1. Background
Recurrent aphthous stomatitis (RAS) is a chronic, inflammatory, and recurrent ulcer in the oral cavity mucosa that occurs in both immunocompromised and immunocompetent individuals (1, 2). The ulcer can be single or multiple, painful, and may present with or without a fibrinous pseudomembrane (3). To date, the etiopathogenesis of RAS remains unclear (4). However, many researchers describe factors such as certain anemias, mechanical trauma to the mucosa, genetic background, stress, as well as some viral, fungal, and bacterial antigens as predisposing disorders (1, 2, 5, 6).
Despite the initial balance of the large microbial population of commensalism (such as some Streptococcal, Actinomycete, and fungal strains) in the oral cavity, as well as the acquisition of tolerance to microbial antigens (7, 8), there is the potential for RAS to be invoked by changes in the homeostasis of the predisposing host and pathological conditions. In fact, microbial agents are responsible for the development of RAS, either as etiopathogens or as antigen sources (9). To date, some bacterial and fungal infections have played a role in RAS (4).
Steroidal and non-steroidal drugs are mainly used in the management of RAS treatment, although they are not always effective. On the other hand, undesirable side effects have limited their use (10). Since no effective therapeutic strategy has been reported so far, the design of novel drugs with natural and plant sources is a positive effort to improve the disease, as plant compounds are safer, more reliable, less toxic, easier to use, more clinically effective, and mostly free of side effects (2). Furthermore, the antimicrobial potential of bioactive substances from many plants has been confirmed (11).
Cassia fistula is an ornamental tree belonging to the Caesalpinioideae family. Mexico, China, Brazil, Asia, and South and East Africa are some prominent areas where C. fistula can be cultivated (12). The extract (Ex) from different parts of the C. fistula plant is used for medical purposes due to its wide range of phenolic bioactive components, such as glycoside derivatives, tannins, flavonoid derivatives, and anthraquinones (12, 13). Antioxidant, anti-inflammatory, antibacterial (14), antifungal, antipyretic, antidiabetic, anticancer, cardioprotective, and blood fat reduction properties are among the outstanding biological roles of this medicinal plant (13, 15). In addition, the broad-spectrum antimicrobial activity of C. fistula Ex against infectious agents has been reported (14).
Rosa x damascena is an ornamental, aromatic, and medicinal plant from the Rosaceae family. Different types of R. x damascena are found all around the world, including Europe, Asia, the Middle East, and North America (16). The rich secondary metabolites found in R. x damascena essential oil (EO) are used in the pharmaceutical industry due to their various biological activities, such as anticancer (16), anti-dysmenorrhea (17), anti-colitis (18), anti-inflammatory, antioxidant (10, 19), and antibacterial (20) properties. Moreover, a double-blind study reported acceptable anti-aphthae activity of R. x damascena EO (21). According to data from some studies, compounds such as citronellol, geraniol, nerol, and phenylethyl alcohol are responsible for the antimicrobial potential of R. x damascena EO, as provided by the phenolic acids in C. fistula Ex (22, 23).
2. Objectives
Since many life-threatening diseases are associated with immunodeficiency, which can predispose individuals to RAS, we hypothesized that Ex combined with EO may exhibit higher antibacterial capacity and stronger therapeutic effects. In this study, for the first time, the antimicrobial activity of an herbal oral gel containing C. fistula Ex and R. x damascena EO for the successful treatment of RAS was evaluated. This research aims to play a small role in alleviating the suffering of these patients.
3. Methods
3.1. Extract/Essential Oil Preparation Process
3.1.1. Plant Materials and Identification
Fresh fruit of C. fistula and flowers of the R. x damascena plant were obtained from Ahvaz, Iran. The plants were confirmed by the Herbarium of the Faculty of Pharmacy, Department of Pharmacognosy, Jundishapur University, Ahvaz, Iran, and herbarium specimen numbers 1399.771 and A252450101FLP were assigned to C. fistula and R. x damascena, respectively. A voucher specimen is deposited in the herbarium of the Faculty of Pharmacy, Department of Pharmacognosy, Jundishapur University, Ahvaz, Iran.
3.1.2. Extraction and Preparation of Extract and Essential Oil
The crude Ex of C. fistula fruit was prepared using the maceration method. Briefly, 500 g of fresh and powdered C. fistula fruit was soaked in 70% ethanol (800 mL) for 72 hours. After filtration through No. 125 mm filter paper (Dorsan Quantitative Filter Paper, Barcelona), the fluid Ex was concentrated under reduced pressure using a rotary evaporator (Heidolph, Labrota 4011, Germany) and finally dried using a freeze dryer (Operon, EDCF 12006, South Korea). Additionally, the EO of 300 g of fresh R. x damascena flowers was separated by the hydrodistillation technique using a Clevenger Pyrex apparatus for 3 hours. Dehydration was performed using anhydrous sodium sulfate (Na2SO4). The EO was then stored in a closed container at -20°C until use.
