1. Background
The Apiaceae family, formerly known as Umbelliferae, is recognized as one of Iran's leading aromatic plant families, encompassing an impressive total of 121 genera and 360 species (1). Members of this family are characterized by distinct features, including their annual or perennial aromatic herbs, hollow stems, and inflorescences that can be either simple or compound umbels. The leaves are arranged alternately, and they produce non-growing fruits or seeds that contain oil ducts (2). This family is highly regarded for its fragrant, medicinal, and culinary contributions, including well-known species such as Foeniculum vulgare (fennel), Petroselinum crispum (parsley), Cuminum cyminum (cumin), Pimpinella anisum (anise), Apium graveolens (celery), and Coriandrum sativum (coriander) (3). The essential oils derived from Apiaceae species are rich in biologically active metabolites, including monoterpene hydrocarbons, oxygenated and aromatic monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, phenylpropanoids, and aliphatic compounds (4). The essential oil components are well-documented for their diverse biological properties, such as antibacterial, antifungal, antioxidant, and antiviral effects, alongside a range of specific medicinal benefits.
Trachyspermum copticum, known in Persian as Zenyan, is an aromatic annual herbaceous plant with white flowers and small brownish fruits. This species is native to the Mediterranean region and Southwest Asian countries (5). The fruits of T. copticum are traditionally used in Iranian folk medicine as a carminative, diuretic, anti-fever, anti-spasm, antiemetic, and stomach tonic (6). It has been reported that the essential oil obtained from the fruits of T. copticum has shown potential in treating gastrointestinal ailments, lack of appetite, and bronchial problems (7). The antimicrobial, antifungal, and antioxidant activity, the cytotoxicity on tumor cells, and the induction of lymphocyte proliferation have been proven for T. copticum essential oil (7-10). The main constituent of the T. copticum essential oil is an aromatic monoterpenoid named Thymol, which is well known for its antioxidant and antibacterial properties (11).
Cuminum cyminum, known in Persian as Zireh Sabz, is an aromatic annual herb used as a spice for flavoring food. This species is native to Egypt, the Mediterranean, and South Asian countries (12). The fruits of C. cyminum are traditionally used in Iranian folk medicine for the treatment of gastrointestinal disorders, diabetes, and epilepsy (13, 14). It has been reported that the essential oil obtained from the fruits of C. cyminum possesses antioxidant, antidiabetic, anti-inflammatory, antibacterial, and anticancer effects (15). Cuminaldehyde, the main constituent of the C. cyminum essential oil, is well-known for its potent antibacterial activity (16).
Heracleum persicum, known in Persian as Golpar, is an aromatic perennial herb widely used in Persian food as a spice. This species is native to the Asian countries of Iran, Iraq, and Turkey (17). The fruits of H. persicum are traditionally used in Iranian folk medicine for the treatment of gastrointestinal, respiratory tract, urinary tract, and rheumatological disorders (17, 18). The antibacterial, anticancer, and antioxidant activities have been proven for H. persicum essential oil (18-20). It has been reported that the essential oil obtained from the fruits of H. persicum contains significant amounts of aliphatic esters and possesses antimicrobial and cytotoxic activity (20).
2. Objectives
Current understanding acknowledges that various environmental influences play a crucial role in shaping the secondary metabolites of medicinal plants, including phenolic, terpenoid, and alkaloid compounds, leading to both quantitative and qualitative modifications in their biosynthesis. This has resulted in a broad spectrum of phytochemical investigations on medicinal plants, potentially revealing instances where a plant synthesizes a specific natural compound or a considerable amount of therapeutically important phytochemicals. Accordingly, the present study aimed to (1) qualitatively and quantitatively analyze the chemical constituents present in the essential oils of T. copticum, C. cyminum, and H. persicum, native to Iran; and (2) evaluate the antioxidant and antimicrobial activities of the aforementioned essential oils.
3. Methods
3.1. Materials
The fruits of T. copticum, C. cyminum, and H. persicum were purchased from local stores and identified by the Department of Biology, Shahid Chamran University of Ahvaz. 2,2'-Diphenyl-1-picrylhydrazyl radical (DPPH) was purchased from Sigma Chemical Co. Butylated hydroxytoluene (BHT) was purchased from Merck. All other chemicals and solvents (Dr. Mojallali Chemical Co.) were of analytical grade. The bacterial strains (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, and Enterococcus faecalis ATCC 49452) were purchased from the Pasteur Institute (Tehran, Iran). Mueller Hinton Broth (MHB) medium was purchased from Condalab Co.
