1. Background
Polycystic ovary syndrome (PCOS) is among the most frequently diagnosed endocrine conditions in women during their reproductive years, with its occurrence varying from 5% to 10% of the population depending on the diagnostic criteria (1-6). Originally described by Stein and Leventhal, it is primarily characterized by hormonal imbalances leading to menstrual irregularities, hyperandrogenism, and ovarian dysfunction (7, 8). Diagnosis involves assessing clinical features and measuring biochemical markers to confirm hormonal imbalances. Key indicators include a higher concentration of luteinizing hormone (LH) compared to follicle-stimulating hormone (FSH), elevated androgen levels, such as total and free testosterone, prolactin, and dehydroepiandrosterone sulfate (DHEAS), as well as disruptions in insulin metabolism (9, 10). Menstrual dysfunction, often presenting as oligomenorrhea or amenorrhea, is frequently accompanied by dermatological manifestations like hirsutism, acne, and alopecia, as well as polycystic ovarian morphology (PCOM) detected through ultrasonography. Additionally, insulin resistance is frequently observed in affected individuals, contributing to metabolic complications such as hyperinsulinemia and a heightened susceptibility to type 2 diabetes mellitus. Although the criteria for diagnosis differ, PCOS is often identified based on a combination of oligomenorrhea, hyperandrogenism, and ovarian morphology (10, 11). Ovarian ultrasonography serves as a key diagnostic approach for the early identification of PCOS, offering critical insights into follicle count, size, and spatial distribution within the ovary (12). Current pharmacological treatments, including metformin, primarily aim to regulate menstrual cycles, restore ovulation, and manage symptoms. However, these medications are associated with side effects such as mood disturbances, musculoskeletal discomfort, and metabolic fluctuations (13). Given these challenges, there is growing interest in exploring alternative therapeutic approaches, particularly plant-based remedies, which may offer symptom relief with fewer adverse effects (14).
Tragopogon graminifolius DC is a perennial plant belonging to the Asteraceae family, widely found in western Iran, where it is traditionally known as “Sheng” (15-17). In traditional medicine, it has been valued for its therapeutic potential, particularly in treating gastrointestinal disorders, wound healing, and liver protection (18). Phytochemical analyses of various Tragopogon species have revealed several bioactive substances, including flavonoids (e.g., luteolin, quercetin, vitexin, and orientin), along with phenolic compounds such as caffeic acid, gallic acid, triterpene saponins, and ferulic acid, which contribute to its anti-inflammatory, antioxidant, and antimicrobial properties (18-21). Additionally, some species within this genus contain vitamins C, E, and K, further supporting their medicinal applications (22).
Despite these promising properties, the potential effects of T. graminifolius in PCOS remain unexplored. Given the crucial role oxidative stress and inflammation play in PCOS pathophysiology, we hypothesized that the hydroalcoholic extract of T. graminifolius could exert beneficial effects in managing PCOS by modulating oxidative stress and hormonal imbalances.
2. Objectives
The study aimed to evaluate its impact on PCOS induced by testosterone enanthate in female Wistar rats.
3. Methods
3.1. Preparation of Tragopogon graminifolius Extract
The T. graminifolius plant was gathered in the spring season from the Kermanshah region and taxonomically verified by a specialist in the Biology Department at the University of Tehran’s Faculty of Sciences, based on the corresponding herbarium reference (Specimen No. 43603). The collected botanical samples were carefully rinsed, air-dried in a shaded area to prevent direct sunlight exposure, and finely powdered using a laboratory mill. Subsequently, 100 grams of this plant powder were immersed in 100 milliliters of a 70% hydroalcoholic solution and left to macerate for 72 hours. Afterwards, the solution was filtered through Whatman filter paper. To isolate the extract, the solvent was gradually removed through rotary evaporation. The resulting extract was stored under refrigerated conditions until use, yielding approximately 29.45%.
3.2. Animals
Twenty-five juvenile female Wistar rats, each aged 21 days and weighing approximately 40 - 70 grams, were procured from the animal facility of Kermanshah University of Medical Sciences. The animals were kept under regulated laboratory conditions, which included consistent temperature (22 ± 2ºC) and humidity (55 ± 5%) settings, along with a 12-hour alternating light/dark cycle. They were given unlimited access to standard chow and water. All experimental protocols were reviewed and approved by the Institutional Ethics Committee of Kermanshah University of Medical Sciences (IR. KUMS. AEC. 1402. 039).