3.1.3. Determination of Total Phenolic Content of the Extract
The phenolic content of the crude Ex of C. fistula fruit was measured using the Folin-Ciocalteu reagent and tannic acid (as a standard with phenolic content of 12.5, 25, 50, 75, and 100 μg/mL). A 0.5 mL sample solution (100 μg/mL) or tannic acid standard (100 μg/mL) was mixed with 2.5 mL of Folin-Ciocalteu reagent and kept at 37°C for 5 minutes. Then, 2 mL of 7.5% sodium carbonate was added and shaken. Subsequently, the absorbance of the sample solutions versus the blank sample (all reagents without sample or tannic acid) was measured after 2 hours of incubation in the dark at room temperature using a UV-visible spectrophotometer (Human X-MA 3200pc, South Korea) at a wavelength of 675 nm. The phenolic content of the test sample was expressed using the tannic acid absorption standard curve (triplicate of each sample).
3.1.4. GC-MS Analysis
The analysis of R. x damascena flower EO ingredients was performed using gas chromatography-mass spectrometry (GC/MS) (Shimadzu, QP2010SE model, Japan) equipped with a SE-30 capillary column (30 m length, 0.25 mm diameter, 0.25 μm film thickness). In this analysis, nitrogen (40 mL/min) was used as the carrier gas. The EO compounds and their Retention Index [calculated based on the retention time (RT) of n-alkanes] were identified based on the Wiley library and published articles (24, 25). Briefly, the column temperature was initially set at 40°C for 2 minutes and then increased to 200°C over 15 minutes at a rate of 10°C/min. The sample was injected at a temperature of 250°C with a detector temperature of 230°C.
3.2. In-vitro Antimicrobial Activity
3.2.1. Microbial Culture
The in-vitro antimicrobial activity of the crude Ex of C. fistula, EO of R. x damascena, the combination of both (Ex/EO), and the gel formulation (collection of samples) were assayed against two bacterial strains, Streptococcus mutans (PTCC 1683), Actinomyces viscosus (PTCC 1202), and one fungal strain, Candida albicans (PTCC 5027) using disc diffusion (DD) and broth microdilution (BMD) inhibitor methods according to protocol M27‑A3 of the Clinical and Laboratory Standards Institute (CLSI). The three microbial strains were purchased from the Iranian Microorganism Collection Center and activated according to the provided instructions. The stock cultures of S. mutans, A. viscosus, and C. albicans were kept on nutrient agar (NA), brain heart infusion (BHI), and sabouraud dextrose agar (SDA) media at 4°C, respectively.
3.2.2. Disc Diffusion Method
Six-millimeter paper discs were immersed in 30 μL of different concentrations (1.6, 1.7, 1.8, 1.9% w/v) of crude Ex of C. fistula or (0.6, 0.7, 0.8, 0.9% v/v) EO of R. x damascena, then allowed to dry at room temperature. A 0.1 mL microbial suspension of S. mutans, A. viscosus, and C. albicans was streaked on the surface of NA, BHI, and SDA, respectively. The discs were placed on them, and the plates were incubated at 37°C, 37°C in an anaerobic jar with 5% CO2, and 28°C for 24 hours, respectively. A diameter of the zone greater than 6 mm was considered indicative of inhibition.
3.2.3. Determination of Minimum Inhibitory Concentration
Minimum inhibitory concentration (MIC) is defined as the lowest concentration of the Ex or EO at which the microorganism is unable to grow. A cell suspension equivalent to 0.5 McFarland (1.5 × 106 CFU/mL) was achieved using sterile physiological saline from a 24-hour culture of each microorganism. Then, a final concentration of 1.5 × 103 CFU/mL was prepared in nutrient broth (NB), BHI, and sabouraud dextrose broth (SDB) for S. mutans, A. viscosus, and C. albicans, respectively. The plant Ex or EO was prepared in the medium in a two-fold dilution. A total of 100 μL of the serial dilution of the Ex or EO and 100 μL of the cell suspension (1.5 × 103 CFU/mL) were added to each well, with a final volume of 200 μL. The incubation time for S. mutans, A. viscosus, and C. albicans was 35°C for 24 hours, 28°C for 48 hours, and 25°C for 72 hours, respectively. Under the same conditions as the samples, norfloxacin for bacteria and fluconazole for fungi (as drug standards), as well as a microorganism-free well, were considered as positive and negative controls. The MIC values were determined using a loop and turbidimeter.
3.2.4. Determination of Minimum Bacterial Concentration
A total of 100 μL from each well without growth in the MIC method was transferred to a plate containing agar medium and incubated under the appropriate conditions as previously mentioned for each microorganism. The highest dilution that showed no growth of the microorganism on agar was considered the minimum bacterial concentration (MBC). We also tested mixing these two compounds according to the method described previously.
3.2.5. Determination of Hole Diffusion for Microbial Test of Gel
The appropriate agar plate with each microorganism was streaked with 100 μL of the prepared microbial suspension (1.5 × 103 CFU/mL), and then 8 mm holes were made in it. A total of 40 μL of the gel formulation was placed into each hole and incubated. Finally, the inhibitory zone diameter was measured using a caliper.