3.2. Isolation of the Essential Oils
The essential oils were extracted using the hydro-distillation method. Briefly, 100 g of dried fruits were ground and hydro-distilled for 3 hours using a Clevenger-type apparatus. The isolated essential oils were dried over anhydrous sodium sulfate, filtered, and stored in the refrigerator before further analyses.
3.3. Gas Chromatography-Mass Spectrometry
The chemical constituents of the T. copticum, C. cyminum, and H. persicum essential oils were identified using a Gas Chromatography (GC) system (7890 A, Agilent Technologies, USA) coupled to a mass spectrometer (5975C VL MSD with Triple-Axis Detector, Agilent Technologies). The gas chromatography system had an Rtx-5 MS column (30 m × 0.25 mm × 0.25 µm film thickness). The ionization energy was set to 70 eV, and helium gas with a flow rate of 1 mL/min was used as the mobile phase. The oven temperature was gradually increased from 40 to 270℃ at a rate of 3℃/min. The injection port and detector temperatures were maintained at 250℃ and 230℃, respectively.
3.4. Antioxidant Activity
The antioxidant activity of the T. copticum, C. cyminum, and H. persicum essential oils was assessed based on the scavenging of the DPPH free radical. A 0.5 mL sample of each essential oil at various concentrations was combined with 2 mL of a freshly prepared methanolic DPPH solution (100 µM) and thoroughly shaken for 30 minutes at room temperature in a dark setting. The absorbance was subsequently measured at 517 nm using a spectrophotometer, and the percentage of DPPH free radical inhibition was calculated using the following formula (21):
The percentage of DPPH free radical inhibition is determined where (A0) represents the absorbance of the control (blank) and (A sample) indicates the absorbance of the test compound. The compound concentration that achieved 50% inhibition (IC50) was derived from a plot of DPPH inhibition percentage versus sample concentration. All tests were performed in triplicate, with essential oils and DPPH dissolved in methanol. Butylated hydroxytoluene was utilized as the positive control.
3.5. Antibacterial Activity
3.5.1. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
The antimicrobial properties of essential oils of T. copticum, C. cyminum, and H. persicum were assessed against E. coli ATCC 25922, P. aeruginosa ATCC 27853, S. aureus ATCC 25923, and E. faecalis ATCC 49452 using a serial dilution technique. Samples were serially diluted in MHB within 96-well plates, achieving a concentration range from 2 to 64 mg/mL. The final concentration of the microorganisms was set to 5 × 10^6 CFU/mL. To determine the minimum inhibitory concentration (MIC) of the aforementioned essential oils, a 0.5% v/v Tween 80 solution was incorporated into the medium. The plates were incubated at 37ºC for 24 hours, and the MIC values were identified as the lowest concentration at which no visible microbial growth was observed. The minimum bactericidal concentration (MBC) was established by incubating 100 µL of broth from wells showing no growth. The broth was then plated onto Mueller Hinton Agar (MHA) and incubated at 37ºC for 24 hours. The MBC was the lowest concentration capable of killing 99.9% of the treated microorganisms (22).
4. Results
4.1. Composition of Essential Oils
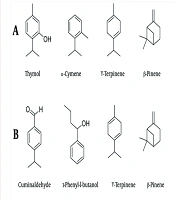
The findings from qualitative and quantitative analyses of T. copticum, C. cyminum, and H. persicum essential oils are presented in Table 1. A total of 15 compounds were identified in the essential oil of T. copticum, representing 99.2% of the overall essential oil composition. In the case of C. cyminum, 43 compounds were identified, while H. persicum essential oil contained 69 compounds, accounting for 98.84% and 92.11% of their total essential oils, respectively. The main constituents of T. copticum essential oil were identified as Thymol (56.20%), o-Cymene (21.17%), and γ-Terpinene (16.66%) (Figure 1A). Consequently, these three monoterpenoids represent 94.03% of the overall composition of the T. copticum essential oil.