3.3. Study Design
To establish a model of PCOS, a testosterone-induced approach was employed using female Wistar rats (23, 24). Beginning at 21 days of age, the animals received daily subcutaneous administrations of testosterone enanthate at a dosage of 1 mg per 100 g of body weight over 35 days (25). Upon completion of the induction phase, the rats were randomly assigned into five distinct groups, with five animals allocated to each group:
1. Healthy control: Received normal saline orally for 28 days.
2. The PCOS model: No treatment after PCOS induction.
3. Metformin-treated group: Given oral doses of metformin at 20 mg/kg body weight each day over a 28-day period (26).
4. Tragopogon graminifolius extract (low dose): Treated orally with 100 mg/kg of the extract on a daily basis for 28 days (27, 28).
5. Tragopogon graminifolius extract (high dose): Received a daily oral dose of 200 mg/kg of the plant extract throughout the 28-day study duration (29).
Following the treatment period, the animals underwent biochemical, hormonal, and histopathological evaluations.
3.4. Hormonal and Biochemical Assessments
On the final day of the experiment, the rats were euthanized using an overdose of sodium pentobarbital (200 mg/kg) (30). Blood was drawn through cardiac puncture, then centrifuged at 3000 rpm for 10 minutes to obtain serum, which was subsequently frozen at -80ºC for further analysis. Levels of FSH, LH, testosterone, and glucose were determined by enzyme-linked immunosorbent assay (ELISA) kits, including FSH ELISA KIT (Cat No. PT-EFS-201), LH ELISA KIT (Cat No. PT-EFS-202), Testosterone ELISA KIT (H66L1L2), and Mouse Glucose ELISA Kit (MBS7200879) from a commercial source, following the manufacturer’s instructions.
Histological examination of ovarian tissue: Ovarian tissues were carefully dissected, fixed in 10% formalin, and subjected to paraffin embedding. Sections of tissue were cut to a thickness of 5 micrometers and stained with hematoxylin and eosin (H&E), as outlined in the procedure by Parr (31). The histopathological assessment was conducted using a light microscope at a magnification of X40 to evaluate ovarian morphology, including follicular development and cystic changes.
3.5. Statistical Analysis
Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS), version 21.0. Differences among the experimental groups were assessed through a one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison procedure. Data are presented as mean values alongside their standard error (SEM), and a P-value below 0.05 was considered to indicate statistical significance.
4. Results
4.1. Testosterone Hormone Analysis Results
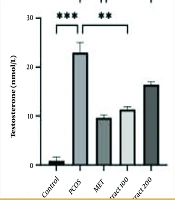
The findings on testosterone levels are presented in Figure 1. In the PCOS group, testosterone levels showed a marked rise compared to the healthy control group (P < 0.001). Meanwhile, metformin treatment in the reference group resulted in a substantial decrease, with statistical significance indicated by P < 0.01.
Results of the evaluation of different doses of hydroalcoholic Tragopogon graminifolius extract on testosterone levels. Control: Healthy group with no polycystic ovary syndrome (PCOS) induction; PCOS: The PCOS group administered saline through ingestion; MET: Reference group given metformin at a daily dosage of 20 mg/kg by ingestion; Extract 100: Group treated with 100 mg/kg of the plant extract via the same route for 28 consecutive days; Extract 200: Group receiving 200 mg/kg of the extract across the same timeframe (data are presented as mean ± SEM; statistical comparisons to the PCOS group are marked by *P < 0.05, **P < 0.01, and ***P < 0.001).
Likewise, administering the hydroalcoholic extract of T. graminifolius at a dose of 100 mg/kg caused a significant decline in testosterone levels (P < 0.01). A comparable reduction was also noted with the 200 mg/kg dose, though the statistical threshold in this case was P < 0.05. Furthermore, a meaningful variation was found when comparing the impact of metformin to that of the higher extract dose (P < 0.05).