3.3. Preparation and Evaluation of Physicochemical Parameters of Gel Formulation
3.3.1. Preparation of Carbomer 940, SCMC, and HPMC Gel
For the design of all three gel formulations, the same amount of Ex from the C. fistula plant and R. x damascena EO was used. The gelling polymers Carbomer 940, CMC, and HPMC, along with other contents, are listed in Table 1. Initially, methylparaben, used as a preservative, was dissolved in two-thirds of the purified water of the formulation, which was heated to 80°C. Then, according to the gel formulation, Carbomer 940, CMC, or HPMC powder was gradually added to the previous solution and allowed to completely homogenize. Separately, C. fistula Ex was dissolved in one-third of the remaining water, and R. x damascena EO was dissolved in PEG. Then, C. fistula Ex and R. x damascena EO were added to the gel composition, respectively. Mixing with a magnetic stirrer was continued until the gel was completely uniform.
| Ingredients (g) | Formulation | ||
|---|---|---|---|
| Carbomer 940 | CMC | HPMC | |
| Carbomer 940 | 1 | ̵ | ̵ |
| CMC | ̵ | 6 | ̵ |
| HPMC | ̵ | ̵ | 2 |
| Methyl paraben | 0.1 | 0.1 | 0.1 |
| Cassia fistula Ex | 1.7 | 1.7 | 1.7 |
| Rosa x damascena EO | 0.7 | 0.7 | 0.7 |
| Triethanolamine | Q.S1 | ̵ | ̵ |
| PEG 400 | 10 | 10 | 10 |
| Water | 86.5 | 81.5 | 85.5 |
| Total | 100 | 100 | 100 |
Abbreviations: CMC, carboxymethyl cellulose; HPMC , hydroxypropyl methylcellulose; Ex, extraction; EO , essential oil; Q.S , quantum satis; PEG 400 , polyethylene glycol-400.
3.3.2. Macroscopic Evaluation
The appearance characteristics of the gel formulation, including color, odor, uniformity, transparency, and consistency, were examined 48 hours after preparation.
3.3.3. pH Determination
The pH of each gel formulation was measured and adjusted using a pH meter (Metrohm, 827 PH lab, Switzerland) and a standard buffer solution. The pH was measured to detect possible fluctuations 48 hours, 1 week, 2 weeks, and 3 weeks after preparation (triplicate for each formula).
3.3.4. Melting and Freezing Evaluation
To evaluate the stability of the gel formulation structure, 15 g of each product was kept for 6 consecutive periods of 48 hours at -8°C, followed by 48 hours at 25°C. After this process, the stability of the gel formulation was checked.
3.3.5. Viscosity Evaluation
The viscosity of each gel formulation was measured using a viscometer (Brookfield, DV-II+Pro, USA) with spindle number 63. The gel was placed into a suitable container, and the spindle was immersed in the gel up to the mark line. The viscosity was then measured at 5 different shear rates at room temperature.
3.3.6. In-vitro Release Test
Franz cells were used to evaluate membrane permeability in vitro. PBS buffer was placed into the receptor cell as a dissolution medium and shaken. Prior to this, pieces of cellulose-acetate membranes were macerated in distilled water. Five grams of each gel formulation was uniformly spread on the membrane using a spatula, and the cap was fixed on the cell. The receptor solution was adjusted to 37°C. Then, 2 mL of the sample was replaced with 2 mL of phosphate buffer at each time interval (15, 30, 60, 90, and 120 minutes). The absorption rate of the samples was measured, and the concentration was determined using the Folin-Ciocalteu method.
In general, an important aspect in the design of different types of drugs is the evaluation of drug release kinetics, as the release and consequently stable and regulated dissolution optimize the effect of the drug. To evaluate the kinetics of drug release in the gel formulation, the data were evaluated using three models: (1) Zero order, (2) first order, and (3) Higuchi model. Their release mechanism was calculated as follows.
1. Qt = K0t
2. Log C = Log C0 - kt/2.303
3. Qt = Kht1/2
3.3.7. Expiration Date
The expiration date of each gel formulation was determined as described by Amirjahid (26). Briefly, three samples of each formula were incubated separately at 45°C, 55°C, and 65°C. The phenolic content was measured using the Folin-Ciocalteu reagent until a 10% reduction was observed during the storage period. Based on the curve of the linear equation, stability was obtained at 25°C.
3.4. Statistical Analyses
Statistical analysis of the data was carried out using one-way ANOVA and SPSS16 software. P-values less than 0.05 were considered significant.