| Compound | Area (%) | ||
|---|---|---|---|
| Trachyspermum copticum | Cuminum cyminum | Heracleum persicum | |
| α-Thujene | 0.38 | 0.45 | - |
| α-Pinene | 0.33 | 1.12 | - |
| Camphene | - | 0.03 | - |
| propyl-2-methylbutanoate | - | - | 0.11 |
| Isoamyl propionate | - | - | 0.08 |
| β-Pinene | 1.59 | 13.06 | - |
| Myrcene | 0.54 | 1.04 | - |
| α-Phellandrene | 0.04 | 0.43 | - |
| 3-Carene | 0.06 | 0.06 | - |
| Hexyl acetate | - | - | 0.67 |
| α-Terpinene | 0.42 | 0.17 | - |
| o-Cymene | 21.17 | - | - |
| p-Cymene | - | 9.11 | - |
| β-Phellandrene | 0.55 | - | - |
| Eucalyptol | - | 0.21 | |
| Butyl 2-methylbutyrate | - | - | 1.09 |
| γ-Terpinene | 16.66 | 16.28 | - |
| trans-Sabinene hydrate | - | 0.06 | - |
| 1-Octanol | - | - | 3.51 |
| Terpinolene | 0.09 | 0.07 | - |
| Sabinene hydrate | - | 0.07 | - |
| Linalool | - | 0.05 | - |
| 2-Methylbutyl 2-methylbutanoate | - | - | 1.88 |
| 1-Terpineol | - | 0.04 | - |
| cis-p-Menth-2-en-1-ol | - | 0.05 | - |
| Hexyl isobutyrate | - | - | 2.03 |
| Terpinen-4-ol | - | 0.23 | - |
| α-Terpineol | 0.08 | 0.1 | - |
| Thymol methyl ether | - | - | 0.12 |
| Cuminaldehyde | - | 23.69 | - |
| Hexyl-2-methylbutanoate | - | - | 4.72 |
| Thymol | 56.2 | - | 1.05 |
| Carvacrol | 1.05 | - | 0.61 |
| Nonyl acetate | - | - | 0.12 |
| Octyl isobutyrate | - | - | 1.39 |
| Dodecanal | - | - | 0.16 |
| Caryophyllene | - | 0.15 | 0.12 |
| α-Bergamotene | - | 0.08 | - |
| Calarene | - | 0.06 | - |
| β-Farnesene | - | 0.4 | - |
| Germacrene D | - | - | 0.26 |
| α-Farnesene | - | 0.07 | - |
| β-Himachalene | - | - | 0.13 |
| β-Bisabolene | - | 0.1 | 0.31 |
| β-Sesquiphellandrene | - | 0.03 | 0.39 |
| (E)-γ-Bisabolene | - | 0.02 | 0.06 |
| Spathulenol | - | - | 0.08 |
| Caryophyllene oxide | - | 0.05 | - |
| Viridiflorol | - | - | 0.2 |
| (2Z,6E)-Farnesol | - | - | 0.34 |
| Palmitic acid | - | - | 2.31 |
| Heptyl acetate | - | - | 0.19 |
| Linoleic acid | - | - | 0.95 |
| 4-Ethyl-2,6-xylenol | - | - | 0.43 |
| Dillapiol | 0.04 | - | - |
| Cumene | - | 0.03 | - |
| Isopropylidenecyclohexane | - | 0.08 | - |
| Pinocarvone | - | 0.03 | - |
| phellandral | - | 0.16 | - |
| p-Mentha-1,4-dien-7-ol | - | 0.24 | - |
| Acoradiene | - | 0.19 | - |