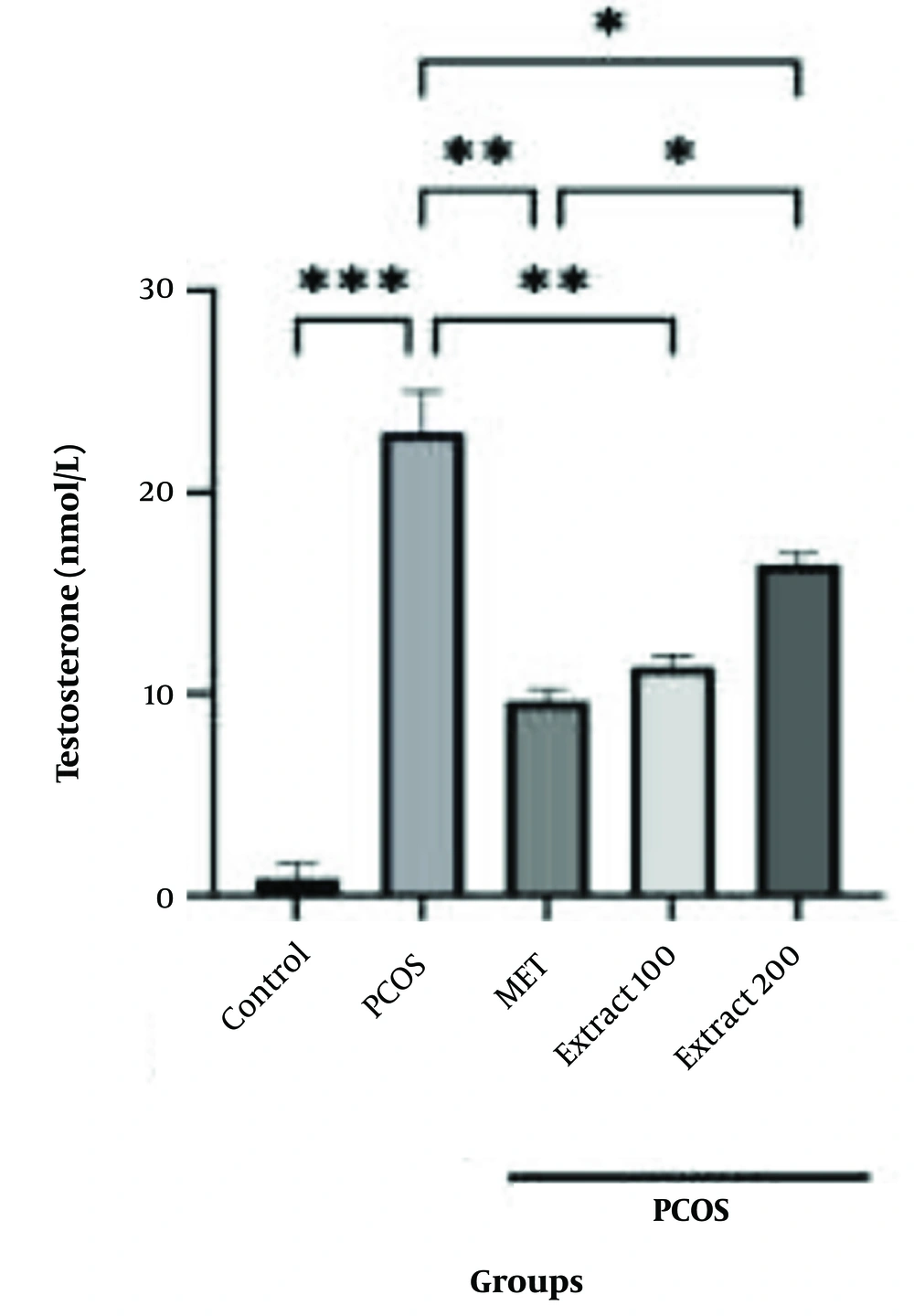
4.2. Luteinizing Hormone Evaluation Results
Alterations in LH concentrations are shown in Figure 2. LH levels were markedly elevated in the PCOS group that received no treatment when compared with the healthy controls, reaching high statistical significance (P < 0.0001). In contrast, substantial reductions in LH concentrations were observed following the administration of metformin and both doses of T. graminifolius extract (100 mg/kg and 200 mg/kg), when compared to the PCOS group, with each intervention showing strong statistical differences (P < 0.001 for the extract groups and metformin). Additionally, treatment with the 200 mg/kg extract yielded a more significant decline in LH levels than both the lower-dose extract and metformin groups (P < 0.005 and P < 0.01 in those comparisons).