4. Results
4.1. Identification of Compounds
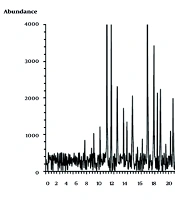
A total of 147 g of hydroalcoholic Ex was obtained from 500 g of C. fistula plant, yielding 29.4% dry Ex. According to the curve of the linear equation and the dilution factor, the phenolic content equivalent to tannic acid per mg of dry Ex was 48.26 mg per gram. Based on the GC/MS chromatogram (Figure 1), 64 peaks with different abundance percentages were identified. Due to the omission of the solvent peak, only the ingredients in R. x damascena EO were presented by the chromatogram. Two compounds, z-5-nonadecene and dibutyl phthalate, with RT of 24.42 and 23.34 minutes, respectively, and four functional groups — ether, ester, acid, and aldehyde — were indicated as the highest peaks. Following these, cosine henei, eugenol, and benzyl tiglate were detected as other important components with RTs of 31.74, 8.95, and 14.90 minutes, respectively. The details of other peaks and their abundance percentages are displayed in Table 2.
Gas chromatography-mass spectrometry (GC/MS) peak intensity chromatogram. Peaks are demonstrated in Table 2.
| Peak IDs | RT (27) | Retention Index | Abundance (%) |
|---|---|---|---|
| Geranyl isovalerate | 4.51 | 1054 | 0.311 |
| Butanoic acid, 3,7-dimethyl-2,6-octadienyl ester, (E)- | 5.72 | 1110 | 0.68 |
| 6-Octen-1-ol, 3,7-dimethyl-, formate | 6.71 | 1153 | 0.506 |
| Citronellol | 7.03 | 1170 | 0.829 |
| Geraniol | 7.94 | 1208 | 0.978 |
| Nonanoic acid | 8.41 | 1231 | 0.395 |
| Eugenol | 8.95 | 1257 | 4.937 |
| Methyleugenol | 9.60 | 1284 | 3.436 |
| Geranic acid | 10.46 | 1323 | 1.857 |
| Caparratriene | 11.39 | 1366 | 1.387 |
| Levomenol | 11.90 | 1388 | 1.085 |
| Alloaromadendrene | 12.71 | 1422 | 1.66 |
| Longifolene | 13.45 | 1459 | 0.532 |
| Fumaric acid, 2-phenethyl 2,2,3,3,4,4,5,5-octafluoropentyl ester | 14.15 | 1489 | 0.705 |
| Benzyl tiglate | 14.90 | 1524 | 4.443 |
| Azulene, 1,2,3,5,6,7,8,8a-octahydro-1,4-dimethyl-7-(1-methylethenyl)-, [1S-(1.alpha.,7.alpha.,8a.beta.)]- | 15.86 | 1565 | 2.767 |
| β-Bisabolene | 16.37 | 1591 | 1.722 |
| Guaia-9,11-diene | 16.81 | 1607 | 1.804 |
| 2-Phenylethyl tiglate | 17.59 | 1646 | 0.591 |
| β-Humulene | 18.30 | 1677 | 0.88 |
| β-Guaiene | 18.65 | 1690 | 1.604 |
| Naphthalene, 1,2,3,5,6,7,8,8a-octa | 19.01 | 1710 | 0.887 |
| Δ-Selinene | 19.55 | 1731 | 1.591 |
| Heptadecane | 20.40 | 1773 | 0.479 |
| α-Farnesene | 21.01 | 1796 | 1.913 |
| β-Myrcene | 21.13 | 1802 | 1.145 |
| Benzyl Benzoate | 21.70 | 1829 | 0.487 |
| Nerolidol | 21.85 | 1835 | 0.392 |
| Octadecane | 22.55 | 1869 | 0.315 |
| Z-5-Nonadecene | 23.34 | 1905 | 12.531 |
| Dibutyl phthalate | 24.42 | 1952 | 11.7 |
| 1,2-Benzenedicarboxylic acid, butyl 2-methylpropyl ester | 24.69 | 1966 | 0.677 |
| Eicosane | 26.05 | 2024 | 1.164 |
| Hexadecanoic acid, ethyl ester | 26.44 | 2040 | 0.601 |
| Octadecanal | 27.06 | 2070 | 1.167 |
| Hexadecanal | 27.19 | 2076 | 0.973 |
| Trans-2-Caren-4-ol | 27.97 | 2109 | 0.292 |
| Phthalic acid, neopentyl 2-phenylethyl ester | 28.89 | 2151 | 0.681 |
| Oxalic acid, decyl 2-phenylethyl ester | 29.61 | 2184 | 0.639 |
| Geranic acid, 2-Phenylethyl ester | 30.50 | 2224 | 0.367 |
| Trans-Farnesol | 30.63 | 2231 | 1.641 |
| 9-Nonadecene | 30.91 | 2245 | 0.326 |
| Heneicosane | 31.74 | 2279 | 5.106 |
| Linoleic acid ethyl ester | 32.28 | 2304 | 0.507 |
| 2,6-Dimethyl-2-trans-6-octadiene | 33.05 | 2340 | 2.283 |
| Squalene | 33.57 | 2365 | 0.708 |
| Docosane | 34.10 | 2389 | 1.201 |
| Butanoic acid, 3-methyl-, 2-phenyl ethyl ester | 34.59 | 2409 | 0.377 |
| 9-Tricosene, (Z)- | 35.23 | 2437 | 0.597 |
| Tricosane | 36.05 | 2473 | 0.86 |
| Pentadecane, 8-hexyl | 36.75 | 2507 | 1.545 |
| Hexadecane, 2,6,10,14-tetramethyl-Octacosane | 36.90 | 2511 | 0.584 |
| Citronellyl palmitoleate | 37.19 | 2526 | 0.663 |
| Tartaric acid, dimenthyl ester | 37.65 | 2549 | 0.376 |
| Tetracosane | 38.45 | 2583 | 1.017 |
| Docosanal | 39.06 | 2611 | 0.792 |
| 1,19-Eicosadiene | 39.17 | 2617 | 0.567 |
| Pentacosane | 40.11 | 2659 | 2.529 |
| Tetratetracontane | 40.30 | 2667 | 0.844 |
| Bis(2-ethylhexyl) phthalate | 41.15 | 2706 | 1.818 |
| Hexacosane | 41.65 | 2729 | 1.811 |
| Felbamate | 42.02 | 2745 | 0.641 |
| Azetidine, 1-chloro-2-phenyl- | 42.20 | 2754 | 0.942 |
| Citronellyl palmitoleate | 42.57 | 2768 | 1.159 |
Abbreviation: RT, retention time.