| Nerolidol | - | 0.02 | - |
| Carotol | - | 0.24 | - |
| 1-Phenyl-1-butanol | - | 17.59 | - |
| 1-Isopropylidene-3-n-butyl-2-cyclobutene | - | 11.53 | - |
| syn-7-Isopropylbicyclo[2.2.1]heptan-2-one | - | 1.39 | - |
| 4,8-Epoxyterpinolene | - | 0.02 | - |
| 1,2,3,4,5-Pentamethylcyclopentadiene | - | 0.02 | - |
| α-Longipinene | - | 0.02 | - |
| Isoprene | - | - | 0.35 |
| 2-Methyl-3-buten-2-ol | - | - | 0.1 |
| Heptane | - | - | 0.05 |
| Nonane | - | - | 0.05 |
| (E)-2-Nonene | - | - | 0.05 |
| Isobutyl 2-methylbutyrate | - | - | 1.84 |
| 2-Methylbutyl isobutyrate | - | - | 0.51 |
| Butyl isovalerate | - | - | 0.05 |
| 2′-Methylacetophenone | - | - | 0.25 |
| 2-Ethylhexyl acetate | - | - | 47.07 |
| 1-Undecanol | - | - | 0.09 |
| Hexyl hexanoate | - | - | 0.31 |
| 1-Methyl-bicyclo[4.1.1]octan-7-one | - | - | 0.37 |
| (Z)-6-Decenyl acetate | - | - | 0.11 |
| Decyl acetate | - | - | 0.72 |
| Octyl 2-methylbutyrate | - | - | 3.69 |
| Cyclodecane | - | - | 0.12 |
| Curcumene | - | - | 0.32 |
| Phenylethyl 2-methylbutyrate | - | - | 0.13 |
| Zingiberene | - | - | 0.34 |
| Curlone | - | - | 0.07 |
| Nonadecane | - | - | 0.05 |
| (6Z,9Z)-6,9-Pentadecadien-1-ol | - | - | 0.94 |
| Heptacosane | - | - | 0.12 |
| Nonacosane | - | - | 0.22 |
| Isobutyl butyrate | - | - | 0.26 |
| Isobutyl isovalerate | - | - | 0.06 |
| Hexyl propanoate | - | - | 0.49 |
| Methyl-4-(E)-Hexenyl Ether | - | - | 0.08 |
| Vinylcyclohexane | - | - | 5.9 |
| cis-5-Octen-1-ol | - | - | 0.99 |
| Pentanoic acid, 4-hexen-1-yl ester | - | - | 0.07 |
| Propanoic acid, 4-hexen-1-yl ester | - | - | 0.21 |
| 10-Dodecen-1-ol | - | - | 0.17 |
| Octyl propionate | - | - | 0.42 |
| 9-Decen-1-ol | - | - | 1.52 |
| Dicyclopentyl | - | - | 0.07 |
| 2-aminomethylbutanoic acid | - | - | 0.21 |
| 4-Isopropenylcyclohexanone | - | - | 0.14 |
| Gossonorol | - | - | 0.1 |
| 4-Methoxyphenyl pivalate | - | - | 0.1 |
| Geranyl linalool | - | - | 0.09 |
The essential oil composition of Trachyspermum copticum, Cuminum cyminum, and Heracleum persicum
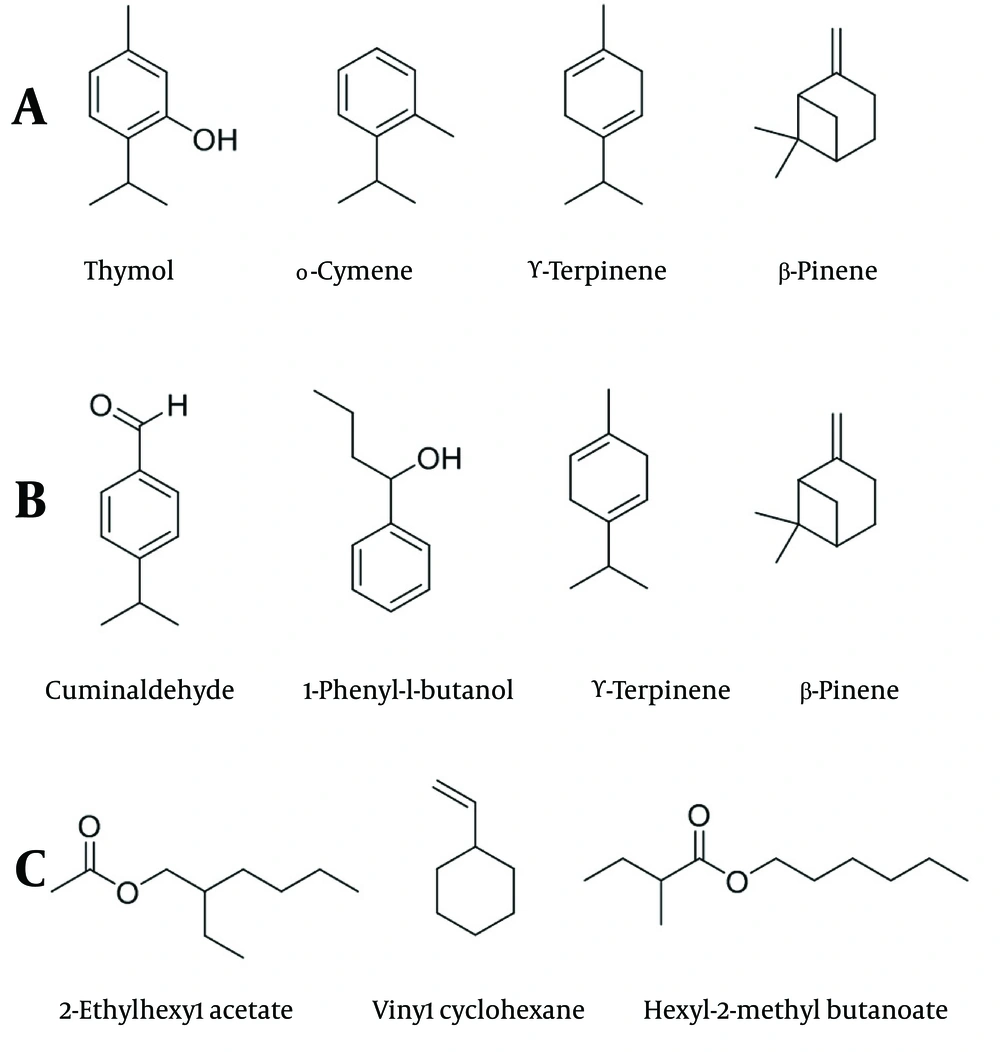