Effects of different doses of hydroalcoholic Tragopogon graminifolius extract on luteinizing hormone (LH) levels. Control: Non-polycystic ovary syndrome (PCOS) group with no induction; PCOS: The PCOS group administered normal saline via ingestion; MET: Reference group receiving oral metformin at a dosage of 20 mg/kg each day; Extract 100: Experimental group treated with 100 mg/kg of the plant extract by mouth over a period of 28 days; Extract 200: Group administered the extract orally at a dose of 200 mg/kg across the same timeframe (values are shown as the mean ± SEM; statistical comparisons with the PCOS group are indicated by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
4.3. Follicle-Stimulating Hormone Analysis Results
The FSH levels across groups are shown in Figure 3. A significant decrease in serum FSH levels was observed in the PCOS group compared to the healthy control group (P < 0.0001), confirming successful induction of PCOS. Treatment with metformin and both doses of the extract (100 and 200 mg/kg) resulted in a statistically significant increase in FSH levels compared to the control group (P < 0.0001). However, none of the treatments fully restored FSH to the levels observed in the control group, but they did increase its level significantly compared to the PCOS group (****P < 0.0001).
Effects of different doses of hydroalcoholic Tragopogon graminifolius extract on follicle-stimulating hormone (FSH) levels. Control: Normal group with no induction of polycystic ovary syndrome (PCOS); PCOS: The PCOS group administered physiological saline by mouth; MET: Group receiving metformin at a daily dosage of 20 mg/kg as a reference treatment; Extract 100: Subjects treated with the plant-derived preparation at a concentration of 100 mg/kg via the oral route over a 28-day period; Extract 200: Subjects administered 200 mg/kg of the extract under the same regimen [results are shown as the average ± standard error (SEM); asterisks indicate statistically significant differences compared to the PCOS group. ****P < 0.0001].
4.4. Blood Glucose Level Analysis Results
The graphical display of blood glucose fluctuations among the experimental groups is shown in Figure 4. Relative to the healthy control animals, those in the PCOS group exhibited a significant elevation in glucose levels (P < 0.001). Treatment with metformin, as well as administration of T. graminifolius extract at 100 mg/kg and 200 mg/kg, led to notable reductions in blood glucose when compared to the PCOS group (P < 0.05 for the lower extract dose). Moreover, the group receiving 100 mg/kg of the extract demonstrated a more substantial decrease in glucose levels than the 200 mg/kg extract group (P < 0.05).
Effects of different doses of hydroalcoholic Tragopogon graminifolius extract on blood glucose levels. Control: Healthy group [without polycystic ovary syndrome (PCOS) induction]; PCOS: The PCOS group that received normal saline through the oral route; MET: Reference group administered metformin at a daily dose of 20 mg/kg; Extract 100: Experimental group treated with 100 mg/kg of the plant extract each day for a duration of 28 days; Extract 200: Experimental group given a daily dose of 200 mg/kg of the extract over the same period (outcomes are reported as mean ± SEM; significant differences from the PCOS group are indicated by *P < 0.05, ***P < 0.001, and ****P < 0.0001).
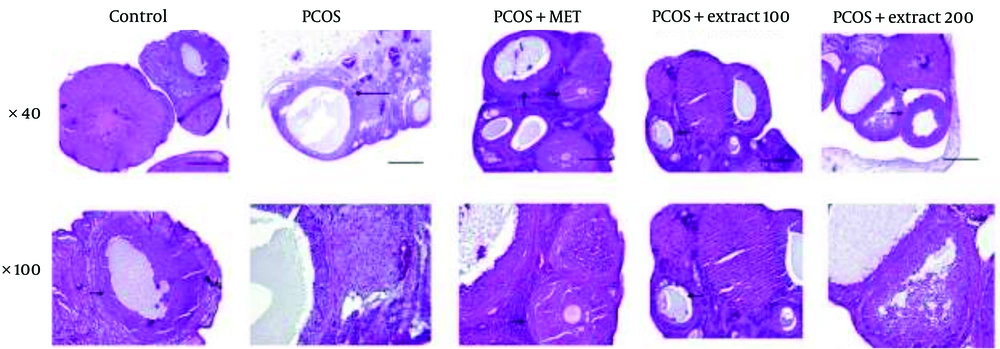
4.5. Histological Analysis Results
Histopathological findings for all groups are summarized in Figure 5. In the healthy control group, ovarian sections revealed numerous corpus luteum (CL) structures, indicating ongoing ovulation. Well-developed Graafian follicles with structurally normal granulosa cells were present, alongside early-stage primary and secondary (antral) follicles, with no signs of morphological disruption. In contrast, the PCOS group (negative control) exhibited severe disruption of normal ovarian architecture. No CL was observed, and large follicular cysts lined by a thin granulosa cell layer dominated the ovarian tissue. Early-stage and mature follicles were absent, confirming impaired folliculogenesis.