4.2. Microbial Test Result
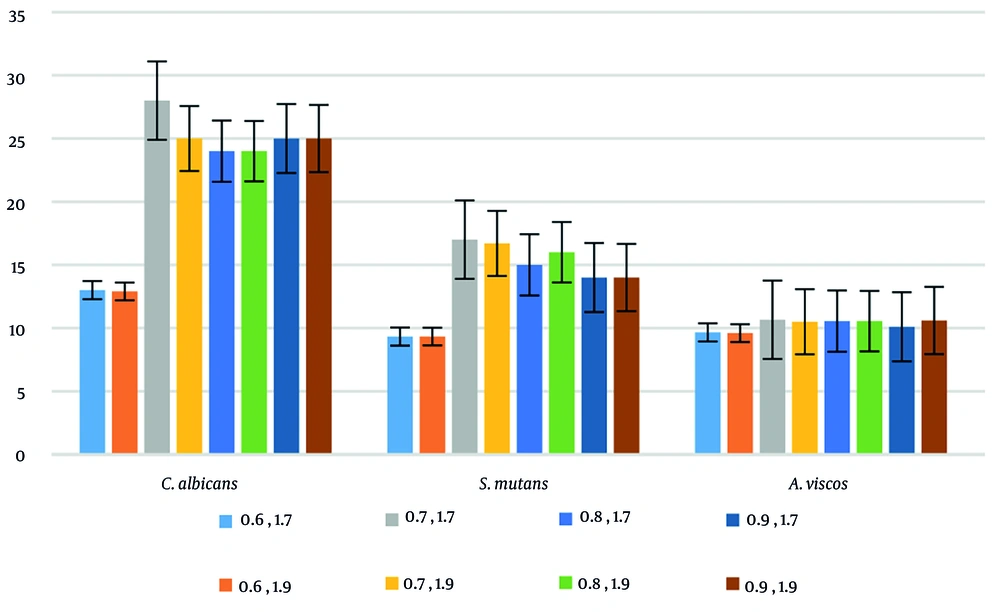
The Ex from C. fistula fruit displayed significant antimicrobial activity against S. mutans, A. viscosus, and C. albicans. Similarly, the test of R. x damascena flower EO yielded excellent results. Notably, an increase in the inhibitory effect with higher concentrations of C. fistula Ex or R. x damascena EO was observed in the results of both the DD and BMD methods. However, no statistically significant difference was observed between the inhibitory effects of two concentrations, 1.7% and 1.9% w/v (P > 0.05) in the former, and 0.7% and 0.9% v/v (P > 0.05) in the latter, for all three microorganisms. Interestingly, the collection of samples showed the highest and lowest inhibitory zone diameters against C. albicans and A. viscosus, respectively (Table 3).
| Microbial Strains | Zone of Inhibition (mm) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EO of R. x damascena | Ex of Cassia fistula | Ex/EO | Gel Formulation (1.7 + 0.7) | |||||||||||
| 0.6 | 0.7 | 0.8 | 0.9 | (Ref ST) | 1.6 | 1.7 | 1.8 | 1.9 | (Ref ST) | 1.7 + 0.7 | ||||
| NOF | FCZ | NOF | FCZ | |||||||||||
| C. albicans PTCC 5027 | 15.33 | 25 | 25 | 25 | 20 | 22 | 18 | 19.66 | 20 | 21 | 20 | 25 | 28 | 23 |
| Streptococcus mutansATCC 1683 | 12.69 | 14.66 | 15 | 16 | 19 | 16 | 11 | 13.33 | 13.66 | 13.74 | 18 | 18 | 17 | 14.66 |
| Actinomyces viscosusPTCC 1202 | 7 | 9.66 | 9.66 | 9.58 | 17 | 17 | 9 | 12.33 | 12.66 | 12.33 | 17 | 16 | 10.66 | 7.50 |
Abbreviations: EO, essential oil; Ex, extract; NOF, norfloxacin; FCZ, fluconazole.