The main constituents of C. cyminum essential oil were identified as Cuminaldehyde (23.69%), 1-phenyl-1-butanol (17.59%), γ-Terpinene (16.28%), and β-Pinene (13.06%) (Figure 1B). 1-Isopropylidene-3-n-butyl-2-cyclobutene (11.53%) and p-Cymene (9.11%) were also identified as other major constituents of the C. cyminum essential oil.
The main constituents of H. persicum essential oil were identified as 2-ethylhexyl acetate (47.70%), vinyl cyclohexane (5.90%), hexyl-2-methyl butanoate (4.72%), octyl-2-methyl butyrate (3.69%), and 1-octanol (3.51%) (Figure 1C). Table 1 clearly indicates that H. persicum essential oil contains a significantly higher variety of chemical compounds compared to T. copticum and C. cyminum essential oils.
4.2. Antioxidant Activity
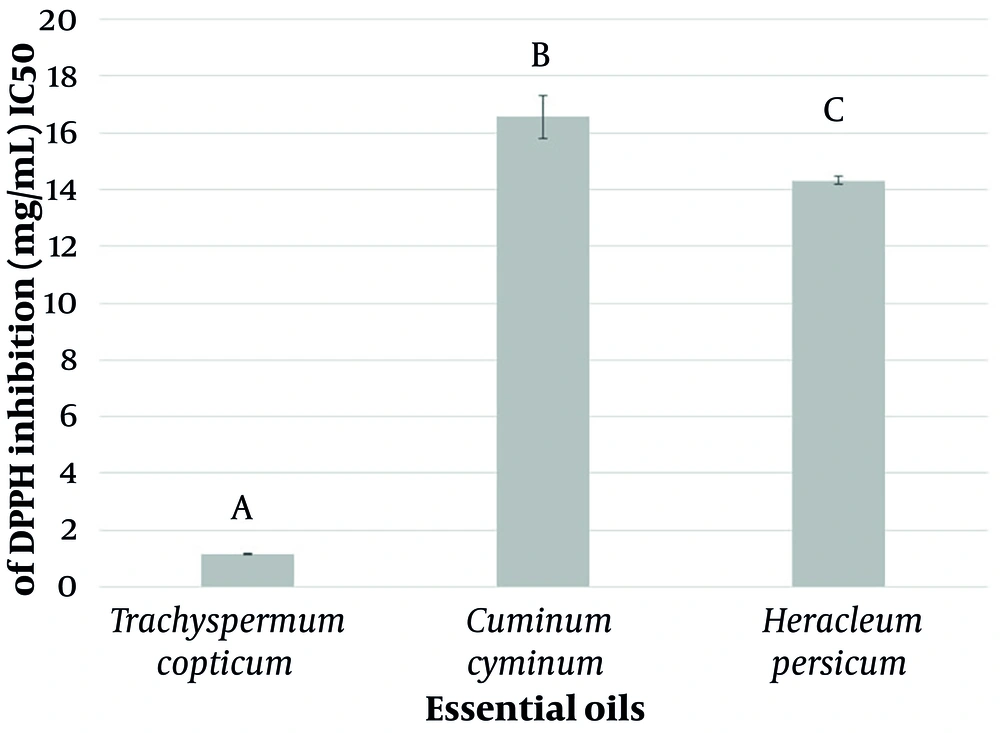
The results of the antioxidant activity of the T. copticum, C. cyminum, and H. persicum essential oils based on the DPPH free radical scavenging assay are represented in Figure 2. The IC50 values indicate that while all three essential oils exhibit weaker antioxidant properties than BHT (as a positive control with an IC50 value of 0.021 ± 0.001 mg/mL), T. copticum essential oil demonstrates a considerably lower IC50 value, which indicates stronger antioxidant capabilities than both C. cyminum and H. persicum essential oils.
4.3. Antibacterial Activity
The results of the antibacterial activity of the T. copticum, C. cyminum, and H. persicum essential oils against two gram-negative bacteria (c ATCC 25922, P. aeruginosa ATCC 27853) as well as two gram-positive bacteria (S. aureus ATCC 25923, and E. faecalis ATCC 49452) were assessed using a serial dilution method.
Table 2 illustrates that the essential oils exhibit antimicrobial properties against the bacteria examined. Notably, the antimicrobial efficacy of C. cyminum and T. copticum essential oils is significantly greater than that of H. persicum essential oil. Furthermore, it is apparent that gram-negative bacteria, particularly P. aeruginosa, exhibit a higher resistance to the essential oils examined, necessitating the use of significantly elevated concentrations of these essential oils to inhibit the growth of this bacterial strain.
| Variables | MIC (mg. mL-1) | MBC (mg. mL-1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus | Enterococcus faecalis | Escherichia coli | Pseudomonas aeruginosa | Pseudomonas aeruginosa | Enterococcus faecalis | |