Histopathological analysis results: Representative histological sections of ovarian tissue stained with hematoxylin and eosin (H&E). Images are presented in two rows: The top row shows sections at 40x magnification, and the bottom row shows the corresponding sections at 100x magnification. Groups include: Healthy control, polycystic ovary syndrome (PCOS) (negative control), metformin-treated (PCOS + MET), extract treated at 100 mg/kg (PCOS + Extract 100), and extract-treated at 200 mg/kg (PCOS + Extract 200) (long arrow: Cyst, short arrow: Follicles; scale bar: 10 μm).
In the metformin-treated group (PCOS + MET), ovarian sections showed partial restoration of normal histology, including primary and pre-antral follicles, visible CL structures, and normalized granulosa cell layers. The group treated with 100 mg/kg of the extract (PCOS + Extract 100) displayed moderate improvement, with organized follicular architecture, oocyte-containing Graafian follicles, and active early-stage folliculogenesis. The group treated with 200 mg/kg of the extract (PCOS + Extract 200) showed the most substantial histological recovery. Large, structurally intact follicles with well-formed granulosa layers were observed, along with the presence of CL and follicular development suggestive of resumed ovulatory activity.
5. Discussion
As a multifactorial endocrine disorder, PCOS is characterized by hyperandrogenism, irregular ovulation, and metabolic disturbances. One of the early indicators of PCOS is an increase in androgen and estradiol production, which leads to disrupted steroidogenesis. Additionally, hormonal irregularities — including increased secretion bursts of the hormone responsible for stimulating gonadotropins (GnRH), a decline in FSH concentrations, and heightened levels of LH and anti-Mullerian hormone (AMH) — suggest impaired signaling along the regulatory pathway between the brain and the ovaries (32). The alteration of normal GnRH pulsatility is primarily driven by an excessive transformation of androgen precursors, including DHEA and testosterone, into estrone within fat tissue. This imbalance is further exacerbated by a decline in sex hormone-binding globulin (SHBG) levels and an increase in leptin secretion from adipose cells (33). The pathogenesis of PCOS is attributed to several mechanisms, including disrupted GnRH pulsatility leading to excessive LH secretion by the pituitary gland, elevated insulin levels, insulin resistance within the ovaries, dysfunction of theca cells, and an overproduction of androgens (34). The PCOS presents with a wide range of clinical features and is categorized into four recognized subtypes. The first type (phenotype A) involves elevated androgen levels, which may be identified through either clinical symptoms or laboratory measurements. The second type (phenotype B) includes increased androgens along with irregular or absent ovulation. In the third type (phenotype C), excess androgen production is observed alongside ovarian changes characteristic of polycystic morphology, though normal ovulatory function is maintained. In contrast, phenotype D is defined by the coexistence of PCOM and ovulatory dysfunction, without any indications of hyperandrogenism (35, 36). Standard approaches to managing PCOS involve changes in daily habits, the use of medications like insulin-sensitizing agents (e.g., metformin) or ovulation inducers [such as clomiphene citrate (CC)], and, when necessary, operative interventions (34, 37, 38). Individuals seeking pregnancy often require medications to stimulate ovulation. Extensive randomized clinical trials have identified letrozole as the most effective ovulation-inducing agent, while CC is recommended as the second-line treatment (39). However, due to the potential side effects and limited long-term efficacy of these treatments, there is growing interest in herbal and plant-based therapies as alternative approaches (40, 41).
Medicinal plants are increasingly recognized for their bioactive compounds, which exhibit antioxidant, anti-inflammatory, and hormone-modulating properties (40). Several botanical extracts, including those from Trifolium pratense (red clover), genistein, and soy, contain phytoestrogenic compounds that have demonstrated antiandrogenic effects in PCOS models (42-45). Phytochemicals such as flavonoids and polyphenols, due to their free radical-scavenging properties, help reduce oxidative damage within ovarian structures, supporting follicular recovery and enhancing ovulatory function. Moreover, certain plant-derived compounds exert negative feedback on LH secretion, thereby reducing androgen synthesis and restoring hormonal balance, a key mechanism in normalizing the ovarian cycle (46, 47). Various natural products and plant-based extracts offer a cost-effective alternative and have shown significant potential in managing PCOS. The therapeutic benefits of several novel nutraceuticals, such as Nardostachys jatamansi, Tribulus terrestris, Serenoa repens, Foeniculum vulgare, and Urtica dioica, have been well-documented in PCOS treatment (48-50). Trigonella foenum-graecum seed extract (Furocyst), enriched with furostanolic saponins, has also been used in reducing ovarian volume and decreasing the number of ovarian cysts (51). In addition, studies on Curcuma longa (turmeric) and Ficus deltoidea have demonstrated significant improvements in insulin resistance and modulation of reproductive hormonal levels in PCOS animal models (52, 53).