The MIC value of the collection of samples against C. albicans was significantly lower compared to the two tested bacterial strains. Meanwhile, the lowest inhibitory effect of C. fistula Ex and R. x damascena EO was observed against A. viscosus. In relation to MBC values, with the exception of the Carbomer 940 gel results, no noticeable changes were observed in other concentrations. The results of the DD test, MIC, and MBC values of the collection of samples are illustrated in Table 4.
| Microbial Strains | MIC (mg/mL) | MBC (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| EO of Rosa x damascena | Ex of Cassia fistula Plant | Ex/EO | Gel Formulation | EO of R. x damascena | Ex of C. fistula Plant | Ex/EO | Gel Formulation | |
| Candidaalbicans PTCC 5027 | 1.56 | 3.125 | 3.125 | 3.125 | 1.56 | 3.125 | 3.125 | 6.25 |
| Streptococcus mutansATCC 1683 | 3.125 | 6.25 | 6.25 | 12.5 | 3.125 | 6.25 | 6.25 | 12.5 |
| Actinomyces viscosus PTCC 1202 | 6.25 | 12.5 | 6.25 | 12.5 | 6.25 | 12.5 | 12.5 | 12.5 |
Abbreviations: MIC, minimum inhibitory concentration; MBC, minimum bacterial concentration; EO, essential oil; Ex, extract.
The results of the combination showed that the most appropriate concentration of Ex in combination with EO against all three microorganisms was 1.7% and 0.7%, which led to stronger inhibitory results (Figure 2).
4.3. Physicochemical Parameters Results
The HPMC formulation lost its stability immediately after adding the C. fistula Ex, so it was excluded from further studies. However, the monitoring of the two Carbomer 940 and CMC formulations for 21 days indicated satisfactory appearance, appropriate consistency, physical uniformity without bubbles, and no change in smell. Other physicochemical properties were tested on these two formulas. The evaluation of Carbomer 940 and CMC formulations for pH changes, according to Table 5, ranged from 7.21 ± 0.03 to 6.87 ± 0.04.
| Time | pH (Mean ± SD ) | |
|---|---|---|
| Carbomer 940 | CMC | |
| 2 day | 7.21 ± 0.03 | 7.21 ± 0.05 |
| 1 week | 6.93 ± 0.02 | 7.21 ± 0.04 |
| 2 week | 6.91 ± 0.01 | 7.10 ± 0.02 |
| 3 week | 6.87 ± 0.04 | 6.98 ± 0.00 |
Abbreviation: CMC, carboxymethyl cellulose.
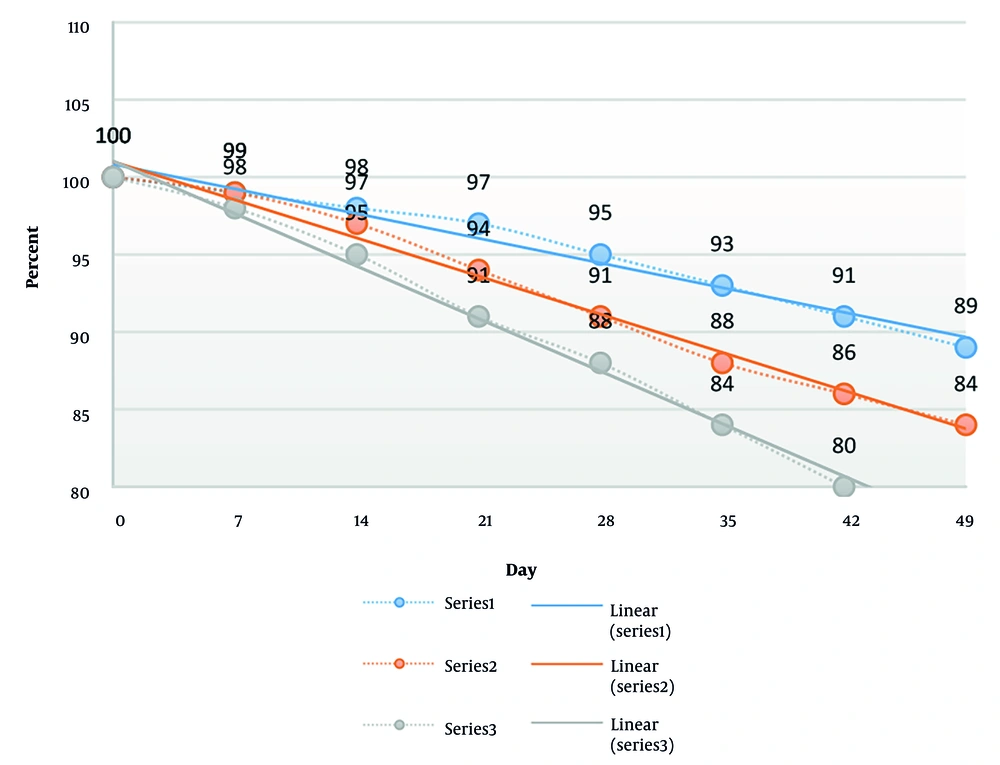
Investigation of the melting and freezing of the two gel formulations over three periods of 48 hours showed no change in appearance. The results of the percentage of the remaining drug versus different temperatures are shown in Figure 3. The expiration date of the Carbomer 940 formulation was calculated to be 65 days. Notably, the CMC formulation was removed from further study due to the loss of stability at 45°C.