| Trachyspermum copticum | 32 | 32 | 8 | 8 | 32 | 32 | 16 | 16 |
| Cuminum cyminum | 8 | 64 | 4 | 4 | 8 | 64 | 8 | 64 |
| Heracleum persicum | > 64 | > 64 | 64 | 64 | > 64 | > 64 | > 64 | > 64 |
Antibacterial Activity of the Trachyspermum copticum, Cuminum cyminum, and Heracleum persicum Essential Oils
5. Discussion
5.1. Composition of Essential Oils
The results obtained from the analysis of the chemical constituents of T. copticum essential oil are in accordance with several reports that highlight Thymol, o-Cymene, and γ-Terpinene as the principal constituents of T. copticum essential oil, with Thymol exhibiting the highest concentration among these three monoterpenoid compounds (23-26). In other words, the T. copticum essential oil can be considered a Thymol-rich essential oil. Reports indicate that Thymol can comprise up to 96.4% of the essential oil derived from this species (11).
The C. cyminum essential oil is made up mainly of Cuminaldehyde, which has been reported in many studies to account for up to 62.7% of the essential oil (27). In addition to Cuminaldehyde, the primary component of C. cyminum essential oil, notable constituents such as 1-phenyl-1-butanol, γ-Terpinene, and β-Pinene have also been identified as significant components of this essential oil (28-30). Identified in C. cyminum essential oil, 1-Isopropylidene-3-n-butyl-2-cyclobutene (11.53%) is an unusual compound that has been highlighted in other studies as a key component, making up to 17% of the total essential oil (31).
The essential oils of T. copticum and C. cyminum are primarily composed of terpenoid compounds, whereas most of the compounds found in H. persicum essential oil are aliphatic esters. According to several reports, the essential oil of H. persicum was found to contain significant amounts of 2-ethylhexyl acetate along with other specified aliphatic esters such as hexyl-2-methyl butanoate and octyl-2-methyl butyrate (32, 33). To the best of our knowledge, this study indicates one of the highest reported contents of 2-ethylhexyl acetate found in the essential oil of H. persicum.
