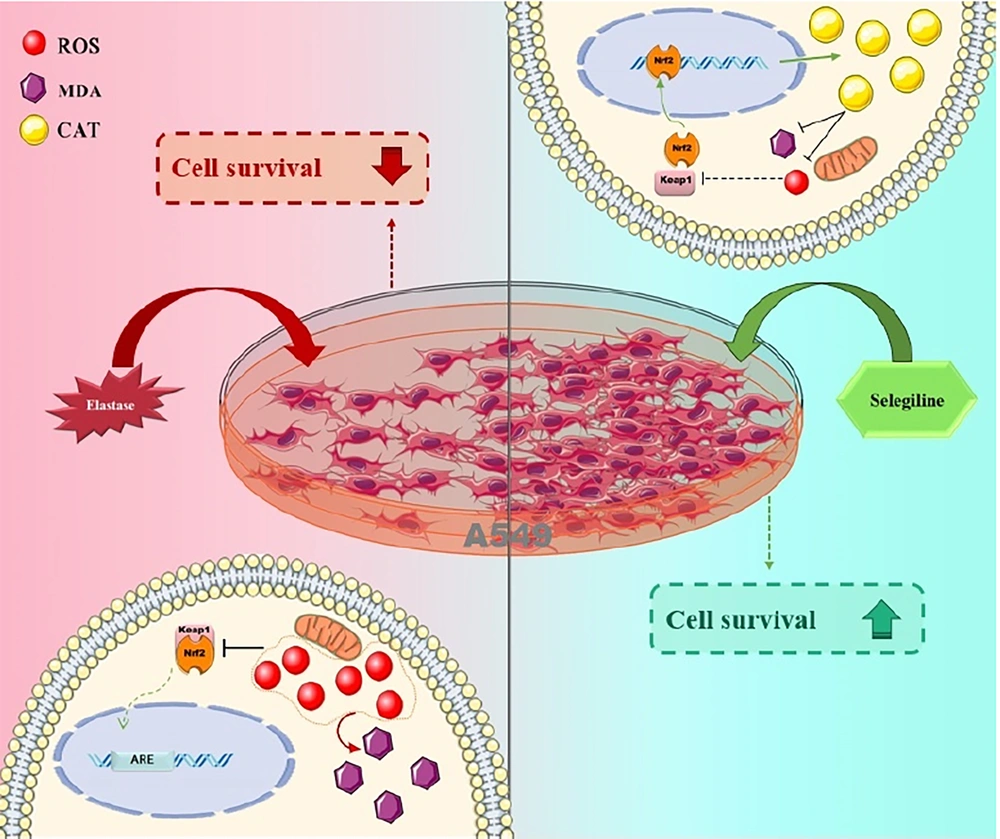
In the current research, we illustrated that the protease-antiprotease imbalance induced by PPE, a key pathogenic mechanism in emphysema, upregulates NRF2 and downregulates Keap1. Selegiline promotes antioxidant gene expression, counteracting oxidative stress in A549 cells (Figure 5). An essential and biologically conserved intracellular defense mechanism that reduces oxidative stress is NRF2 and its intrinsic regulator Keap1. NRF2 is normally targeted for proteasomal destruction because cytoplasmic Keap1 binds it. However, NRF2 dissociates from Keap1 during oxidative stress and migrates to the nucleus, forming heterodimers with small Maf proteins. Antioxidant response elements (AREs), activator sequences found in the regulatory regions of NRF2 target genes, may be detected by these heterodimers, playing a crucial role in recruiting essential transcription factors (20).
Numerous studies have demonstrated the interaction between NRF2 and NF-κB, emphasizing the significance of the NRF2/Keap1 pathway in the pathophysiology of various system dysfunctions, particularly in oxidative and reductive stress (21-23). Additionally, research has indicated that ROS regulates the NRF2/Keap1 pathway through intracellular silencing of information regulatory factors. Mice with a knockout of the gene for these regulatory factors exhibited elevated ROS levels and diminished NRF2 expression, suggesting a close relationship between ROS and NRF2 (20). Under conditions of PPE exposure, ROS production is stimulated in cell mitochondria, which modulates the NRF2/Keap1 signaling pathway. The interplay between NRF2 and Keap1 is vital for the cellular response to oxidative stress. A decline in NRF2 levels typically leads to an elevation in Keap1, which acts as a negative regulator of NRF2. This dynamic results in diminished antioxidative responses, as NRF2 is critical for activating genes responsible for detoxification and cellular protection. On the other hand, the activation of NRF2 can reduce Keap1 levels, thereby enhancing the antioxidant defense mechanisms (24).
Research conducted by Reisman et al. demonstrated that heightened NRF2 activation in the livers of Keap1-knockdown mice significantly boosts the expression of cytoprotective genes that are more effective in detoxifying electrophiles than in neutralizing reactive oxygen species (25). Additionally, a study by Naidu et al. indicated that in lung cancer cells with a loss-of-function mutation in Keap1, NRF2 promotes the degradation of mitolysosomes, ensuring the efficient removal of damaged mitochondria (26). Furthermore, treatment with selegiline was found to increase NRF2 gene expression while simultaneously decreasing Keap1 gene expression in PPE cells. In this context, the in vitro antioxidant research conducted by Nakaso et al. indicated that the cytoprotective properties of selegiline are partially reliant on the NRF2-mediated activation of antioxidative proteins (27).
Furthermore, other studies reported that selegiline promotes the nuclear translocation of NRF2, thereby augmenting its binding to the antioxidant response element and facilitating the expression of antioxidant thioredoxin superfamilies. Additionally, selegiline enhances the activities of glutathione-dependent antioxidant enzymes and anti-peroxidative enzymes such as catalase and superoxide dismutase, aligning with the present study's findings (3, 28). In this in vitro study, low-dose selegiline showed the most notable protective effect against elastase-induced damage in lung epithelial cells. These findings support the idea that initiating treatment with minimal doses may optimize therapeutic outcomes and reduce adverse effects, as suggested by another study (29).
While various pharmaceutical and traditional therapies address respiratory system damage, many alternative treatments have complex, poorly understood mechanisms, underscoring the need for continued research. A key challenge in evaluating their protective effects is the limited availability of clinical and in vivo data. Although animal studies often demonstrate efficacy, translating these findings to human clinical settings remains difficult. This study investigated PPE-induced cellular damage and the protective role of selegiline via the NRF2/Keap1 pathway in human lung epithelial cells. However, in vitro models cannot fully replicate in vivo complexity, and the clinical relevance of selegiline’s protective effects remains unconfirmed. Furthermore, given that selegiline functions as a selective MAO-B inhibitor, it may exert off-target effects, including alterations in neurotransmitter levels, which should be carefully considered in future investigations. Therefore, further in vivo studies and clinical trials are needed to validate these findings and explore their translational potential.
4.1. Conclusions
In conclusion, this study demonstrates that selegiline mitigates elastase-induced injury in lung epithelial cells by enhancing antioxidant defense mechanisms via the NRF2/Keap1 pathway. Given its prior clinical approval, selegiline may represent a promising adjunct therapy for COPD. However, its translational potential must be validated through comprehensive in vivo investigations and clinical trials, particularly in COPD patients with elevated oxidative stress.