This research aimed to evaluate the impact of a hydroalcoholic extract derived from T. graminifolius on PCOS, using a rodent model developed through androgen administration. Our findings demonstrated that the extract significantly reduced testosterone levels and lowered LH concentrations, indicating a positive effect on endocrine function. Although an increase in FSH levels was observed, this change did not reach statistical significance. This discrepancy may reflect the differential sensitivity of gonadotropin regulation pathways to therapeutic intervention and highlights the complexity of fully restoring hypothalamic-pituitary-ovarian axis function in PCOS models. Similar patterns have been reported in prior studies, where LH and testosterone responded significantly to treatment while FSH changes remained modest or non-significant (54, 55).
Furthermore, a marked reduction in blood glucose concentration was noted, aligning with previous findings on the hypoglycemic effects of plant-based compounds (56). Histopathological analysis of ovarian tissues further revealed structural improvements, including the normalization of granulosa cells and the presence of mature follicles with intact layers, indicating enhanced ovarian function. Comparable studies have reported similar effects of plant-based interventions on PCOS-associated metabolic and hormonal abnormalities. For instance, research on Bryonia dioica and B. laciniosa has shown their efficacy in modulating reproductive hormones and improving glucose homeostasis in experimental models. Specifically, the ethanolic extract of B. laciniosa demonstrated hormonal modulation in PCOS rats by normalizing LH and FSH levels and improving insulin sensitivity; findings that mirror the hormonal improvements observed in our study. Prior studies have also highlighted the lipid-lowering properties of plant extracts, including reductions in low-density lipoprotein (LDL) and improvements in the LDL/high-density lipoprotein (HDL) ratio (57, 58). Our study supports these findings, as T. graminifolius extract was associated with improved metabolic parameters in PCOS rats.
The observed hormonal regulation aligns with prior studies on herbal treatments for PCOS. For example, Vitex agnus-castus has demonstrated promising antiandrogenic properties and the ability to normalize menstrual cycles in PCOS models by modulating pituitary GnRH. Likewise, Glycyrrhiza glabra (licorice) has been shown to significantly reduce testosterone levels and improve ovarian morphology in rodent models of PCOS (59, 60). While several studies have demonstrated the potential benefits of V. agnus-castus in managing PCOS symptoms, some findings suggest a more nuanced effect. For instance, a study observed that the extract significantly modulated progesterone and testosterone levels but did not influence estrogen and DHEA levels in PCOS-induced rats (61). Similarly, Norii et al. reported that the extract increased estrogen and progesterone levels without significantly affecting testosterone levels (62). Cinnamomum verum (cinnamon) has also been explored for its insulin-sensitivity effects, with clinical studies showing improvement in fasting glucose and insulin resistance in women with PCOS (63, 64). This aligns with our findings, where a significant decrease in blood glucose was observed following treatment with the extract. While cinnamon supplementation has been associated with improvements in insulin sensitivity and hormonal profiles in some studies, findings are not universally consistent. For instance, a pilot study reported reductions in ovarian volume and abdominal fat but no significant changes in Body Mass Index (BMI), lipid profiles, insulin resistance, or androgen levels following cinnamon supplementation in women with PCOS (65). Additionally, a meta-analysis highlighted significant reductions in insulin resistance markers with cinnamon intake; however, the heterogeneity among studies suggests variability in outcomes (66).
Although direct studies on T. graminifolius remain scarce, related species from the same genus have demonstrated anti-inflammatory and antioxidant activity, suggesting potential mechanisms underlying its observed therapeutic effects (67, 68). However, it is important to acknowledge that not all herbal interventions yield consistent results across studies. For example, a study on Stachys lavandulifolia in a PCOS rat model reported that while the extract brought certain endometrial tissue parameters closer to normal, these changes were not statistically significant. This highlights the variability in responses to herbal treatments and underscores the need for further research to elucidate their efficacy and mechanisms of action (69).