5.2. Antioxidant Activity
Research into the antioxidant characteristics of essential oils indicated that T. copticum essential oil possesses markedly superior antioxidant properties compared to C. cyminum and H. persicum essential oils. The potent antioxidant characteristics of T. copticum essential oil can be attributed to the elevated Thymol content in this essential oil. The antioxidant capabilities of Thymol have been demonstrated in earlier studies through various assays, including DPPH and ABTS free radical scavenging tests. Additionally, essential oils derived from plants that contain substantial levels of Thymol as the main constituent have exhibited notable antioxidant effects (34, 35). Reports indicate that Thymol's antioxidant properties may inhibit proliferation in human cancer cell lines (36).
In addition, it has been reported that Cuminaldehyde, the main compound of C. cyminum essential oil, has weaker antioxidant properties than other components in the essential oil, such as γ-Terpinene (37). Cuminaldehyde lacks the hydroxyl moiety present in Thymol that contributes to the antioxidant activity (38). Moreover, the presence of predominantly aliphatic esters in the H. persicum essential oil suggests that its antioxidant properties are likely less pronounced than T. copticum essential oil, which is rich in the antioxidant monoterpenoid, Thymol.
5.3. Antibacterial Activity
Extensive research has confirmed the antibacterial effects of T. copticum essential oil on multiple bacterial strains, such as E. coli, S. enterica, S. aureus, and B. subtilis, among others. It has been observed that T. copticum essential oil effectively inhibited E. coli growth and promoted the release of cell constituents, proteins, and potassium ions from the bacteria (10, 39).
Thorough investigations have demonstrated the antibacterial effects of C. cyminum essential oil against a range of bacterial strains, including S. typhimurium, L. monocytogenes, S. aureus, and B. cereus. Reports suggest that the antibacterial properties of essential oils are linked to the hydrophobicity of their components, which can penetrate the lipid bilayer of bacterial cell membranes, increasing permeability and leading to the release of critical cellular contents (27).
The discrepancy in the antibacterial activity of the tested essential oils can be attributed to the distinct chemical composition of H. persicum essential oil, which is primarily composed of aliphatic esters, leading to an anticipated variation in antibacterial effectiveness. The enhanced antibacterial activity observed in C. cyminum and T. copticum essential oils is likely due to the substantial presence of monoterpenoid compounds within these oils. The antibacterial properties of monoterpenoid components present in the essential oils of T. copticum and C. cyminum, such as Thymol (40, 41), γ-Terpinene (42), β-Pinene (43, 44), and Cuminaldehyde (45-47), have been thoroughly examined in previous studies. The antibacterial properties of phenolic monoterpenoids, such as Thymol, are linked to their capacity to disrupt and depolarize the cytoplasmic membrane (48). Research indicates that the monoterpenoids α-Pinene and β-Pinene can inhibit the formation of bacterial biofilms (43). Cuminaldehyde has also been observed to enhance the permeability of the S. aureus membrane, resulting in noticeable alterations in cell morphology (46).
The comprehensive analysis of the results indicates that gram-negative bacteria, particularly P. aeruginosa, exhibit lower sensitivity to essential oils compared to gram-positive bacteria. This reduced sensitivity is likely attributed to the more intricate membrane structure found in gram-negative bacteria.
5.4. Conclusions
Essential oils obtained from Apiaceae species are rich in biologically active metabolites with several outstanding biological properties, including antibacterial, antifungal, antioxidant, and antiviral effects. This study highlighted the chemical compositions of the essential oils of three indigenous Iranian Apiaceae species, namely T. copticum, C. cyminum, and H. persicum, and their antioxidant and antibacterial properties. The essential oils' major components were Thymol for T. copticum, Cuminaldehyde for C. cyminum, and 2-ethylhexyl acetate for H. persicum. While both T. copticum and C. cyminum essential oils consist primarily of terpenoid compounds, the essential oil from H. persicum comprises aliphatic esters. The remarkable antioxidant and antibacterial properties of T. copticum and C. cyminum essential oils certainly recommend their future uses in many fields, particularly culinary applications.