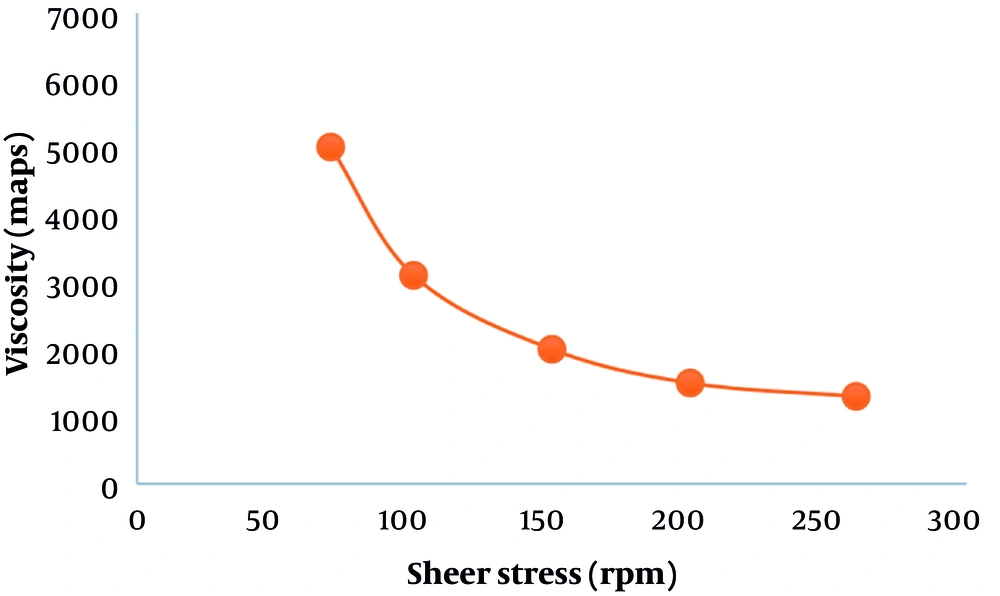
Based on Figure 4, with the increase in shear speed, the viscosity of the Carbomer 940 formulation decreased, which explains the lack of initial stress.
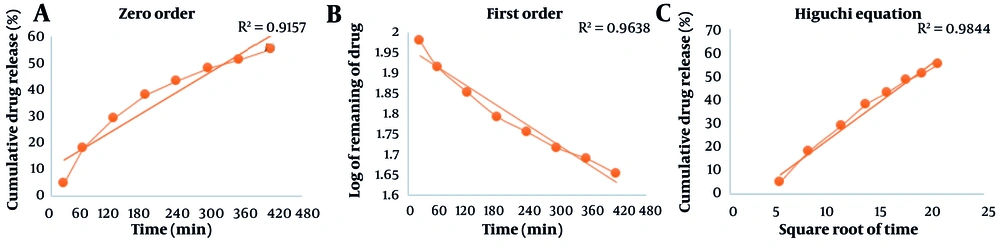
The results of the release of the active ingredient were depicted using three diagrams: Zero order (% of substance released over time), first order (logarithm of remaining drug percentage over time), and the Higuchi model (% of drug released based on the square root of time) in the release kinetics (Figure 5).
5. Discussion
Up to 25% of the world population has been infected with oral aphthous more than once in their lifetime (1, 2). According to several manuscripts, ulcer healing takes about 10 - 14 days, and symptomatic treatment is generally aimed at reducing the duration of the ulcer presence in the oral mucosa (1, 3, 28, 29). Hence, it is necessary to search for and provide novel drugs with a longer half-life, better adhesion, free from significant side effects, and the ability to adapt to the physicochemical conditions of the oral cavity to increase therapeutic performance.