5.1. Conclusions
Our results indicate that the T. graminifolius hydroalcoholic extract may offer therapeutic benefits in a rat model of PCOS by improving ovarian tissue architecture, reducing blood glucose concentrations, and modulating reproductive hormones. The observed reduction in testosterone and LH levels suggests a potential role in restoring hormonal balance, although the increase in FSH levels did not reach statistical significance. Importantly, the extract significantly lowered elevated glucose levels, highlighting its relevance to the metabolic aspects of PCOS. Additionally, the normalization of ovarian histology in the treated groups supports its potential as a therapeutic agent for PCOS-related ovarian dysfunction. Further research, including molecular studies and clinical trials, is necessary to confirm these findings and elucidate the underlying mechanisms.
5.2. Limitations and Future Directions
Although the findings of this research support the potential of T. graminifolius in alleviating PCOS symptoms, certain constraints should be considered. The number of subjects involved was limited, and since the investigation was conducted using an animal model, additional confirmation through human clinical studies is required. Additionally, molecular analyses of insulin signaling pathways and androgen receptor activity could provide deeper insights into the extract’s mechanism of action. Future studies should also explore long-term effects, optimal dosing, and potential interactions with conventional PCOS treatments.
![Effects of different doses of hydroalcoholic <i>Tragopogon graminifolius</i> extract on follicle-stimulating hormone (FSH) levels. Control: Normal group with no induction of polycystic ovary syndrome (PCOS); PCOS: The PCOS group administered physiological saline by mouth; MET: Group receiving metformin at a daily dosage of 20 mg/kg as a reference treatment; Extract 100: Subjects treated with the plant-derived preparation at a concentration of 100 mg/kg via the oral route over a 28-day period; Extract 200: Subjects administered 200 mg/kg of the extract under the same regimen [results are shown as the average ± standard error (SEM); asterisks indicate statistically significant differences compared to the PCOS group. ****P < 0.0001]. Effects of different doses of hydroalcoholic <i>Tragopogon graminifolius</i> extract on follicle-stimulating hormone (FSH) levels. Control: Normal group with no induction of polycystic ovary syndrome (PCOS); PCOS: The PCOS group administered physiological saline by mouth; MET: Group receiving metformin at a daily dosage of 20 mg/kg as a reference treatment; Extract 100: Subjects treated with the plant-derived preparation at a concentration of 100 mg/kg via the oral route over a 28-day period; Extract 200: Subjects administered 200 mg/kg of the extract under the same regimen [results are shown as the average ± standard error (SEM); asterisks indicate statistically significant differences compared to the PCOS group. ****P < 0.0001].](https://services.brieflands.com/cdn/serve/3170c/f81864e2fea597f1ed861ba8f495ae4524f170ec/jjnpp-20-3-161729-i003-preview.webp)
![Effects of different doses of hydroalcoholic <i>Tragopogon graminifolius</i> extract on blood glucose levels. Control: Healthy group [without polycystic ovary syndrome (PCOS) induction]; PCOS: The PCOS group that received normal saline through the oral route; MET: Reference group administered metformin at a daily dose of 20 mg/kg; Extract 100: Experimental group treated with 100 mg/kg of the plant extract each day for a duration of 28 days; Extract 200: Experimental group given a daily dose of 200 mg/kg of the extract over the same period (outcomes are reported as mean ± SEM; significant differences from the PCOS group are indicated by *P < 0.05, ***P < 0.001, and ****P < 0.0001). Effects of different doses of hydroalcoholic <i>Tragopogon graminifolius</i> extract on blood glucose levels. Control: Healthy group [without polycystic ovary syndrome (PCOS) induction]; PCOS: The PCOS group that received normal saline through the oral route; MET: Reference group administered metformin at a daily dose of 20 mg/kg; Extract 100: Experimental group treated with 100 mg/kg of the plant extract each day for a duration of 28 days; Extract 200: Experimental group given a daily dose of 200 mg/kg of the extract over the same period (outcomes are reported as mean ± SEM; significant differences from the PCOS group are indicated by *P < 0.05, ***P < 0.001, and ****P < 0.0001).](https://services.brieflands.com/cdn/serve/3170c/5d1720a606382e9586deb24f6b4f58086321882a/jjnpp-20-3-161729-i004-preview.webp)