In the present research, we evaluated Carbomer 940, CMC, and HPMC polymers in combination with C. fistula Ex and R. x damascena EO to achieve an ideal formulation. Prior to this, we also investigated the antimicrobial aspect. Overall, a relatively higher antifungal role of C. fistula Ex was observed compared to its antibacterial activity. In agreement with our study, Yadava and Verma reported that the antifungal activity of C. fistula was higher compared to its antibacterial activity (30). Additionally, several studies have shown good antimicrobial activity of C. fistula Ex against some bacterial and fungal strains (31, 32). Based on GC/MS data analysis, we identified various ingredients in R. x damascena EO, such as citronellol, geraniol, and nerol, whose antimicrobial potential has been mentioned in previous research (22, 23). In two studies by Fajdek-Bieda et al. and Wei et al., antioxidant, anti-inflammatory, and antimicrobial activities of citronellol, geraniol, and nerol were observed against fungi and a wide range of gram-negative and gram-positive bacteria (33, 34) However, the quantity of these ingredients was negligible compared to the major ingredients detected, such as z-5-nonadecene, dibutyl phthalate, cosine henei, eugenol, and benzyl tiglate. On the other hand, the inhibitory zone diameter, MIC, and MBC values showed significant antimicrobial activity from R. x damascena EO against the studied microorganisms, suggesting that in addition to the aforementioned ingredients, some other compounds in the EO may have antimicrobial capacity.
A study by Ozkan et al. reported the antimicrobial capacity of R. x damascena Ex against all the studied bacteria except one (35). Since the antimicrobial activity of Ex or EO mainly depends on chemical compounds, the differences in results can be attributed to the preparation method, the parts of the plant used, and the plant origin, which subsequently affect the quality and quantity of the effective ingredients. Additionally, it may be due to the different sensitivity of target microbes (6, 36).
In comparing the behavior of our studied microorganisms versus Ex and EO, R. x damascena EO was more effective than C. fistula Ex. Candida albicans (with an inhibitory zone diameter of about 18 - 20 mm for Ex and 15.3 - 20 mm for EO) appeared more sensitive than the two bacterial strains. Furthermore, the lowest inhibitory reaction in the present research was with A. viscosus, with an inhibitory zone diameter of about 9 - 12.66 mm for Ex and 7 - 9.66 mm for EO. Notably, there was an acceptable similarity between the results of the MIC and DDT methods (Table 3). In this regard, some studies have pointed to the structural differences in the outer membrane of gram-positive and gram-negative bacteria, which can be generalized to the presence of ergosterol in the plasma membrane of fungi (37, 38).
We mixed different concentrations of Ex and EO to achieve synergism, as we found that the antimicrobial activity of both Ex and EO was dose-dependent. We considered a concentration of the Ex/EO mixture that had a higher inhibitory effect than either EO or Ex alone as synergistic. Following this, we combined the highest synergy concentration of Ex/EO (1.7/0.7) with Carbomer 940, CMC, or HPMC polymers. Despite the observed additive antimicrobial effect of the Ex/EO mixture, when the mixture was combined with the gel, the zone of inhibition values decreased from 28, 17, and 10.66 mm to 23, 14.66, and 7.5 mm for C. albicans, S. mutans, and A. viscosus, respectively. However, it was stronger compared to the EO or Ex individually. This may be due to the interaction between the Ex/EO mixture and the gel structure, which leads to a slow release of the active ingredient, as increased contact time, slow release, and increased drug absorption are prominent features of the gel (11).
Notably, the physical mixture of HPMC and Ex/EO illustrated the formation of two separate phases and a lack of adaptability, so this formulation was removed from further research. The range of pH changes of the remaining two formulations was between 7.21 ± 0.03 and 6.87 ± 0.04. Since the recommended pH under normal conditions for the oral cavity is 6.2 - 7.6 (39), these formulations were safe to use and did not irritate the oral cavity. The two formulations presented an acceptable and unchanged appearance in the cooling and heating test. However, when checking the thermal stress to calculate the expiration date, we encountered the instability of the CMC formulation. Hence, due to the lack of required quality, the CMC formulation was discarded from further research. Other tests were carried out on the Carbomer 940-based gel.
We analyzed the viscosity of the Carbomer 940 formulation. Increasing the shear rate led to pseudo-plastic behavior and reduced viscosity. Clearly, the decrease in viscosity is associated with the increase in drug release (6). Additionally, we found that based on the three models — zero-order, first-order, and Higuchi — the release process of the active ingredient from the matrix in vitro follows the diffusion mechanism.
This present study has some limitations. The study was conducted on standard strains, while microorganisms isolated from patients under clinical conditions may show different results. Therefore, it is suggested that this study be conducted as a comparison between clinical isolates and standard strains.
5.1. Conclusions
The combination of Ex/EO showed relatively good antibacterial and antifungal activity against the tested bacterial and fungal strains, which requires further studies to isolate and highlight the main compounds more effective in antimicrobial activity. According to the present study, the gel formulation based on Carbomer 940, with a completely uniform appearance, pH compatible with the oral cavity, suitable viscosity, acceptable dissolution and diffusion, maintaining stability, and an expiration date of 65 days, was the only ideal candidate with evident and acceptable antimicrobial performance in vitro. However, focusing on the clinical use of this formulation, due to genetic diversity and differences in pathogenic factors, requires the design of a new in vivo study in this field.